INTRODUCTION
Pneumonia is a leading cause of hospitalization and death in the USA, and persons aged ⩾65 years shoulder the greatest burden of pneumonia-related morbidity and mortality [Reference Jackson1]. Limited population-based data on adult community-acquired pneumonia (CAP) are available from industrialized or developing countries; where they exist, they are provided by research studies with varying surveillance methods and case definitions, and thus suffer from a lack of comparability [Reference Jokinen2–Reference Almirall6]. We previously described a novel surveillance method for measuring the incidence of clinical and radiographic CAP, which was developed and implemented on the White Mountain Apache reservation in the Southwestern United States in 2002 [Reference Watt7]. In our study, the incidence rates of clinical CAP and of X-ray-confirmed CAP were 26·7/1000 person-years and 16/1000 person-years in adults aged ⩾40 years, respectively. These rates are 2–3 times higher than estimates from other United States or European populations.
Streptococcus pneumoniae (pneumococcus) is known to cause a large proportion of pneumonia cases in both children and adults [Reference Oseasohn, Skipper and Tempest4, Reference Almirall6, Reference Vuori8, Reference Forgie9]. However, the exact fraction of pneumonia attributable to pneumococcus cannot be easily established because there are no diagnostic methods with sufficiently high sensitivity and specificity. Practical challenges hinder routine use of lung fluid cultures [Reference Vuori-Holopainen and Peltola10–Reference Scott12]. Further, lung fluid can only be obtained from a subset of pneumonia patients which may not be representative of all cases. Blood culture has limited sensitivity because bacterial pneumonia may not be associated with bacteraemia, bacteraemia may not be present at the time of blood collection, and culture outcome may be compromised by prior antibiotic use or inappropriate specimen processing. Sputum cultures may be contaminated by pathogens colonizing the upper-respiratory airways and therefore lack specificity [Reference Barrett-Connor13, Reference Ewig14]. Moreover, many patients cannot produce optimal sputum specimens. Serological markers of pneumococcal infection, such as antibodies against pneumococcal surface protein A (PspA, interferes with complement deposition on the bacterial surface) and pneumococcal surface antigen A (PsaA, an important element in pneumococcal adhesion to respiratory mucosa), and urine antigen testing have been evaluated in a number of settings with varying results [Reference Scott15–Reference Genne, Siegrist and Lienhard21]. Better diagnostic methods are needed both to improve disease burden measurement and to facilitate the evaluation of the impact of pneumococcal vaccines on pneumonia. Our objectives were to estimate a minimum proportion of pneumonia cases that are likely to be caused by pneumococcus and to evaluate the utility of several new diagnostic tools.
METHODS
Study design
This was a prospective, cross-sectional study of patients who presented for a chest X-ray to the radiology departments of three Indian Health Service clinics in the Southwestern United States, between 1 May 2003 and 22 December 2004. Patients were eligible for inclusion if they were at least 40 years old, were registered as a Navajo or White Mountain Apache tribal member, resided in an area where follow-up was possible, and were available for a convalescent blood draw 4–6 weeks after enrolment. Patients who had been enrolled previously, had been hospitalized for more than 3 days, or had been discharged from the hospital in the past 7 days were excluded. Patients were eligible for enrolment regardless of duration of symptoms or previous antibiotic treatment.
Specimen collection
Study nurses obtained 12 ml venous blood and 20 ml urine in a standard manner within 48 h of consent. A convalescent venous blood specimen was collected at home or at the clinic. Up to three home visits were made in an effort to obtain convalescent blood within 6 weeks of the initial visit for all subjects. At the time of enrolment, nasopharyngeal (NP) swabs were obtained by inserting a flexible calcium-alginate swab to the rear of the nasopharynx and gently rubbing the area to saturate the tip of the shaft. Oropharyngeal (OP) specimens were obtained in a similar fashion, by rubbing a calcium-alginate swab on the back of the patient's throat. Swabs were placed in skim milk/tryptone/glucose/glycerine (STGG) transport vials and stored at −70°C until processing. Blood and sputum cultures were obtained at the discretion of the treating physician.
Specimen processing
Blood cultures and sputum cultures were performed at the hospital microbiology laboratories according to their routine practice. Urine specimens were maintained at room temperature and processed within 24 h using the BinaxNOW® S. pneumoniae immuno-chromatographic test (Binax Inc., USA), following the manufacturer's instructions [22]. Quality control tests were performed as recommended by the manufacturer. BinaxNOW results were read by a single reader according to the manufacturer's instructions.
Antibody titres for pneumococcal PsaA and PspA antigens were performed at the Sanofi-Pasteur clinical serology department in Swiftwater, Pennsylvania. NP and OP specimen cultures were conducted at the Centers for Disease Control and Prevention in Atlanta. Samples were inoculated onto blood agar plates and incubated for 7 days at 37°C under 5% CO2. Alpha haemolytic colonies were identified as pneumococci by optochin sensitivity and bile solubility testing and then serotyped using the Quellung reaction.
Medical record review
A study nurse reviewed each participant's medical record and completed a standardized data collection form which included demographic data, clinical information, laboratory results, medical history, vaccination history, and radiology results.
Chest X-ray interpretation
Chest X-rays were read by two radiologists trained according to a modified version of the World Health Organization (WHO) guidelines for paediatric X-ray interpretation [Reference Cherian23] and performed in a standardized fashion as described elsewhere [Reference Watt7]. Discrepant readings were adjudicated by a third WHO-trained radiologist, whose interpretation was accepted as definitive.
Case definitions
We defined two case groups as follows:
• Clinical CAP. Clinical diagnosis consistent with pneumonia and at least one objective sign and one subjective symptom or two objective signs of pneumonia. Objective signs: respiratory rate >20, altered mental status, temperature >38°C or <36°C, decreased breath sounds, rales. Subjective symptoms: new or worsening dyspnoea, subjective fever, new or worsening cough, pleuritic chest pain, increased sputum production.
• Radiographic CAP. Presence of an acute infiltrate on standardized X-ray review and clinical findings as described above.
In addition, we defined two control groups, where group 1 was a subset of group 2:
• Control group 1. No clinical diagnosis consistent with pneumonia, no acute infiltrate on standardized X-ray review, and none of the objective signs or subjective symptoms of pneumonia listed above.
• Control group 2. No clinical diagnosis of pneumonia, no acute infiltrate on standardized X-ray review.
Statistical analysis
We calculated the proportion of subjects with pneumococcus identified in blood culture, sputum culture, or pharyngeal specimen culture (positive if either the NP or the OP culture was positive), pneumococcal antigen identified in urine by urine antigen test, and two-, three- and fourfold rise in PsaA and PspA titres in the four groups of subjects defined above. We then compared each case group to both control groups using two-sample t tests for proportions. We generated reverse cumulative distribution curves of the fold rise in PsaA and PspA titres in all four groups of subjects, including convalescent blood draws obtained per-protocol (28–42 days after acute blood draw), within 1 week of per-protocol limits (21–49 days post-acute), and at any time. All analyses were conducted in Stata 9.0 (StataCorp, USA).
Ethics
The study was approved by the Navajo Nation, the National Indian Health Service, and the Johns Hopkins Bloomberg School of Public Health institutional review boards and by the White Mountain Apache Health Board. Written informed consent was required for all participants.
RESULTS
Study subjects
We enrolled 515 subjects. Table 1 shows the distribution of study subjects by group and selected demographic and clinical characteristics of cases and controls. Cases were similar to controls in gender and age, but were significantly more likely to be hospitalized than controls. A majority of subjects (68%) had received the 23-valent pneumococcal polysaccharide vaccine an average of 5·1 years prior to enrolment as part of routine medical care. The clinical CAP group was more likely to be vaccinated than other groups (Table 2). Blood cultures were performed on 113 subjects (22%), of which four (3·5%) were positive for S. pneumoniae. Of 68 sputum cultures, seven (10·3%) were positive for pneumococcus.
Table 1. Demographic and clinical characteristics of Native American adults with community-acquired pneumonia (CAP) and control groups
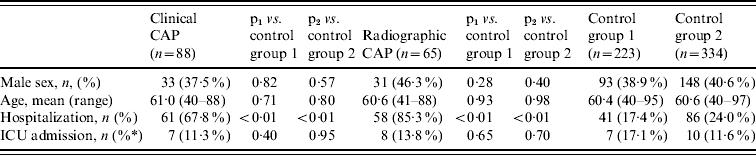
ICU, Intensive care unit.
* % of hospitalized patients.
Table 2. Proportion of patients with community-acquired pneumonia (CAP) and of control subjects with a history of vaccination with pneumococcal polysaccharide vaccine
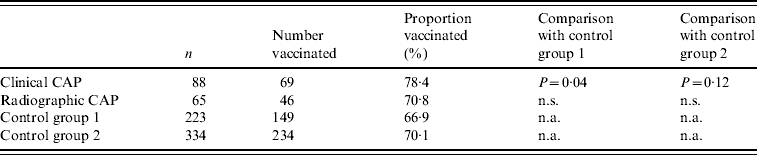
n.s., Not significant; n.a., not applicable.
Subjects with pneumococcal bacteraemia
Of the four subjects with pneumococcal bacteremia, three had clinical and radiological CAP and one had a radiological diagnosis of pneumonia using the study definition but no clinical diagnosis or clinical signs of pneumonia. All three pneumococcal CAP patients had a positive urine antigen test and the two with serological testing done had a ⩾twofold rise in PsaA and ⩾threefold rise in PspA titres; one had a positive sputum culture. All other laboratory tests were negative for the bacteraemic patient without CAP.
Serology, urine antigen testing, blood and sputum cultures
Paired sera were available from 452 subjects. Of these, 236 were drawn per protocol (convalescent blood drawn 28–41 days after the acute blood draw) and an additional 88 were within 1 week of the protocol stipulation. Differences between the CAP groups and control groups were variable depending on antigen and change in titre. When ⩾twofold rises in PsaA or PspA were considered, we obtained yields of about 15% in CAP patients and significantly lower yields in controls (4–7%). When ⩾threefold rises in PsaA or PspA were considered, the proportion positive in all groups declined. There was a significant difference for a ⩾threefold rise in PsaA between radiographic CAP and the control groups, but not for clinical CAP or PspA comparisons. Higher cut-offs (⩾fourfold) for rises in PspA and PsaA did not discriminate between CAP patients and controls. Reverse-cumulative distribution graphs show that the greatest difference between cases and controls was seen between a one- to twofold rise in PsaA or PspA (Fig. 1). The curves were similar for specimens collected with per-protocol timing, specimens collected per-protocol ±1 week, and all specimens.
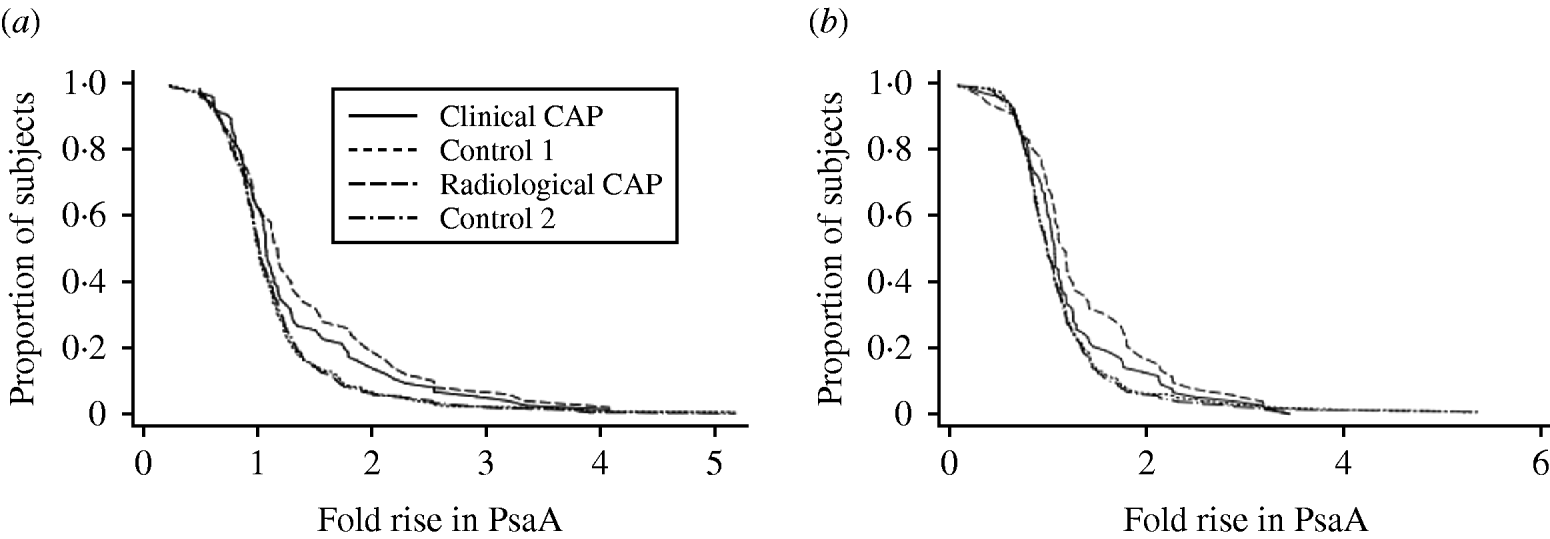
Fig. 1. Reverse cumulative distribution of rise in (a) PsaA titre and (b) PspA titre for all patients with acute and convalescent sera available, by study group.
As shown in Table 3, blood cultures had relatively low yields in CAP cases. Sputum culture yields were higher, but were not statistically different between cases and controls, probably because of the small number of specimens available for testing. Urine antigen test yields were 12·7–17·0% in CAP cases and only 2% in controls (P<0·001 for all pair-wise comparisons). Pneumococcal colonization had a significant impact on urine antigen testing results in controls: 7% of those colonized with pneumococcus had a positive urine antigen test compared with 1% of those who were not colonized (P=0·05 in control group 1, P=0·02 in control group 2). We assessed agreement between the urine antigen test and serology in clinical and radiographic CAP cases. In clinical CAP cases, the kappa statistics comparing the urine antigen test to a ⩾twofold rise in PsaA, ⩾threefold rise in PsaA, ⩾twofold rise in PspA and ⩾threefold rise in PspA were 0·51, 0·49, 0·46 and 0·25, respectively. In radiographic CAP cases, these values were 0·42, 0·36, 0·33 and 0·16.
Table 3. Diagnostic test results for clinical community acquired pneumonia (CAP), radiographic CAP and control group patients
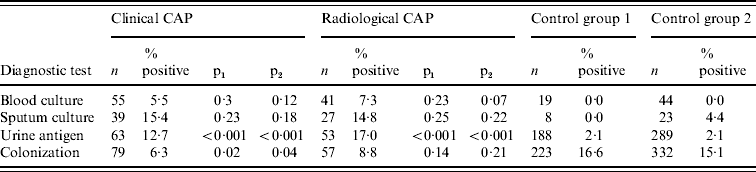
p1, P value for comparison with control group 1; p2, P value for comparison with control group 2.
Pneumococcal colonization
At least one pharyngeal specimen was collected from 464 subjects, of which 60 (12·9%) showed colonization with S. pneumoniae. Subjects in both control groups were more likely to be colonized by pneumococci than in the CAP groups; no information was available on prior antibiotic use. Colonization rates were highest in patients aged ⩾80 years (24·4%); significant differences in carriage between cases and controls were only seen in the 50–64 years group (18·9% vs. 2·9%, P=0·02 for clinical CAP vs. control group 2).
DISCUSSION
In this study, we enrolled American Indian adults presenting for a chest radiograph at three Indian Health Service clinics and compared the outcomes of a series of diagnostic tests for identifying pneumococcus in patients with and without community-acquired pneumonia. We found that only 2% of control subjects tested positive using the BinaxNOW S. pneumoniae urinary antigen test. This test also showed a larger difference in proportion positive between CAP patients and control patients than all serological comparisons tested. This observation is in line with several previous studies from Europe, the USA and Japan [Reference Smith19, Reference Gutierrez20, Reference Roson24]. Control subjects with pneumococcal colonization were significantly more likely to test positive for S. pneumoniae urinary antigen than those without colonization. We found that 12·7% of clinical CAP and 17·0% of radiographic CAP cases had a blood culture or a urine antigen test positive for pneumococcus. After subtracting the background prevalence of positive urine antigen tests in controls, we estimated that 10·6% of clinical CAP and 14·9% of radiographic CAP patients had pneumococcal pneumonia based on blood culture and urine antigen test results.
This estimate is at the lower end of previous estimates of the proportion of CAP caused by pneumococcus. A recent review found that different studies have reported between 11% and 48% of hospitalized CAP to be due to pneumococcus [Reference File25]. Two studies of outpatient CAP reported that 20% and 28% were due to pneumococcus [Reference Bochud26, Reference Falguera27]. Direct comparison between studies is difficult due to the variability in study methods. Few studies have included healthy controls to assess the specificity of diagnostic tests. Some studies have used serology as part of their diagnostic algorithm, while we have found serology to have limited specificity. Other studies have used diagnostic tests that we have not included, such as PCR. There are several possible ways in which we may have underestimated the proportion of disease due to pneumococcus. Our estimate of the proportion of CAP due to pneumococcus is based primarily on the urine antigen test. Because there is no gold standard diagnostic test, we are unable to estimate the sensitivity of the urine antigen test. Any limitations in sensitivity would result in an underestimation of the proportion of CAP due to pneumococcus. Second, this population has a high level of pneumococcal vaccination, which could also reduce the proportion of CAP due to pneumococcus. Third, we did not exclude patients with prior antibiotic treatment, and the sensitivity of blood culture may have been reduced if cases had been previously treated.
Our estimates of the proportion of CAP cases attributable to pneumococcus have other inherent limitations. First, the patients we enrolled may not be representative of all pneumonia cases. We aimed to systematically recruit all patients who had a chest radiograph from three Indian Health Service clinics. Since informed consent was required for participation, hospitalized patients or outpatients who could be easily traced were more likely to be included in the study. This may lead to biased estimates of the proportion of pneumonia cases due to pneumococcus, although the direction of this bias is unknown. Moreover, this population has two- to threefold higher rates of CAP than other populations in the USA and Europe, which limits the generalizability of our estimates [Reference Watt7]. The high levels of pneumococcal polysaccharide vaccination in all groups may also affect the generalizability of our estimates. Pneumococcal polysaccharide vaccine should have no direct effect on either the urine antigen or serological tests. Pneumococcal polysaccharide vaccination could affect the tests indirectly through a reduction in pneumococcal carriage, leading to a reduction in non-specific antigenuria or a reduction in prior exposure to pneumococcal antigens and therefore in acute antibody levels. However, existing data show that polysaccharide vaccines have little impact on carriage.
Because there is no gold standard diagnostic test for pneumococcal pneumonia, it has been difficult to estimate the proportion of CAP caused by pneumococcus. To calculate the proportion of CAP cases caused by pneumococcus we assumed that the prevalence of a positive urine antigen test in control patients represented the proportion of false-positive tests in case-patients. However, we have limited data with which to test this assumption. We found that control patients with pneumococcal carriage were more likely to have pneumococcal antigenuria than those without pneumococcal carriage. Since case-patients had lower levels of pneumococcal carriage, it is possible that there was a lower prevalence of false-positive pneumococcal antigen tests in case-patients than in control patients and we have underestimated the proportion of CAP due to pneumococcus. On the other hand, it is possible that we were less able to detect pneumococcal carriage in case-patients due to unidentified prior antibiotic treatment and that case-patients had a higher level of false-positive urine antigen tests than control patients. In this situation, we may have overestimated the proportion of CAP due to pneumococcus. In this analysis, agreement between urine antigen and serological test results was fairly poor. The highest kappa (of 0·51) was obtained for the comparison of urine antigen with a ⩾twofold rise in PsaA in clinical CAP patients. The low levels of agreement suggest that serology, urine antigen testing, or both are likely to misclassify a large proportion of cases. Given poor agreement between these methods and the high proportion of controls (>5%) with a ⩾twofold rise in PsaA, we did not use serological results to estimate the proportion of CAP due to pneumococcus, and focused solely on urine antigen results. PsaA has been reported to be a promising diagnostic tool in other settings [Reference Scott15], while other serological markers have displayed a lack of specificity, possibly associated with transient pneumococcal colonization [Reference Musher17, Reference Scott, Hall and Leinonen28, Reference Musher29]. Because of difficulties with patient recall and the overlap in clinical presentation between pneumococcal pneumonia and other respiratory diseases, we did not limit specimen collection based on patient-defined symptom onset. It is possible that delays in patients seeking healthcare and thus delays in acute specimen collection could have limited the sensitivity of serological testing. However, our approach reflects what is possible in clinical settings. Further evaluation is needed to clarify the causes of changes in PsaA and PspA antibody titres.
Pneumococcal colonization rates were high in this study, and as observed previously, collecting OP in addition to NP swabs yielded additional isolates [Reference Watt30]. Carriage was lower in CAP cases than in controls; we conjectured that this may be due to increased prior antibiotic treatment in pneumonia patients, although data were not available to test this hypothesis.
This study suggests that the BinaxNOW S. pneumoniae urine antigen test can be useful for diagnosing pneumococcal pneumonia and estimating the incidence of pneumococcal CAP in a high-risk American Indian population. These incidence estimates may underestimate the true burden of disease if urine antigen testing has limitations in sensitivity for pneumococcal pneumonia. However, given the ability of the urine antigen test to discriminate between pneumonia cases and controls, it might prove useful for both clinical management and vaccine trials. In vaccine trials with a pneumonia endpoint, diagnostic tests with high specificity and greater sensitivity than blood culture for pneumococcus would allow a reduction in the sample size needed to measure vaccine efficacy.
ACKNOWLEDGEMENTS
We thank the White Mountain Apache tribe and the Navajo Nation for their support of this project. We also thank Lora Lavender, Deborah McGinty, Carol Tso, Brenda Benally, Reanna Bowie, Stella Cly, Monica Johnson and the staff of the Center for American Indian Health for collection of study data. This research was supported in part by Sanofi-Pasteur Ltd, and by the White Mountain Apache Native American Research Center for Health (National Institute of General Medicine RO1 grant U26 94 00012-01). BinaxNOW® test kits were provided by Binax, Inc. The opinions expressed are those of the authors and do not necessarily reflect the views of the Indian Health Service or the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.






