Carotenoids are a class of >700 tetraterpenoid compounds found in nature. These plant pigments contribute to a plethora of biological functions, due to their unique chemical features(Reference Britton1), where they play important roles in both plants (e.g. the regulation of light in oxygenic photosynthesis(Reference Young and Frank2)) and animals (e.g. precursors in the formation of vitamin A(Reference Harrison3)).
Carotenoids contain a conjugated system of double bonds, known as a polyene backbone, which is capped with two end groups. The backbone is responsible for their respective photochemical properties (short-wavelength light absorption) and chemical reactivity (antioxidant capacity), whereas the carotenoids are identified and defined primarily by their respective end groups. One or both end groups can undergo cyclisation as well as substitution with oxygen-containing groups (i.e. keto, hydroxy or epoxy groups), which is why there is such a large variety of carotenoids in nature. Oxygen-containing carotenoids are referred to as xanthophylls (e.g. lutein (L), zeaxanthin (Z) and meso-zeaxanthin (MZ)), while the true hydrocarbon carotenoids are referred to as carotenes (e.g. α- and β-carotene) (Fig. 1).

Fig. 1 Chemical structures of the three macular carotenoids lutein, zeaxanthin and meso-zeaxanthin and β-carotene.
L, Z and MZ are the predominant xanthophyll carotenoids found in the macula(Reference Bone, Landrum and Hime4), the central part of the retina responsible for fine detail vision(Reference Hirsch and Curcio5). The macula accumulates these yellow carotenoids and excludes all the other thirty or so circulating carotenoids(Reference Khachik, Spangler and Smith6), and are collectively known as macular pigments (MP) in this specialised tissue(Reference Bone, Landrum and Tarsis7).
There is a consensus that the macular carotenoids play an important role in protecting the macula from the damaging effects of reactive oxygen species(Reference Beatty, Koh and Henson8). Reactive oxygen species, which can exist as either free radicals or non-radical sub-groups, are produced as a result of oxygen metabolism, and their generation is increased when the tissue is exposed to short-wavelength (blue) light and to other environmental (pollution) and lifestyle (smoking) factors(Reference Snodderly and Hammond9). Interestingly, the polyene chains of L, Z and MZ have the ability to quench reactive oxygen species(Reference Trevithick-Sutton, Foote and Collins10, Reference Pintea, Socaciu and Rugina11) and absorb short-wavelength (blue) light(Reference Britton1, Reference Trevithick-Sutton, Foote and Collins10, Reference Junghans, Sies and Stahl12). Of interest, a study by Li et al. reported that a mixture of L, Z and MZ in vitro, in a ratio of 1:1:1 (the ratio of these carotenoids typically seen in normal MP)(Reference Bone, Landrum and Friedes13), can quench more singlet oxygen than the individual carotenoids at the same total concentration, and may explain the exquisite biological selectivity and spatial distribution of these pigments within this specialised retinal tissue(Reference Li, Ahmed and Bernstein14). The aforementioned properties of the macular carotenoids are believed to confer protection against age-related macular degeneration (AMD), the most common cause of blind registration in the developed world(Reference Bressler15, Reference Loane, Kelliher and Beatty16). Also, the optical (short-wavelength filtering) properties of the macular carotenoids suggest that they play a role in visual function, by reducing the effects of chromatic aberration (and therefore improving image quality) and light scatter (and therefore reducing the symptoms of glare)(Reference Loughman, Davison and Nolan17–Reference Stringham, Garcia and Smith19).
L and Z cannot be synthesised de novo in mammals and must be obtained from the diet. L and Z are found in common foodstuffs such as fruits (e.g. kiwi) and vegetables (e.g. spinach and maize)(Reference Perry, Rasmussen and Johnson20), whereas MZ has not been identified in these foods, although it is important to point out that there has been no published study on MZ concentrations in foods of a typical diet (including fruits and vegetables) as yet. However, Maoka et al. (Reference Maoka, Arai and Shimizu21) have shown MZ to be present in some unusual foods, such as fish skin and turtle fat. Interestingly, MZ accounts for one-third of MP at the macula, and simian experiments suggest that it is produced by isomerisation of L(Reference Johnson, Neuringer and Russell22).
Many studies have reported serum responses to supplemental L and Z, but only three trials have reported on responses to supplemental MZ(Reference Connolly, Beatty and Thurnham23–Reference Thurnham, Tremel and Howard25), as this carotenoid was only identified as being present at the macula in 1993(Reference Bone, Landrum and Hime4). However, a recent clinical trial by Connolly et al. (Reference Connolly, Beatty and Loughman26) reported a rapid serum and MP response to a supplement containing all three macular carotenoids and, importantly, none of these trials reported any adverse effects associated with consumption of the macular carotenoids.
The present study was designed to investigate serum carotenoid responses to supplements containing at least two of the three macular carotenoids (i.e. a macular carotenoid supplement comparison study) in subjects with and without AMD.
Methods
Subjects
This was a randomised and double-blind study. All subjects signed an informed consent document and the experimental measures conformed to the Declaration of Helsinki. The study was reviewed and approved by the Research Ethics Committees, South East Region, Waterford Regional Hospital, and the Ethics Committee of the Waterford Institute of Technology, Waterford, Ireland.
We were interested in studying two different subject populations, those with and without AMD. These two populations were recruited under the following criteria. Normal subjects were those with no ocular pathology and in good health. Subjects suffering from AMD were defined as those with signs of early AMD, exhibiting drusen and pigmentary changes. The AMD subjects were identified at a pre-project enrolment and screening visit, conducted by an ophthalmologist with a special interest in retinal disease and experienced in the classification of AMD for research purposes (S. B.)(Reference Neelam, Muldrew and Hogg27). Exclusion criteria comprised past or present use of supplemental macular carotenoids and/or pregnancy. Subject BMI was calculated (kg/m2). Height (m) was measured using a Leichester Height Measure and weight (kg) was measured using SECA weighing scales (SECA). Smoking status was identified as one of three positions: current smoker, ex-smoker and never smoker. A subject's weekly intake of carotenoid-rich foods (eggs, broccoli, maize and dark leafy vegetables) were inputted into the ‘L/Z screener’ to give a carotenoid diet ‘score’. Values were weighted for frequency of intake of the food and for the bioavailability of L and Z within these foods, and a ranking score reflecting the relative intakes was generated. The range of scores from the L/Z screener is 0 to 75. After adding foods with known concentrations of L and Z into the screener, the following estimates were made. Low dietary carotenoid intake score is from 0 to 15 (i.e. ≤ 2 mg/d); medium dietary carotenoid intake score is from 16 to 30 (i.e. between >2 and 13 mg/d); and high dietary carotenoid intake score is from 31 to 75 (i.e. >13 mg/d).
Originally, seventy-two subjects were enrolled into the study. However, as the use of supplemental macular carotenoids was an exclusion criterion, and following analysis of baseline serum data (performed only after final study visit), we noted that eight subjects had substantial amounts of MZ in their serum. Given that these subjects suffered from AMD (and were based in the Republic of Ireland, where a supplement containing MZ is widely available for patients with AMD)(Reference Loughman, Nolan and Stack28), we suspected that they had in fact been supplementing with the macular carotenoids, but had failed to disclose this fact at enrolment.
This was subsequently confirmed by a phone call to each of these volunteers, and data relating to these subjects were excluded from all analyses. Of the remaining sixty-four subjects, ten (one AMD, nine normal) did not attend all three study visits, and were therefore also excluded from the analysis, thus leaving twenty-one, twenty and thirteen subjects in the carotenoid intervention Groups 1, 2 and 3, respectively (see later).
Of these fifty-four subjects, twenty-seven had no ocular pathology (normal subjects) and twenty-seven had previously been diagnosed with AMD (AMD subjects). The fifty-four subjects were split into three different carotenoid intervention groups as follows: Group 1: (n 21; eleven normal, ten AMD) 20 mg of L and 2 mg of Z (‘Ultra Lutein™’, provided by Nature's Plus, Natural Organics, Inc.); Group 2: (n 20; ten normal, ten AMD) 10 mg L, 2 mg Z and 10 mg MZ (Macushield™, provided by MacuVision Europe Limited); Group 3: (n 13; six normal, seven AMD) 3 mg L, 2 mg Z and 17 mg MZ (customised MZ formulation provided by Industrial Organica (not available commercially)). All supplements used in the present study consisted of oil-suspended, unesterified carotenoids provided in gelatine capsules. Each subject was required to consume one capsule daily, with the main meal, for the duration of the 8-week study period, with serum samples taken at baseline, and at 4 and 8 weeks. Significant efforts were made to ensure compliance to the study intervention. Compliance was monitored closely at the bi-weekly study visits. In addition, subjects were requested to return their supplement packs at their exit visit, and compliance was checked by tablet counting at this visit.
Standards and solvents
DSM Nutritional Products supplied the L and Z reference standards. The MZ standard was supplied by Industrial Organica as a soyabean oil oleoresin. The internal standard (IS) α-tocopheryl acetate and all solvents (HPLC grade) used for extraction and HPLC analysis were supplied by Sigma-Aldrich.
Serum carotenoid extraction
Non-fasting blood samples were collected in 9 ml vacuette tubes containing a ‘Z Serum Sep Clot Activator’, usually in the morning when subjects arrived at the clinic. The blood samples were allowed to clot at room temperature for approximately 1 h and then centrifuged at 725 g for 15 min in a Gruppe GC 12 centrifuge (Desaga Sarstedt) to separate the serum from the whole blood. The resulting serum samples were stored at − 70°C until the time of extraction (maximum 12 months).
Serum (0·4 ml) was micropipetted into clear 1·5 ml Eppendorf tubes labelled according to subject and visit number. IS (0·2 ml), α-tocopheryl acetate (250 mg/l ethanol) and 0·3 ml of butylated hydroxyltoluene (250 mg/l ethanol) were added and extracted into 0·5 ml of heptane using a Vortex Genie-2 (Scientific Industries) at the highest setting for 2 min, followed by centrifugation with a AccuSpin Micro 17 (Fisher Scientific Ireland) for 5 min at 400 g.
An aliquot of the upper heptane layer (0·4 ml) was removed to a light-resistant Eppendorf tube, and the heptane extraction was repeated once more, adding a further 0·5 ml of heptane to the original residue. The combined extracts were dried under N2 and stored at − 70°C until analysis.
HPLC analysis of serum L and total zeaxanthin (total Z) (Assay 1)
The HPLC system used for the study was an Agilent 1200 Series (Agilent Technologies Limited) consisting of a quaternary pump, autosampler, thermostat column compartment and a photodiode array detector monitoring a wavelength of 450 nm for serum carotenoids and 292 nm for the IS. Sample analysis was carried out in order of subject number and time of visitation. In other words, subjects were batch assessed.
The dried samples were reconstituted in 0·2 ml of the isocratic mobile phase, vortexed at the lowest setting for 1 min and pipetted into 2·5 ml vials containing 0·35 ml glass inserts (Agilent Technologies Limited). The sample (0·1 ml) was injected via autosampler onto a Phenomenex Ultracarb ODS 3μ(Reference Perry, Rasmussen and Johnson20) C18 column, 250 × 4·6 mm (part number: 00G-025-E0) with a guard column (Phenomenex) and a 0·5 μm in-line filter (Upchurch; Sigma-Aldrich). The column was shown to separate the carotenoids of interest in previous studies(Reference Thurnham, Tremel and Howard25, Reference Bone, Landrum and Guerra29).
The method used a premixed isocratic mobile phase consisting of 85 % acetonitrile, 15 % methanol and 0·1 % triethylamine, with a stepwise dichloromethane gradient initiated at 15 min with 10 % dichloromethane over 1 min and increased to 50 % dichloromethane between 25 and 27 min. The initial flow rate was set at 1 ml/min, and then increased to 2 ml/min at 15 min and remained as such for the duration of the dichloromethane gradient. The system resumed initial settings at 34 min. The L and total Z (co-eluted Z and MZ) peaks eluted at approximately 9·9 and 10·5 min, respectively. The IS eluted at 17·8 min. The system temperature throughout was maintained at 15°C. The mixed Z fraction was collected manually by switching the waste tube to a collection tube a couple of seconds after the peak was observed to start on the monitor. The eluent was dried under N2 and stored for no more than a few days at − 70°C for further analysis (Assay 2).
HPLC analysis of serum meso-zeaxanthin (Assay 2)
The enantiomers Z and MZ present in the total Z peak were separated using a 5 μm chiral column (Chiralpak™ AD column (250 × 4·6 mm)), a guard column (Apex Scientific Limited) and 2 μm filter(Reference Thurnham, Tremel and Howard25). The total Z fractions were reconstituted by vortex in 0·1 ml of mobile phase (n-hexane–isopropanol, 90:10) and 0·05 ml was then injected using normal-phase chromatography and a linear gradient during which the proportion of hexane increased to 95 % over 30 min. MZ, Z and L eluted at approximately 15·8, 18 and 20 min, respectively.
The absolute concentrations of L and total Z were calculated directly from the peak areas obtained in Assay 1. The concentrations were quantified using their respective response factors determined by UV–VIS spectroscopy analysis of the individual L and Z standards in absolute ethanol. Individual Z and MZ concentrations were then quantified from Assay 2, by calculating the percentage proportion of Z and MZ and applying the resulting ratio to the corresponding total Z value (obtained in Assay 1). All chromatographic peaks of interest were manually integrated using the Agilent ChemStation software (Agilent Technologies Ltd).
Absolute carotenoid concentrations were calculated as μmol/l. For purposes of interpretation, we also report the responses of the individual carotenoids as the changes in concentrations per milligram of supplement carotenoid provided. That is, the response was calculated as the average of the 4-and 8-week concentrations, or saturation plateau minus baseline values. This allowed for direct comparison between the interventions (Groups 1, 2 and 3), in terms of individual and total serum macular carotenoid responses and controls for the amount of supplement provided.
Capsule carotenoid analysis
The carotenoid content of the three supplements used in the present study were analysed using the following protocol. A stock solution was made by dissolving the contents of one capsule in 250 ml of acetone. A 0·5 ml aliquot was taken from this stock solution and made up to 25 ml with acetone to give the working solution for the analysis. The working solution was analysed in triplicate.
A measure of 0·4 ml of the working solution was transferred to a glass tube and dried under N2. A measure of 0·1 ml of IS (ethanolic echinenone, 0·4 mg/500 ml) and 0·4 ml methanolic KOH (50 g/100 ml) was added to the sample. The samples were stoppered and allowed to saponify at 45°C for 1 h in a shaking incubator. The samples were removed and allowed to cool to room temperature. The remaining KOH was neutralised using 1·5 ml 1 m-HCl. Butylated hydroxyltoluene in hexane (1 ml; 25 mg/100 ml) was added to the sample and mixed by vortex for 2 min. The sample layers were allowed to separate under gravity for 5 min and a 0·5 ml aliquot of the organic layer was removed to an evaporation tube. A measure of 1 ml of hexane was added to the remaining sample, which was vortexed and allowed to separate as earlier. A measure of 1 ml of the hexane layer was removed and combined with the initial extract, which was dried in a solvent concentrator and stored at − 80°C until the time of analysis. These samples were analysed using the HPLC method described earlier in order to quantify L, Z and MZ concentrations in each capsule. Of note, tablet carotenoid assessment of the three supplements used in the study was also performed by Industrial Organica (supplier of Intervention 3) and the data are concordant.
Statistical analysis
Means and standard deviations are presented in the text and tables (SPSS version 17; SPSS, Inc., used for data analysis). SigmaPlot (version 8; SyStat Software) was used for graphical presentations. All data were tested using the non-parametric Kolmogorov–Smirnov test, and they exhibited normal distribution. Between-group differences for numeric data (age, BMI, diet score and serum carotenoid levels) were calculated using ANOVA. Between-group differences for categorical variables (sex, smoking habits, sex and ocular status (normal or AMD)) were calculated using the standard χ2 test. Difference between baseline and saturation point (i.e. average of weeks 4 and 8) was investigated for L, Z and MZ using paired-sample t tests. Repeated measures analysis was used to test for differences in response of each carotenoid between normal subjects and subjects with AMD, by testing for a time/subject group (i.e. normal subjects v. AMD subjects) interaction effect (Greenhouse-Geisser significance values were used and presented in the results section). A 5 % level of significance was implemented throughout the analysis.
Results
The demographic, lifestyle, ocular disease status (normal or AMD) and baseline serum carotenoid data for all three groups are presented in Table 1. There were no statistically significant differences between groups for baseline parameters, with the exception of significantly higher mean serum Z concentration for subjects in Group 3.
Table 1 Demographic, lifestyle, ocular status (normal or early age-related macular degeneration (AMD)) and baseline serum carotenoid data for the three intervention groups (Mean values and standard deviations or number of subjects)

L, lutein; Z, zeaxanthin; MZ, meso-zeaxanthin.
* High L group (20 mg L/d and 2 mg Z/d).
† Combination group (10 mg L/d, 2 mg Z/d and 10 mg MZ/d).
‡ High MZ group (3 mg L/d, 2 mg Z/d and 17 mg MZ/d).
§ Significance difference between the three intervention groups.
∥ Significance (P) values calculated using the standard χ2 test.
¶ Significance (P) values calculated using ANOVA.
** Significant difference with respect to age between AMD and normal subjects in Group 1 (P= 0·024).
†† Group 3 baseline serum Z was significantly greater than Groups 1 and 2; BMI, sex, smoking habits and diet scores information did not differ between AMD and normal subjects and were therefore reported as combined values.
There was no significant difference between normal subjects and AMD subjects with respect to any of the known possible confounders for carotenoids (e.g. BMI, sex and smoking habits), with the exception of a significant difference between these populations for age, which was principally due to the older AMD subjects in Group 1 (P= 0·024). Also, there were no significant differences between normal subjects and AMD subjects in terms of baseline serum concentrations of the macular carotenoids or in terms of the responses to any of the three carotenoids (with one exception, Group 3 response to MZ, discussed later). Therefore, data for normal and AMD subjects were combined for the main analyses reported here (Table 2). Furthermore, there were no differences in the serum carotenoid responses between weeks 4 and 8, and these data were therefore averaged to provide data on the serum response at saturation point.
Supplement assessment
Table 3 presents the findings of the capsule analysis for each intervention used in the trial. The chromatograms in Fig. 2 show the presence of MZ in the UltraLutein™ (intervention Group 1) supplement.

Fig. 2 (a) HPLC analysis of the UltraLutein™ (intervention used by Group 1) supplement and (b) HPLC analysis of meso-zeaxanthin (MZ) reference standard. L, lutein; Z, zeaxanthin. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Serum lutein response
There was no significant difference with respect to serum L response between normal subjects and subjects with AMD (Group 1: P= 0·409; Group 2: P= 0·843; and Group 3: P= 0·571). Serum L concentrations (for normal and AMD subjects combined) increased significantly in Group 1 (0·036 μmol/l per mg (269 %) increase; P< 0·001) and Group 2 (0·079 μmol/l per mg (340 %) increase; P< 0·001), with no significant change seen in Group 3 (0·006 μmol/l per mg (7 %) increase; P= 0·466). Serum L concentrations at the three study visits and the saturation point responses are presented in Tables 2 and 4, respectively.
Table 2 Concentrations of serum lutein (L), zeaxanthin (Z), meso-zeaxanthin (MZ) and total macular carotenoids for each of the three carotenoid intervention groups investigated* (Mean values and standard deviations)†

* Repeated measures analysis found no differences in response of each carotenoid between normal subjects and subjects with AMD except in Group 3 (see text).
† Mean values were in response to each carotenoid component for each group.
‡ Significance (P) values represent paired-sample t tests significance for the increase between baseline and 8 weeks. There were no differences in carotenoid concentrations between weeks 4 and 8.
§ Group 1 (n 21): high L group (20 mg L/d and 2 mg Z/d).
∥ Group 2 (n 20): combination group (10 mg L/d, 2 mg Z/d and 10 mg MZ/d).
¶ Group 3 (n 13): high MZ group (3 mg L/d, 2 mg Z/d and 17 mg MZ/d).
** There were no differences in baseline concentrations, except for Z, in Group 3 (see Table 1).
†† Mean values were in response to each carotenoid component for all three carotenoids in each supplement.
Table 3 Declared and measured carotenoid content of the three study supplements
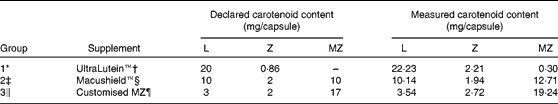
L, lutein; Z, zeaxanthin; MZ, meso-zeaxanthin.
* Group 1 (n 21; eleven normal and ten age-related macular degeneration (AMD)).
† Provided by Nature's Plus, Natural Organics, Inc., Melville, NY, USA.
‡ Group 2 (n 20; ten normal and ten AMD).
§ Provided by MacuVision Europe Limited, Solihull, UK.
∥ Group 3 (n 13; six normal and seven AMD).
¶ Provided by Industrial Organica, Monterrey, Mexico (not available commercially).
Table 4 Serum lutein (L), zeaxanthin (Z), meso-zeaxanthin (MZ) and total carotenoid response in μmol/l per mg of supplemental carotenoid to carotenoid saturation point (Mean values and standard deviations)

* Significance values refer to ANOVA testing with post hoc analysis for significant difference (Tukey honestly significant differences) in serum carotenoid response between intervention groups.
† Significance (P) values represent paired-sample t tests for the increase in serum carotenoids between baseline and the average concentration at visits 4 and 8 weeks.
‡ High L group (20 mg L/d and 2 mg Z/d).
§ Combination group (10 mg L/d, 2 mg Z/d and 10 mg MZ/d).
∥ High MZ group (3 mg L/d, 2 mg Z/d and 17 mg MZ/d).
¶ The intervention taken by Group 1 did not contain MZ, therefore an MZ response per mg of supplemental MZ could not be calculated.
** ANOVA calculated using mean concentrations as μmol/l for comparisons with Group 1 results. Total macular carotenoid, combined L, Z and MZ response calculated in μmol/l per mg for total carotenoid dosage in each intervention.
Serum zeaxanthin response
There was no significant difference with respect to serum Z response between normal subjects and subjects with AMD (Group 1: P= 0·198; Group 2: P= 0·626; and Group 3: P= 0·404). Serum Z concentrations (for normal and AMD subjects combined) increased significantly in Group 1 (0·037 μmol/l per mg (69 %) increase; P= 0·001) and Group 2 (0·015 μmol/l per mg (75 %) increase; P< 0·001), with no significant change seen in Group 3 ( − 0·0002 μmol/l per mg (6 %) decrease; P= 0·384). Serum Z concentrations at the three study visits and the saturation point responses are presented in Tables 2 and 4, respectively.
Serum meso-zeaxanthin response
There was no significant difference with respect to serum MZ response between normal subjects and subjects with AMD for either Group 1 (P= 0·675) or for Group 2 (P= 0·985). However, there was a significant difference for subjects in Group 3, with AMD subjects demonstrating a significantly greater serum MZ response than normal subjects following supplementation with this carotenoid (AMD subjects: 0·094 μmol/l at saturation point (0·006 μmol/l per mg increase); normal subjects: 0·036 μmol/l at saturation point (0·002 μmol/l per mg increase); P= 0·014) (Fig. 3).

Fig. 3 Serum meso-zeaxanthin (MZ) response (μmol/l) observed for Group 3, divided by ocular status (normal subjects (![]() ) v. age-related macular degeneration subjects (
) v. age-related macular degeneration subjects (![]() )).
)).
Although there was no MZ declared in the Group 1 supplement, a small concentration of MZ appeared to be present in serum at weeks 4 and 8 (0·009 μmol/l at week 8; P= 0·015). Serum MZ concentrations increased significantly in Group 2 (0·005 μmol/l per mg increase; P< 0·001) and Group 3 (0·004 μmol/l per mg increase; P< 0·001). As MZ was not included as a supplemental carotenoid in Group 1, the MZ response can only be presented as the absolute value of MZ in μmol/l (Table 2). Serum MZ concentrations at the three study visits and the saturation concentration are presented in Tables 2 and 4, respectively.
Total macular carotenoid serum response
There was no significant difference with respect to total serum carotenoid response between normal subjects and subjects with AMD (Group 1: P= 0·393; Group 2: P= 0·842; and Group 3: P= 0·152). Total serum carotenoid concentrations (for normal and AMD subjects combined) increased significantly in Group 1 (0·036 μmol/l per mg (242 %) increase; P< 0·001), Group 2 (0·040 μmol/l per mg (321 %) increase; P< 0·001) and Group 3 (0·004 μmol/l per mg (24 %) increase; P= 0·030). Total serum macular carotenoid response over the three study visits and to saturation point is presented in Tables 2 and 4, respectively.
Discussion
The present study was conducted to investigate serum responses in normal subjects and in those with early AMD to three different macular carotenoid interventions, and is the first study to do so. We believe detailed investigation into serum macular carotenoid response is required, given that serum is the transporter of these carotenoids to their target tissues, including the retina. Moreover, the question as to whether there are differences in response between normal subjects and subjects with AMD is important, and was uniquely answered in the present study.
To this point, we report that subjects with AMD are comparable with normal subjects in how they respond to macular carotenoid supplements in serum, with the exception of a difference between these subject populations in response to supplementation with the central macular carotenoid, MZ. Of note, we report no significant difference in serum L or serum Z response between these subject populations for any of the interventions tested. However, the Group 3 formulation (i.e. very high MZ group) yielded significantly different serum MZ responses between the AMD and normal populations, with the MZ response amongst AMD subjects significantly greater than that observed for normal subjects. This seemingly counterintuitive observation is difficult to explain, but may reflect enhanced absorption of this macular carotenoid in subjects who exhibit tissue deficiencies of MZ, reflected in the absence of a typical central peak in MP optical density spatial profile in association with risk factors for AMD(Reference Kirby, Beatty and Loane30). However, the main finding from the present study is that normal and AMD subjects responded comparably to the three different macular carotenoid interventions. This is an important finding, as it confirms that the known lack of MP seen in subjects afflicted with(Reference Bone, Landrum and Mayne31), or at high risk of developing(Reference Nolan, Stack and O'Donovan32), AMD is not due to an inability of such subjects to respond to carotenoid consumption, and is therefore due to either a defective capture of circulating carotenoids by, or stabilisation within, the central retina. Our data are consistent with a publication by Wang et al. (Reference Wang, Connor and Johnson33) who studied subjects with and without AMD following dietary modification (increased consumption of spinach, maize, cabbage, circa 11 mg/d of L and Z (combined), in the high-carotenoid-fed group) and reported no difference between these group in terms of serum concentrations of L and Z.
Given that, by and large and with the exception of response to supplementation with high-dose MZ, there were no differences between subjects with and without AMD in terms of response to supplementation with MP's constituent carotenoids, we elected to treat our dataset as a single and merged set of data.
We examined the literature to determine the carotenoid responses reported by other researchers, in order to allow comparison with our findings. Examination of these studies has shown that it has been a common practice for other researchers to report carotenoid response in terms of concentrations and/or percentage increases. Presenting the data as percentages and/or concentrations, however, makes it very difficult to interpret the data, as baseline concentrations can vary considerably between subjects. It was for this reason that we constructed and present Table 5. Table 5 presents data on serum L, Z and MZ responses of these other published studies, but we have converted their data to the unit reported in the present study, which is serum response per mg of supplemental carotenoid (controlling for the amount of carotenoid given and the baseline values). Interestingly, upon examination of this table with respect to L response of the other published studies (and excluding our own data), we found that the mean response was 0·066 (sd 0·042) with a range of 0·01–0·17 μmol/l per mg. Also, for Z response (and excluding our own data), the mean response was lower at 0·050 (sd 0·035) with a range of 0·004–0·15 μmol/l per mg. Thus, the range of serum macular carotenoid response reported in the literature is very wide. Of note, the responses observed in the present study fall comfortably within the ranges reported in these other studies (see Table 5).
Table 5 Serum carotenoid response per mg of supplemental carotenoid, following supplementation with the macular carotenoids

L, lutein; Z, zeaxanthin; MZ, meso-zeaxanthin; JN, Journal of Nutrition; ABB, Archives of Biochemistry and Biophysics; EER, Experimental Eye Research; IOVS, Investigative Ophthalmology and Visual Science; AJCN, American Journal of Clinical Nutrition; JID, Journal of Infectious Diseases; VR, Vision Research; CER, Current Eye Research; BJN, British Journal of Nutrition; NM, Nutrition and Metabolism; AMD, age-releated macular degeneration.
* Data unavailable.
† Free (unesterified) carotenoid supplement.
‡ Includes MZ supplementation.
As seen in Table 5, studies investigating serum carotenoid response to MZ are few, although Connolly et al. (Reference Connolly, Beatty and Thurnham23) and Thurnham et al. (Reference Thurnham, Tremel and Howard25) did study and report on MZ response in normal men and women to a supplement similar in composition to that used in Group 2 here. Although it is known that there is considerable inter-individual variation in terms of MP response to any dietary/supplement intervention(Reference Hammond, Wooten and Snodderly34), there are no published reports of studies designed to investigate serum response to differing macular carotenoid formulations.
Serum lutein response
L was present in each of the three carotenoid group interventions. However, Group 1 (the high L group) contained approximately double that of Group 2 and six times that of Group 3. As expected, Groups 1 and 2 demonstrated the greatest serum response to L (0·036 μmol/l per mg (269 %) and 0·079 μmol/l per mg (340 %), respectively), with Group 3 demonstrating no response (0·006 μmol/l per mg (6 %)).
The Group 1 response is consistent with a previous study by Johnson et al. (Reference Johnson, Hammond and Yeum35), who used a similar formulation. Of interest, the Group 2 supplement, which contained only half the amount of L compared with that of Group 1, but also contained 10 mg of MZ and 2 mg of Z, achieved a significantly greater serum L response than that observed for Group 1 (0·079 v. 0·036 μmol/l per mg, respectively). The only other previous study that reported on serum response to a formulation similar to Group 2 was conducted by Thurnham et al. (Reference Thurnham, Tremel and Howard25) in 2008. In that study, nineteen subjects were supplemented with 10 mg L, 1·2 mg of Z and 8 mg of MZ (LuteinPlus™), and a lower L response (0·056 μmol/l per mg) was reported than that reported in the present study (0·079 μmol/l per mg). In a different study, Thurmann et al. (Reference Thurmann, Schalch and Aebischer36) reported serum responses to two different dosages of almost pure L (4·1 and 20·5 mg/d). In that 42 d study, the authors found that the rate of increase in serum L was greater for the low-dose L supplement when compared with the high-dose L supplement (0·093 and 0·064 μmol/l per mg, respectively). However, as expected, supplementation with high-dose L did result in a significantly higher absolute L concentration in serum than seen following supplementation with low-dose L. Indeed, these findings are consistent with the results of the present study.
While a lower serum response to L in Group 3 (the high MZ group) was expected, reflecting its lower dosage (only 3 mg L), the lack of any response was not anticipated and did not compare well with other studies(Reference Bone, Landrum and Guerra29, Reference Thurmann, Schalch and Aebischer36). Bone et al. (Reference Bone, Landrum and Guerra29) reported that only 2·4 mg of L/d achieved a 0·100 μmol/l per mg response in serum L. However, our data (for Group 3) are in agreement with a more recent study by Bone et al. (Reference Bone, Landrum and Cao24), which found that a supplement containing 5·5 mg L, 1·4 mg Z and 14·9 mg MZ achieved only a 0·001 μmol/l per mg response in serum L, while 0·012 μmol/l per mg was observed for the total Z fraction (the dominant carotenoids in their formulation).
Interpretation of our data for serum L response suggests that uptake of L may be inhibited when MZ is present in the supplement formulation in very high amounts (e.g. Group 3 where MZ was 77 %), but uptake of L may be unaffected or facilitated when the combined amounts of MZ and Z are present in more comparable amounts to L (i.e. as in Group 2). Whether high amounts of Z in a supplement has the same effect on L uptake into serum, as seen here with MZ, has not yet been studied, but warrants attention. Thus, in the present study, our data demonstrate a greater serum response to supplemental L when the macular carotenoids are provided in a L:Z:MZ ratio of 10:2:10, possibly representing an interactively additive relationship that is dependent on a ratio approximating the Group 2 formulation.
Serum zeaxanthin response
Z was present in all three intervention groups as follows: Group 1 containing 2 mg Z, and Groups 2 and 3 each containing 2 mg Z in their respective formulations. Interestingly, the increase in serum Z is comparable between Groups 1 and 2. Also, the Z response for all groups in the present study is lower, when compared with reports by Hartman et al. (Reference Hartmann, Thurmann and Spitzer37) and Thurnham et al. (Reference Thurnham, Tremel and Howard25) (0·088 μmol/l per mg and 0·087 μmol/l per mg, respectively).
Surprisingly, the Group 3 intervention did not achieve any serum Z response, suggesting that very high amounts of MZ (i.e. 17 mg, as in Group 3) in a supplement inhibits uptake of Z and is also consistent with previously published data(Reference Connolly, Beatty and Thurnham23). Further, Bone et al. (29) and Schalch et al. (39) have each reported maximum serum Z response when this carotenoid is supplemented in the absence of other carotenoids, but this does not agree with the observations of Hartmann et al. (Reference Hartmann, Thurmann and Spitzer37) and Thurnham et al. (Reference Thurnham, Tremel and Howard25), who obtained high Z responses with very different supplement compositions. Nevertheless, several other studies report a reduced Z response in the presence of high amounts of L, as observed in the present study in Group 1 (see Table 5 for comparison)(Reference Bone, Landrum and Guerra29, Reference Johnson, Hammond and Yeum35, Reference Johnson, Chung and Caldarella38, Reference Schalch, Cohn and Barker39).
Interpretation of our data for serum Z response suggests that uptake of Z is inhibited when supplemented with very high quantities of MZ (Group 3). It is possible that the inhibitions seen in Group 3 are due to the structural and chemical similarities between Z and MZ, which might cause them to compete for absorption. Differences between the present study and other studies with respect to absorption of Z may also be explained by differences in the supplement components (e.g. type of carotenoid suspension) and/or differences in dietary habits of the subjects.
Serum meso-zeaxanthin response
MZ was present in two of the three interventions as follows: Group 2 containing 10 mg of MZ and Group 3 containing 17 mg of MZ. Of interest, both groups that were supplemented with MZ demonstrated a serum response to this carotenoid. This finding is important given that MZ has yet to be found in a typical diet in large amounts, and therefore uptake into serum of this carotenoid warranted investigation. Indeed, our observation in this regard is consistent with a previous report by our group(Reference Connolly, Beatty and Thurnham23). Further, serum MZ increases for both Groups 2 and 3 were comparable, but four to five times lower than that previously reported by Thurnham et al. (Reference Thurnham, Tremel and Howard25). It is important to point out that, in terms of absolute values, circulating concentrations of MZ were lower than those of either L or Z, in spite of the high doses given. However, we propose that, as MZ is not known to be present in a typical diet, newly absorbed MZ could be directed to potential storage tissues and, as a result, concentrations in the blood may take many weeks to reach saturation, thereby explaining the low circulating concentrations of this carotenoid when compared with either L or Z following supplementation. Previous studies have reported that MZ was less readily absorbed than other Z isomers(Reference Schiedt, Glintz and Vecchi40), but nevertheless the amount present in blood is likely to be sufficient to supplement the macula, where the average amount of MZ measured within 24 h of death was 7·7 ng(Reference Khachik, de Moura and Zhao41). Also, it is possible that we simply have, and need, less MZ in serum compared with the other macular carotenoids, as its presence in tissue (with the exception of the macula) is unknown. In other words, it is possible that, in human subjects, MZ is found only at the macula, and study is warranted to investigate its presence, or absence, in other tissues (including the brain).
An interesting finding was the unexpected observation of a peak with the spectrophotometric characteristics of MZ in the serum of Group 1 subjects at 4 and 8 weeks. Subjects in Group 1 received no MZ (at least according to the box label claim). Given that this was an unexpected finding, we reanalysed random serum samples from Group 1 in order to confirm or refute our observation, and confirmed that MZ was, indeed, present in the serum of these subjects. We then tested the Group 1 intervention formulation (Ultra Lutein® from Nature's Plus®: L provided by FloraGLO® which is a registered trademark of Kemin Health, L.C.) and determined that this formulation did, in fact, contain MZ (0·3 mg per capsule), which we believe explains the observation that Group 1 subjects exhibited a trace peak with the spectrophotometric characteristics of MZ in serum at 4 and 8 weeks. We then went on to test the composition of the other interventions used in the present study and found that they were concordant with their respective label claims (see Table 3). These findings have implications on the ongoing research surrounding carotenoid supplementation. Indeed, any discrepancy between actual and alleged concentrations of the respective macular carotenoids in commercially available preparations is particularly important when such formulations are used for research. Consequently, the concentration of all three macular carotenoids in a wide array of commercially available formulations and foods, with particular attention directed towards MZ, will be the subject of further study by our laboratory.
Conclusion
The present study has yielded important and novel findings, such as the presence of MZ in the serum of normal and AMD subjects following supplementation with high doses of L, and the significantly greater serum MZ response amongst subjects with AMD v. normal subjects following supplementation in the high MZ group. A limitation of the present study rests on the absence of a placebo arm, and further research should therefore include a placebo control group. Moreover, a head-to-head trial of supplemental MZ v. supplemental Z (in comparable amounts) would also enhance our understanding of serum response to supplementation with the macular carotenoids.
We conclude that all three macular carotenoid interventions resulted in significant serum carotenoid response, albeit to varying extents. Group 2, an intervention containing 2 mg Z, 10 mg L and 10 mg MZ achieved the greatest composite serum response for these carotenoids (i.e. total macular carotenoid response). In other words, it appears that a formulation containing all three macular carotenoids was more efficacious in terms of achieving a higher concentration of circulating levels of total macular carotenoids, thereby potentially optimising the bioavailability of these compounds for capture by the target tissue (retina).
Acknowledgements
The authors acknowledge the support given by the Howard Foundation, Cambridge, UK. J. M. N. is a Fulbright Scholar and is funded by the European Research Council (ERC). K. A. M. carried out the experimental procedures, the data interpretation and statistical analysis, and drafted the manuscript. D. I. T. helped with data interpretation and statistical analysis, and helped to draft the manuscript. S. B. helped to the draft the manuscript. A. N. H. helped with the design of the trial and to draft the manuscript. E. C. carried out the recruitment of subjects, managed the clinical visitations and collected the serum samples. W. C. supported the experimental procedures. J. M. N. designed and supervised the study, helped with data interpretation and statistical analysis and helped to draft the manuscript. All authors have read and approved the manuscript. J. M. N. and S. B. do consultancy work for nutraceutical companies, in a personal capacity, and as directors of Nutrasight Consultancy Limited. D. I. T. is a consultant to the Howard Foundation and receives consulting fees for the same. All other authors report no potential conflicts of interest.










