Cryptosporidiosis, a parasitic disease that causes watery diarrhoea, affects 1–2 persons/100 000 population annually in the USA [Reference Yoder and Beach1]. Faecal–oral transmission of Cryptosporidium occurs through ingestion of contaminated drinking or recreational water, consumption of contaminated food, or contact with infected persons or animals [Reference Dillingham, Lima and Guerrant2, 3]. The typical incubation period for cryptosporidiosis is 7 days (range 1–12 days). Cryptosporidium oocysts are highly resistant to chlorine disinfection and can survive for days in water with recommended residual chlorine levels (1–3 ppm) [Reference Korich4]. Because of its chlorine resistance, environmental stability, and low infectious dose, Cryptosporidium is the leading cause of gastroenteritis outbreaks associated with chemically treated swimming venues in the USA [Reference Dillingham, Lima and Guerrant2, Reference Dziuban5]. Nitazoxanide, the only licensed treatment for Cryptosporidium-induced diarrhoea, was Food and Drug Administration-approved for use in children aged 1–11 years in 2002 and in persons aged ⩾12 years in 2004 [Reference Fox and Saravolatz6, 7].
In August 2006, a parent notified Tri-County Health Department (TCHD), Colorado, USA, of children experiencing gastrointestinal symptoms; the only common exposure was a birthday party held at an indoor community swimming pool. We investigated the outbreak to identify the illness aetiology and implement environmental control measures. After Cryptosporidium was identified, TCHD received additional notifications about household transmission and persistent symptoms after treatment. In response, we conducted a descriptive follow-up survey to assess disease burden and treatment response. This report describes the outbreak investigation and follow-up survey.
A cohort study was conducted among 37 persons from eight households who were invited to the pool party. Initial telephone inquiries determined that those not attending had experienced no gastrointestinal illness. All 21 attendees completed an internet-based questionnaire assessing food and pool exposures and illness status; 95% completed the survey within 2–3 weeks of the pool party. A primary cryptosporidiosis case was defined as diarrhoea (three or more loose stools per day), vomiting, or abdominal pain/cramping that occurred 1–12 days after the pool party in an attendee. Twelve (57%) primary cases were identified in 21 attendees with a median incubation period of 6 days (range, 3–9 days) (Fig. 1). Eighty-three percent of primary cases occurred in children (median age 7 years, range 5–38 years). According to the initial questionnaire, the median illness duration was 6·5 days, but 7/12 patients reported still being sick. Self-reported symptoms included watery diarrhoea (100%), abdominal cramps (92%), nausea (83%), fatigue (83%), loss of appetite (83%), fever (50%), vomiting (33%), and body aches (33%). One patient aged 7 years was immunocompromised.
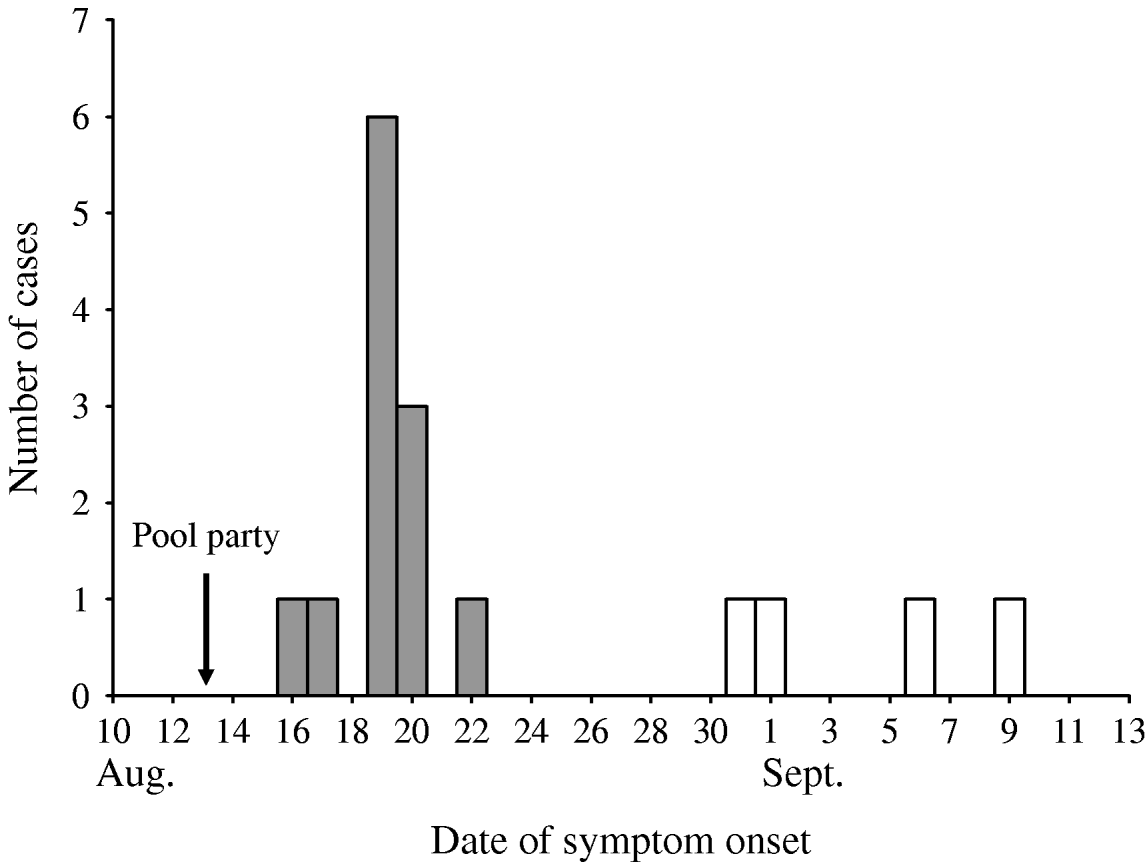
Fig. 1. Date of symptom onset for primary (
![]() ) and secondary (□) cryptosporidiosis cases in persons invited to the pool party, August–September 2006.
) and secondary (□) cryptosporidiosis cases in persons invited to the pool party, August–September 2006.
To assess risk factors for cryptosporidiosis, we calculated unadjusted relative risks (RR) by using SAS® version 9.1 (SAS Institute Inc., USA) and P values and 95% confidence intervals (CI) by using exact non-parametric procedures available in StatXact® version 7 (Cytel Inc., USA). The risk for illness was greater in attendees who had entered the pool and in swimmers who got water in their mouth or swallowed water (Table 1). Illness was not associated with any food items served at the party.
Table 1. Risk for primary cryptosporidiosis infection in pool party attendees, August 2006
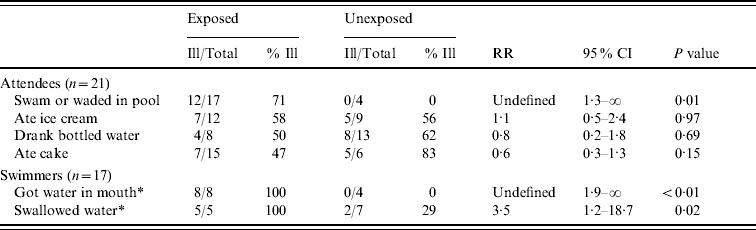
CI, Confidence interval; RR, relative risk.
* Total number of exposed and unexposed persons does not add up to 17 because of missing data.
A follow-up survey of the cohort was conducted 7–8 weeks after the pool party. Thirty-three (89%) of 37 persons from 7/8 households completed a telephone-based questionnaire assessing secondary transmission, symptom duration, and treatment. A secondary cryptosporidiosis case was defined as diarrhoea, vomiting, or abdominal pain/cramping that occurred >12 days after the pool party in a non-attendee. Four (25%) secondary cases (three adults, one child) from two households were identified in 16 non-attendees with onset 18–27 days after the pool party (Fig. 1).
Of the 15 (11 primary, four secondary) cryptosporidiosis patients who completed a follow-up survey, 80% reported having recurring symptoms and the median illness duration including relapses was 26 days (range 10–42 days). Thirteen patients (four adults, nine children) who sought medical care were treated with age-appropriate dosages of nitazoxanide [7]; none were hospitalized. Of nine immunocompetent patients (four adults, five children) who were symptomatic at the start of treatment, the median time from treatment initiation to diarrhoea resolution was 5·0 days (range 3–18 days). Six of nine immunocompetent patients reported diarrhoea resolution by day 7 of treatment for a 67% clinical response rate; two of the remaining three patients received a second round of nitazoxanide. The immunocompromised child had diarrhoea resolution on day 14 of treatment.
Bulk stool samples from seven primary and two secondary cases were tested at the Colorado Department of Public Health and Environment (CDPHE) or a commercial laboratory. Four of seven samples from primary cases were sent to the Centers for Disease Control and Prevention (CDC) for species analysis by polymerase chain reaction and genotyping based on gp60 gene sequencing [Reference Xiao8]. Cryptosporidium was detected in all nine samples. Four samples tested at CDC contained Cryptosporidium hominis genotype IbA10G2.
An environmental inspection revealed that the pool had consistently met appropriate chlorination standards and had used UV light irradiation disinfection procedures and high-rate sand filtration. Pool logs indicated non-diarrhoeal faecal incidents 8 days before and 5 days after the pool party. A 1-litre backwash sample of pool water and sand filter was collected 17 days after the pool party (before hyperchlorination) and tested at CDC. No parasites were identified in the sample.
This report describes a cryptosporidiosis outbreak epidemiologically linked to contaminated pool water at a community swimming pool. Identification of the same genotype of C. hominis implies a single, human source of faecal contamination. We surmise that a faecal incident occurred but was not reported to the pool management. Undetected faecal incidents (including diarrhoeal accidents and swim diaper leakage) continue to be problematic for maintenance of a healthy swimming environment; thus, patrons should be educated to refrain from swimming while ill with diarrhoea and to report all faecal incidents to pool staff.
This study provides two unique contributions to the literature. First, this cryptosporidiosis outbreak occurred despite a well-maintained, state-of-the-art pool operation system that included UV light irradiation, a supplemental disinfection technology that inactivates Cryptosporidium [Reference Bukhari9, Reference Rochelle10]. We are not aware of other published studies describing cryptosporidiosis outbreaks at recreational water facilities that used supplemental disinfection. UV disinfection occurs in line with the pool filtration system, so the speed by which Cryptosporidium oocysts are inactivated is dependent upon the pool water recirculation rates. Thus, UV light technology reduces the duration of Cryptosporidium transmission from several days to a few hours compared to chlorination alone. Since supplemental disinfection does not eliminate Cryptosporidium transmission, pool operators should adhere to newly revised CDC disinfection recommendations immediately after a diarrhoeal faecal incident (http://www.cdc.gov/healthyswimming/).
Second, we were able to conduct a follow-up study that provided detailed information on disease burden and treatment response, neither of which are commonly reported in outbreak investigations. During this outbreak, a high disease burden was observed despite the availability and use of a therapeutic agent. The 6·5-day symptom duration reported during the initial outbreak investigation is in agreement with the 4- to 9-day duration reported during previous outbreaks [Reference MacKenzie11, Reference Wheeler12]; however, the follow-up survey revealed that the majority of patients experienced recurring symptoms lasting more than 3·5 weeks. The 25% secondary attack rate observed in this study is considerably higher than the 5–10% reported previously [Reference Insulander13, Reference MacKenzie14], but was derived from a small sample size. The elevated occurrence of secondary transmission in this outbreak is probably a reflection of Cryptosporidium's high infectivity and the parents' reported use of chlorine bleach and other chlorine-based cleaning products, which are ineffective against Cryptosporidium. To reduce household transmission, health departments should inform the public that cleaning with hydrogen peroxide [Reference Weir15] or boiling water is recommended to inactivate Cryptosporidium.
A 67% clinical response rate for nitazoxanide was observed during our study. Two clinical trials in immunocompetent children and adults in Egypt reported a 80–96% clinical response rate [Reference Rossignol, Ayoub and Ayers16, Reference Rossignol17]. Additionally, the time from treatment initiation to diarrhoea resolution was 2 days longer in our study compared with published data [Reference Rossignol, Ayoub and Ayers16]. Although our cohort has a small sample size and our methodology is not directly comparable to the clinical trials mentioned above, we believe that reports from the field on practical applications of drugs in outbreak settings can be useful and that the efficacy of nitazoxanide in immunocompetent cryptosporidiosis patients in the USA should be examined in future outbreaks and in clinical settings.
This study is subject to at least two limitations. First, additional case ascertainment might have better determined the extent of the outbreak beyond the cohort of party invitees. Second, the follow-up study was limited by sample size and lack of a comparison group. Nevertheless, results from our study contribute to the literature by documenting disease burden and treatment response during a cryptosporidiosis outbreak linked to a community swimming pool that used supplemental UV disinfection in addition to standard procedures.
ACKNOWLEDGEMENTS
The authors thank the following persons for their contributions and assistance during this investigation: Michael Beach, PhD, Centers for Disease Control and Prevention; Alicia Cronquist, MPH, Colorado Department of Public Health and Environment; Cynthia Bruso, Dan Collins, MA, Donna Hite-Bynum, Laura DeGolier, MPH, Jill Swope, Jennifer Patnaik, MHS, Gary Sky, Anita Watkins, MPH, Stacy Weinberg, MA, Lloyd Williams, MA, and Bruce Wilson, MPA, Tri-County Health Department; and Chris Helm and Sarah Rodriguez from the swimming pool facility.
N. B. Alden is currently with Boulder County Public Health, Boulder, Colorado.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.




