Introduction
Cancer is the leading cause of death worldwide, accounting for more than 10 million deaths each year (Zaimy et al., Reference Zaimy, Saffarzadeh, Mohammadi, Pourghadamyari, Izadi, Sarli, Moghaddam, Paschepari, Azizi, Torkamandi and Tavakkoly-Bazzaz2017). The burden of cancer continues to increase, placing a large economic, healthcare and financial burden on society (Fane and Weeraratna, Reference Fane and Weeraratna2020; Lin et al., Reference Lin, Li, Yan, Liu, Yang and Li2021). Number of new cases of cancer is expected to reach 28.4 million by 2040 (Sung et al., Reference Sung, Ferlay, Siegel, Laversanne, Soerjomataram, Jemal and Bray2021). Thus, identifying the death risk associated with patients having cancer has implications for clinically targeted interventions and treatments.
Suicide has become the 10th leading cause of death in North America (Fazel and Runeson, Reference Fazel and Runeson2020). First primary cancer (FPC) not only places a huge burden on society but also causes great psychological distress to patients (Dewar et al., Reference Dewar, Ahn, Eraj, Mahal and Sanford2021; Schofield et al., Reference Schofield, Butow, Thompson, Tattersall, Beeney and Dunn2003). Statistics show that the incidence of suicide death among patients with cancer was disturbingly high, with 39.72 per 100,000 person-years (Du et al., Reference Du, Shi, Yu, Liu, Jin, Yan-Qian, Fu, Song, Cai and Chen2020). Another overall analysis revealed an 85% increased suicide mortality rate among patients with cancer compared with the general population (standard mortality ratio (SMR) = 1.85, 95% CI = 1.55–2.20) (Heinrich et al., Reference Heinrich, Hofmann, Baurecht, Kreuzer, Knüttel, Leitzmann and Seliger2022). Besides, cancer treatment and management have changed considerably over the past decades (Carioli et al., Reference Carioli, Malvezzi, Bertuccio, Hashim, Waxman, Negri, Boffetta and La Vecchia2019; Riley et al., Reference Riley, June, Langer and Mitchell2019; Vanneman and Dranoff, Reference Vanneman and Dranoff2012), contributing to a significant increase in the survival rate among patients with FPC as well as the incidence of second primary cancer (SPC). SPC is not a phenomenon of cancer recurrence or metastasis but rather the occurrence of another cancer distinct from FPC (van der Waal and de Bree, Reference van der Waal and de Bree2010). The standardized incidence risk for any SPC was 1.16 (95% CI 1.12–1.19) (Rombouts et al., Reference Rombouts, Hugen, Elferink, Feuth, Poortmans, Nagtegaal and de Wilt2017). Therefore, more attention should be given to the risk of suicide death among patients with SPC.
Few studies have been conducted on suicide death among patients with SPC. Previous studies among patients with cancer have mainly focused on the suicide death of patients with FPC or a single cancer (Dalela et al., Reference Dalela, Krishna, Okwara, Preston, Abdollah, Choueiri, Reznor, Sammon, Schmid, Kibel, Nguyen, Menon and Trinh2016; Kam et al., Reference Kam, Salib, Gorgy, Patel, Carniol, Eloy, Baredes and Park2015; Zaorsky et al., Reference Zaorsky, Zhang, Tuanquin, Bluethmann, Park and Chinchilli2019), and suicide death has not been assessed in a large sample of patients with SPC. SPC may be a stronger stressor, leading to an increase in suicide death (Yang et al., Reference Yang, Qu, Shang, Wang, Wang, Lu and Song2022), or it may account for a decrease in suicide death because of the stronger psychological power acquired from surviving an FPC (Brinkman et al., Reference Brinkman, Recklitis, Michel, Grootenhuis and Klosky2018). As a result, relevant studies concerning suicide death among patients with SPC are urgently needed.
To identify and characterize subgroups of patients with SPC with a higher risk of suicide death, we conducted a population-based cohort study comparing them with patients with FPC. These results are expected to provide a scientific basis for developing specific suicide death management strategies tailored to the needs of patients with SPC, as well as providing insights for the reduction of suicide occurrence among this population.
Methods
Data source
Patients with cancer in this retrospective population-based study were screened from the SEER database using SEER*Stat Version 8.4.0.1. The SEER 17 database provides cancer cases from specific geographic areas, covering approximately 26.5% of the U.S. population. The SEER research data include incidence and population data, including patient age, sex, race, year of diagnosis and geographic areas (by SEER registry and county). No ethical approval was necessary as this study was based on a public database (Sturgeon et al., Reference Sturgeon, Deng, Bluethmann, Zhou, Trifiletti, Jiang, Kelly and Zaorsky2019).
Study population
A total of 5,643,421 patients with cancer were screened from 2000 to 2019 according to the International Classification of Disease Oncology (ICD-O) third edition codes. In our study, FPC was defined as ‘one primary only’, and SPC was defined as ‘1st of 2 or more primaries’ in the SEER database. The inclusion criteria were as follows: (1) cancer types defined by ICD-O third edition codes; (2) pathologically diagnosed; (3) cancer sequence numbers of ‘one primary only’ or ‘1st of 2 or more primaries’; and (4) 6 months after the diagnosis of FPC diagnosis. The exclusion criteria were as follows: (1) unknown survival time and (2) unknown race (Supplemental Figure S1). We conducted separate analyses on system-based cancer sites in the sub-analysis according to the ICD-O third edition codes. The results in current study are presented for patients with cancers of the lymphoma, digestive system, respiratory system, bones and joints, soft tissue including heart, skin excluding basal and squamous, breast, female genital system, male genital system, urinary system, eye and orbit, brain and other nervous system, endocrine system, oral cavity and pharynx, myeloma, leukaemia, mesothelioma, Kaposi sarcoma and miscellaneous.
Patient variables and outcome assessment
Patient variables were age at diagnosis (years), sex, race, year of diagnosis, socio-demographic factors (i.e., median household income, marital status), tumour characteristics (i.e., stage, cancer site and grade) and cancer treatment (i.e., chemotherapy, radiotherapy and cancer-directed surgery). In the present study, the primary endpoint event was suicide death among patients with FPC and SPC, defined as ‘suicide and self-inflicted injury’ in the SEER database, corresponding with the International Classification of Diseases (ICD) – 10th edition (ICD-10) codes as U03, X60-X84 and Y87.0. Causes of death were determined on the basis of a death certificate recorded and confirmed by the doctor in charge of the patient. The causes of death in the SEER database were categorized according to ICD-10 codes and documented through the National Center for Health Statistics. The follow-up times were from the date of cancer diagnosis to the date of death, loss to follow-up or the last follow-up date (31 December 2019).
Statistical analysis
Categorical variables at baseline were compared using the chi-square test. As the most popular model, the Cox proportional-hazards regression model is used to examine the predicted values of survival based on covariates such as treatment, age, sex, race and income to predict survival among patients under certain medical conditions. The exponent of the Cox model coefficient provides the instantaneous relative risk of a one unit increase in the relevant covariate. We used univariate Cox proportional hazards (PHs) regression analyses to identify risk factors for suicide death among patients with cancer, and multivariate analyses were employed to examine factors that were significant in univariate analyses (P < 0.05) as well as the system-based cancer sites. The PH assumption was also checked. We used Kaplan–Meier (KM) survival curves to show the cumulative incidence of suicide events in patients with cancer with different clinical characteristics. Data analyses were performed using IBM SPSS Statistics (version 21.0.1). All calculations were completed with R software (version 4.1.2). P values <0.05 were statistically significant.
Results
Study population
A total of 5,643,421 patients with cancer were screened from the SEER database, including 4,931,695 patients with FPC and 711,726 patients with SPC (Table 1). The median follow-up time was 8.32 [interquartile range (IQR) 3.75–13.42] and 12.67 (IQR 7.83–16.58) years for patients with FPC and SPC, respectively. Compared with patients with FPC, patients with SPC were more likely to be male (53.70% versus 50.60%), moderately differentiated (32.20% versus 25.20%), localized stage (61.10% versus 48.40%) and receive cancer-directed surgery (71.10% versus 60.20%). Notably, breast cancer (19.00%) was responsible for the highest number of suicides among patients with SPC, while digestive system cancer (17.70%) had the highest number of suicides among patients with FPC. The suicide death distribution by cancer site and age at diagnosis among FPC and SPC is shown in Supplemental Figure S2. Overall, patients with male genital system cancer had the highest proportion of suicide deaths (>25.00%), followed by digestive system cancers in patients with FPC and SPC of any age group.
Table 1. Demographics and clinical characteristics of the study population

FPC = first primary cancer, SPC = second primary cancer.
a includes American Indian/Alaska Native and Asian/Pacific Islander.
Cumulative incidence of suicide death among patients with cancer with KM analysis
The number of suicide death for FPC and SPC were 6801 and 851, respectively. When considering death by suicide as the outcome, the 5-year survival probability for patients with FPC and SPC was found to be 99.86% and 99.94%, respectively. We further analysed the cumulative incidence of suicide death with the KM test. Figure 1 presents the results for suicide deaths in patients with SPC. Overall, the risk of suicide increased year by year in patients with SPC. Older age at diagnosis was associated with a higher incidence of suicide death among patients with SPC (P < 0.001) (Fig. 1a) and the suicide death was not significantly different for patients undergoing chemotherapy (P = 0.118) (Fig. 1k). The year of diagnosis was not statistically significant for the risk of suicide among patients with FPC (P > 0.05) (Supplementary Figure S3). In addition, we observed a significantly lower suicide death for patients with SPC than for patients with FPC (P < 0.001) (Fig. 2).

Figure 1. Kaplan–Meier survival analysis of patients with second primary cancer.
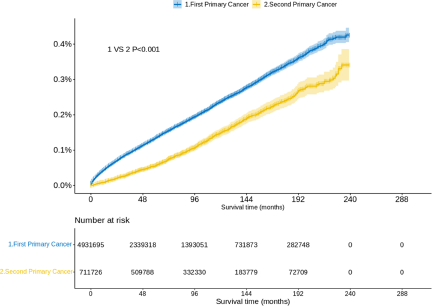
Figure 2. Probability of suicide among patients with first and second primary cancer.
Cox PHs regression analysis among patients with FPC and SPC
We used a Cox PHs regression model to estimate risk factors for survival time. In the univariate Cox analysis, older age, male sex, white race, unmarried status, advanced stage, poorer differentiation and untreated (including cancer-related surgery and radiotherapy) patients had a higher risk of suicide in both the FPC and SPC populations. Notably, chemotherapy was statistically significant for patients with FPC (p < 0.001) but not for patients with SPC (P = 0.118) in the univariate Cox analysis (Supplemental Tables S1 and S2). Multivariate Cox PHs regression further included cancer sites and statistically significant factors in univariate Cox analysis (P < 0.05). Figure 3 depicts a forest plot of multivariate Cox PHs regression analysis of patients with SPC. The results were basically consistent with single factors. Notably, cancer grade (P > 0.05), as well as radiotherapy (P = 0.115), were not statistically significant for suicide death in patients with SPC. Patients aged ≥80 years had a higher suicide death than patients with cancer aged ≤39 years (HR, 1.73; 95% CI, 1.08–2.77). Compared with patients with lymphoma, patients with female genital system cancer (HR, 3.04; 95% CI, 1.82–6.36) had the highest risk of suicide. In addition, there was no significant difference in suicide death among patients with different malignancies of the female genital system, including cervix uteri, uterus, ovary, vagina and vulva (Supplemental Table S3). Among patients with FPC, patients with mesothelioma (HR, 3.17; 95% CI, 2.02–4.98) had the highest suicide death, followed by patients with oral cavity and pharynx cancer (HR, 2.63; 95% CI, 2.24–3.09) (Supplemental Figure S4).
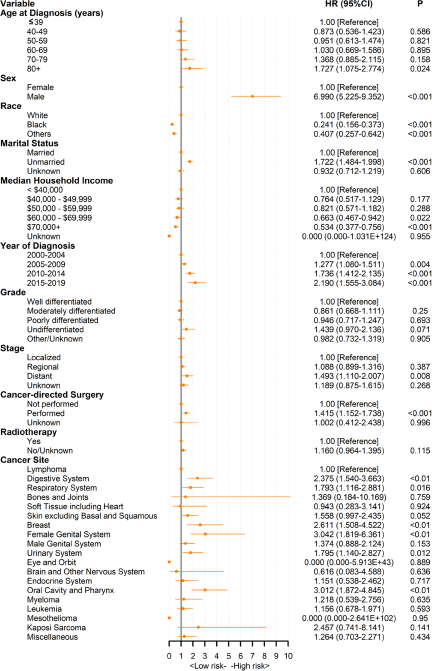
Figure 3. Multivariate Cox proportional hazards regression analyses of patients with second primary cancer. Lymphoma is used as the reference for Cox proportional hazard model.
Suicide distribution of cancer site and survival time
In the suicide death distribution of patients with SPC, suicide events occur mainly within 5 to 15 years after diagnosis. Respiratory system cancer had the highest suicide proportion within 5 years (Fig. 4a). Strikingly, patients with FPC accounted for a significantly higher proportion of suicides within the first year and within 5 years, and more than 50.00% of patients presented with suicidal behaviour during 5 years of follow-up (Fig. 4b).

Figure 4. Follow-up trends of patients with first and second primary cancer from cancer diagnosis to suicide.
Discussion
This is a large-scale study exploring suicide death among patients with SPC and FPC based on U.S. registry data. After adjusting for several potential confounders, we observed a reduced risk of suicide death among patients with SPC compared to patients with FPC throughout the follow-up period. However, patients with SPC had an increasing risk of suicide as the diagnosis year increased. Our study explored the risk of suicide in patients with SPC by comparison with patients with FPC, providing a scientific basis for suicide death management strategies for patients with SPC.
Previous studies have mainly focused on suicide by individual cancer type (Chen et al., Reference Chen, Jiang, Yang, Cai, Liu, Wu and Lin2021a; Reference Chen, Lin, Xu, Liu, Cai, Yang, Lv and Jiang2021b), or in patients with FPC (Chen et al., Reference Chen, Yu, Xiong, Zhang, Zhou, Dai, Wu and Wang2022; Ma et al., Reference Ma, Wu, Fu, Zheng, Bai and Lyu2021), which has limited our knowledge of the suicide death among patients with SPC with extensive cancer types. Our study found a reduction in suicide death with an SPC diagnosis compared to an FPC diagnosis. Although there is a lack of similar research, our study provides direction for future exploration. Previous related studies differ in part from our study. Yang et al. reported a higher risk of suicide among patients with SPC compared with patients with FPC (Yang et al., Reference Yang, Qu, Shang, Wang, Wang, Lu and Song2022). This disparity could be attributed to the inconsistency in the year of diagnosis within their sample. Their study included data from 1975 to 2016, while our study covered the period from 2000 to 2019. Notably, during earlier times, patients with SPC had poor access to good care, which likely contributed to an higher risk of death by suicide. Consequently, the overall suicide death reported by Yang et al. for patients with SPC would be likely influenced by the poor access to good care before 2000. Additionally, the survival time of patients from SPC diagnosis to suicide increased significantly, which may be associated with greater psychological power and adaptation to cancer-related treatment (Schofield et al., Reference Schofield, Butow, Thompson, Tattersall, Beeney and Dunn2003). Samson and Zerter pointed out that the experience of cancer is an experience that promotes great personal and spiritual growth, which can provoke personal growth and transformation (Samson and Zerter, Reference Samson and Zerter2003).
Notably, the risk of suicide increased year by year in patients with SPC, despite the reduction in the risk of suicide and the increase in survival time from diagnosis to suicide. Of note, we found that patients with FPC have a decreased risk of suicide as the year of diagnosis increases, while it increases among patients with SPC. Our results are in line with previous studies. For example, Ma, Wen et al. reported a significant decrease in age-adjusted suicide death from 1975 to 2017 in almost all patients with solid tumours (Ma et al., Reference Ma, Wu, Fu, Zheng, Bai and Lyu2021). Improvements in living standards and medical technology may help reduce suicide death in patients with FPC (D’Anci et al., Reference D’Anci, Uhl, Giradi and Martin2019) . For patients with SPC, the diagnosis of SPC is devastating and causes great psychological stress (Kazak et al., Reference Kazak, Derosa, Schwartz, Hobbie, Carlson, Ittenbach, Mao and Ginsberg2010; Mitchell et al., Reference Mitchell, Chan, Bhatti, Halton, Grassi, Johansen and Meader2011). At the same time, the treatment modality that patients receive after the treatment of FPC may have an effect on the occurrence of SPC (Chaturvedi et al., Reference Chaturvedi, Engels, Gilbert, Chen, Storm, Lynch, Hall, Langmark, Pukkala, Kaijser, Andersson, Fosså, Joensuu, Boice, Kleinerman and Travis2007; El-Gamal and Bennett, Reference El-Gamal and Bennett2006), which may lead to patients’ disappointment with the treatment modality (Schmid et al., Reference Schmid, Spießl and Cording2005), causing higher suicide death year by year. Although the overall risk of suicide decreased over the year of diagnosis for patients with SPC compared with those with FPC, patients with SPC have an increased risk of suicide as the year of diagnosis increases, underscoring the importance of continuous suicide interventions.
Similar to the results of previous FPC studies (Misono et al., Reference Misono, Weiss, Fann, Redman and Yueh2008; Saad et al., Reference Saad, Gad, Al‐Husseini, AlKhayat, Rachid, Alfaar and Hamoda2019; Yang et al., Reference Yang, Zhang and Hou2021), our study found characteristics associated with high suicide death among patients with SPC. Older patients (85+) had a higher risk of suicide, which may be related to poorer health status, low resistance, low quality of life, loneliness and depression among older patients with SPC (Kam et al., Reference Kam, Salib, Gorgy, Patel, Carniol, Eloy, Baredes and Park2015; Gaitanidis et al., Reference Gaitanidis, Alevizakos, Pitiakoudis and Wiggins2018; Yang et al., Reference Yang, He, Chen, Pan, Zhang, Li and Lyu2019). However, it is worth noting that regardless of cancer, older people, particularly those over 80 years of age, are at higher risk of suicide, especially when facing conditions such as chronic pain, dependence on others, loneliness, feelings of abandonment and loss of meaning. The goal of SPC suicide prevention may be included in a broader one to improve the quality of life for elder and eliminate factors that contribute to depression (Leo, Reference Leo2022). In addition, the suicide death is markedly higher among men with SPC than women, which may be due to influence by conventional masculine social norms and the worse emotion regulating than women (Möller-Leimkühler, Reference Möller-Leimkühler2002; Nolen-Hoeksema, Reference Nolen-Hoeksema2012). White patients were more likely to commit suicide than patients of other races, which may be attributable to the majority of study subjects being white (84.9%) and to the religious beliefs of various ethnic groups (Yu et al., Reference Yu, Tao, She, Liu, Wu and Lyu2022). Additionally, the marital status of patients with SPC was also linked to suicide death. We found that unmarried patients with SPC had a higher risk of suicide. Studies have revealed that married patients could receive more care and socioeconomic support from their partners, who are less likely to be depressed and commit suicide (Aizer et al., Reference Aizer, Chen, McCarthy, Mendu, Koo, Wilhite, Graham, Choueiri, Hoffman, Martin, Hu and Nguyen2013). Our study also found that patients with SPC with a median household income of over $60,000 have a lower risk of suicide. This finding corresponded to another population-based study (Suk et al., Reference Suk, Hong, Wasserman, Swint, Azenui, Sonawane, Tsai and Deshmukh2021), which may be due to the lack of mental healthcare for patients with cancer living in low-income areas.
Intriguingly, cancer grade, chemotherapy and radiotherapy had no significant effect on suicide death among patients with SPC. This may be due to the tolerance of cancer and related treatments and psychological preparation after FPC treatment. This result indicates that radiotherapy and chemotherapy can be recommended more positively in treating patients with SPC. Strikingly, patients with female genital system cancer harboured the highest suicide death among patients with SPC in the current study. The sexual impact of gynaecologic cancer treatment on physical aspects is well documented (Abbott-Anderson and Kwekkeboom, Reference Abbott-Anderson and Kwekkeboom2012; Audette and Waterman, Reference Audette and Waterman2010; Boa and Grénman, Reference Boa and Grénman2018). Ward et al. reported that patients with gynaecologic malignancies had a 1.3 times higher incidence of suicide than patients with other cancer types (Ward et al., Reference Ward, Roncancio and Plaxe2013). The high risk of suicide in patients with female genital system cancer may be multifactorial. Similar to patients with other cancers, patients with female genital system cancer bear a significant psychological burden after a cancer diagnosis (Boa and Grénman, Reference Boa and Grénman2018; Pignata et al., Reference Pignata, Ballatori, Favalli and Scambia2001). In addition, previous studies have shown that 50% of women with female genital system cancer may experience anatomical changes in their genitalia while undergoing treatment, which may have an irreversible impact on sexual function and may lead to changes in relationships with sexual partners, resulting in anxiety and depression (Andersen and van Der Does, Reference Andersen and van Der Does1994; Stead et al., Reference Stead, Fallowfield, Selby and Brown2007). Furthermore, several previous studies have shown that patients with female genital system cancer express concerns related to negative body image (Carmack Taylor et al., Reference Carmack Taylor, Basen-Engquist, Shinn and Bodurka2004). Above all, the combination of these emotional and psychological stressors may increase the risk of suicide death in women with genital system cancer.
These results further elucidate the psychological stress associated with a diagnosis of SPC and call for attention to the mental health status of patients with SPC, particularly those diagnosed in recent years who have female genital system cancer, are 85+ years of age, are white and are unmarried. Those patients need psychological interventions such as effective psychosocial care and effective suicide death screening. Given the increased risk of suicide in patients with SPC over the years, continued psychological support is of greater significance than early psychological support for these priority patients.
The major strength of our study is the large population sample of patients with SPC and FPC in the United States. The large sample size enables us to perform detailed analyses of all subgroups, increasing the reliability of our results. Using ample information on cancer characteristics and treatment modality, we are able to analyse the factors related to suicide in patients with cancer more deeply in our analysis.
Our study has some limitations. First, the specific cause of death is often difficult to identify, and the ICD-10 cause of death code is unique, so there may be a possibility of misclassification bias for death causes in the SEER database (Horn et al., Reference Horn, Stoltzfus, Mackley, Lehrer, Zhou, Dandekar, Fox, Rizk, Trifiletti, Rao and Zaorsky2020; Zaorsky et al., Reference Zaorsky, Churilla, Egleston, Fisher, Ridge, Horwitz and Meyer2017). Second, the comorbid medical and psychiatric conditions were not assessed, including factors that could influence the incidence of cancer, such as alcohol and tobacco use, which may be associated with their own risk of suicide. Likewise, supportive psychosocial care was not assessed, although combining cancer treatments with supportive care can improve prognosis while reducing adverse effects like suicide death. Third, we are unable to affirm whether the suicide event is secondary to the diagnosis of cancer, other diseases or dramatic changes in life circumstances that occurred in the interval after the cancer diagnosis. Fourth, it is difficult to estimate the effect of time-varying trends, such as therapeutic advances and people’s perceptions of cancer over the past 20 years (de Vries et al., Reference de Vries, Schaapveld, Janus, Daniëls, Petersen, van der Maazen, Zijlstra, Beijert, Nijziel, Verschueren, Kremer, van Eggermond, Lugtenburg, Krol, Roesink, Plattel, van Spronsen, van Imhoff, de Boer and van Leeuwen2021). Finally, caution should be exercised when generalizing the findings to other countries, as the SEER 17 database provides data from specific geographic area.
Conclusions
Compared with patients with FPC, patients with SPC in general had a lower risk of suicide. However, patients with SPC have an increasing risk of suicide as the year of diagnosis increases. Therefore, oncologists and related health professionals need to provide continuous psychological support to reduce the incidence of suicide, especially for those diagnosed in the most recent year, age of 85+ years, white race and unmarried patients. The results of the current study may provide some scientific basis for the development of a comprehensive suicide death scoring system for screening suicide death in patients with SPC. Larger and longer follow-up cohort studies are needed to validate the SPC–suicide correlation in the future.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796023000690.
Availability of data and materials
The datasets are publicly available from the SEER database (http://seer.cancer.gov).
Acknowledgements
We thank the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) for providing these data.
Author contributions
YJ, FZ and SY designed the study. YJ, YW, XC and ZZ downloaded and analysed data. JW, HY, GY, ZL and XC controlled the quality of data and algorithms. ZZ, JW and HY prepared for the manuscript. YJ, YW and XC wrote the manuscript. FZ and SY edited and reviewed the manuscript review. All authors read and approved the final manuscript. Yanting Jiang and Yiqi Wang contributed equally to this study. Senxiang Yan can also be contacted for correspondence, [email protected].
Financial support
This study has been supported by the National Natural Science Foundation of China (82171890 and 81701683) and Key Research and Development Projects of Zhejiang Provincial Science and Technology Department (2021C03122).
Competing interests
The authors have no conflict of interest, financial or otherwise.
Ethical standards
We obtained summary data from publish database. Therefore, no further sanction was required.







