Vitamin and mineral supplements (VMS) are widely used and available throughout the world with highest use in Canada, Asia, Australia and the USA(Reference Harris, Cooper and Relton1–Reference Steel, McIntyre and Harnett3). VMS are classified as dietary supplements in the USA with expenditure rising from USD9·6 billion in 1994 to 41·1 billion in 2016 and predicted to reach 294 billion by 2021(4–6). An estimated 50 % of American adults use dietary supplements(Reference Kantor, Rehm and Du7) including pregnant and lactating women(Reference Barnes, Barclay and McCaffery8), children(Reference Madden, DeBias and Cook9) and those living during chronic health conditions(Reference Foley, Steel and McIntyre10). The pattern of use in Australia is similar. The Australian Therapeutic Goods Administration (TGA) classify VMS as complementary medicines (CM). The TGA are responsible for regulating the quality and safety of products(11). A 2017 report found VMS to be the most used complementary medicine product (47·8 %) and the most frequently recommended by all types of health practitioners including general practitioners, specialist doctors and pharmacists(Reference Steel, McIntyre and Harnett3). However, the majority of VMS use remains self-directed use. The estimated annual expenditure on complementary medicine products by the adult Australian population in 2006 was A$1·86 billion and increased to AUD$2·9 billion in 2017(Reference Harnett, McIntyre and Steel12).
There are various motivations for VMS use, including to supplement dietary intake and to prevent diet-related diseases or deficiencies(Reference O’Brien, Malacova and Sherriff13) The increased use and sales of VMS is likely attributable to perceptions of VMS safety, a growing interest in preventative health, and marketing(Reference Burnett, Livingstone and Woods14,Reference Dwyer, Coates and Smith15) .
An under-recognised potential impact of increasing use of VMS is the potential for poisoning. The rise of palatable formulations (e.g. multivitamin ‘gummies’/chews), and marketing for children raises concerns about accidental paediatric exposures. Risk assessment for VMS poisoning can be difficult, with dietary supplement databases revealing a wide variety of products on the market(Reference Durazzo, Camilli and D’addezio16), as well as inaccuracies in labelling of stated dosing identified in some countries(Reference Andrews, Gusev and McNeal17). While most VMS products have low acute toxicity, high-dose Fe products are of particular concern. Fe exposures > 60 mg/kg can cause severe symptoms such as gastrointestinal bleeding, multi-organ failure, convulsions, coma and death(Reference Chang and Rangan18,Reference Manoguerra, Erdman and Booze19) . Given that Fe toxicity is dose-dependent and these products are easily accessible by the public, Fe products present a potential risk.
There are limited reports on VMS poisoning exposure trends worldwide. The US study using Poisons Centre data showed an overall increase in dietary supplement exposures, 2000–2012, with the majority of exposures occurring in young children(Reference Rao, Spiller and Hodges20). A study focusing on vitamin D exposures in the USA revealed a 1600 % increase in exposures, 2005–2011; however, no increase in major outcomes(Reference Spiller, Good and Spiller21). Young children account for one-fifth of US emergency department presentations related to dietary supplements, with Fe supplements being the second most commonly implicated product type(Reference Geller, Shehab and Weidle22). Up until the 1990s, Fe was the leading cause of drug-related deaths in US children(Reference Litovitz and Manoguerra23).
To our knowledge, there are no studies reporting on VMS poisoning exposures in Australia.
The present study aims to (i) identify time trends in VMS exposures, July 2014 to June 2019, (ii) identify substances most commonly implicated in VMS exposures, and (iii) describe basic demographics and disposition of VMS exposures. We report on all VMS, with a focus on Fe, due to the high risk of toxicity from Fe supplements.
Methods
Data source: New South Wales Poisons Information Centre
Australian Poisons Information Centres were established over 50 years ago and provide 24/7 advice to members of the public and healthcare professionals, taking ∼205 000 calls annually. New South Wales Poisons Information Centre (NSWPIC) is Australia’s largest PIC, taking approximately 50 % of the national call volume, including calls from New South Wales, the Australian Capital Territory and Tasmania on a near full-time basis, and after-hours calls from other jurisdictions as part of the shared on-call system of Australian PIC. Data are entered at the time of call by specialists in poisons information (with pharmacy and medical science backgrounds, and additional toxicology training). Data collected include details on substance, type of exposure, dose, basic demographics and recommended management.
Data extraction, cleaning and analysis
We searched the NSWPIC database from 1 July 2014 to 30 June 2019 for exposures to vitamins and minerals. The NSWPIC database codes substances within a taxonomy, and thus broad product categories of ‘vitamins’ and ‘minerals’ were queried. This yielded the following individual substance codes which were included in the study: multivitamins, vitamin D, Fe, Mg, B-group vitamins, Ca, vitamin C, folic acid, K, Zn, vitamin E, vitamin A, Se salts, folinic acid, phytomenadione/menadione and other mineral salts.
Basic demographic information (age and sex) is reported in categories captured by PIC, including the following age categories: neonate (birth to 4 weeks), infant (1–12 months), toddler (1–4 years), child (5–14 years), adolescent (15–19 years), adult (20–74 years) and elderly (75 + years). Disposition is recorded by PIC based on recommended management (e.g. at-home management, hospital referral and general practitioner referral), or location of the call (e.g. already in hospital or attending general practitioner). Exposure type is collected at the time of call and includes accidental exposures (e.g. paediatric exploratory behaviours), therapeutic errors (e.g. incorrect dose, incorrect time, incorrect medication, incorrect patient), adverse drug reactions (unpleasant responses to therapeutic doses), intentional (includes self-harm exposures, intentional misuse) and other/unknown.
For our detailed analysis of Fe exposures, free-text substance fields (containing information on brand/formulation) were reviewed and classified into ‘high strength’ and ‘low strength’. Products containing > 45 mg of elemental Fe dose unit (solid preparation) or per mL of liquid preparation were classified as high strength as they were above the upper level intake for Fe(24). From the calls regarding Fe supplements, we calculated the poisoning risk in each exposure, based on reported elemental Fe ingested and the patient’s body weight data (where available). Patients were classified as high risk if their calculated dose (mg/kg) was > 40 mg/kg (based on the American Association of Poisons Control Centers’ consensus guideline(Reference Manoguerra, Erdman and Booze19)).
Basic descriptive statistics (frequencies and percentages) were calculated with Microsoft Excel. For analysis of time trends, we used generalised linear modelling using R.4.0.2(25). Trends in yearly poisoning counts were fitted using Poisson modelling. Where data were over-dispersed, quasi-Poisson regression was used.
Ethical approval
Ethical approval for the present study was obtained from the Human Research Ethics Committee of the Sydney Children’s Hospitals Network (LNR/16/SCHN/44).
Results
There were 10 944 VMS exposures reported to NSWPIC during the study period, increasing by 9·6 % per year (95 % CI, 7·2, 12·1 %). The VMS most commonly involved were multivitamins (3610), followed by vitamin D (2080), Fe (1533) and Mg (804). Overall, toddlers were most commonly exposed (4546, 41·5 %), followed by adults (4169, 38·1 %). There were slightly more females than males (Table 1). Most exposures were accidental, followed by therapeutic errors, and intentional exposures (e.g. self-harm). The vast majority (77 %) could be managed at home; however, 17·7 % had hospital assessment (call originated from hospital, or PIC referred patient to hospital).
Table 1. Characteristics of 10 944 vitamin and mineral supplement exposures reported to the New South Wales Poisons Information Centre, July 2014 to June 2019
(Number and percentages)
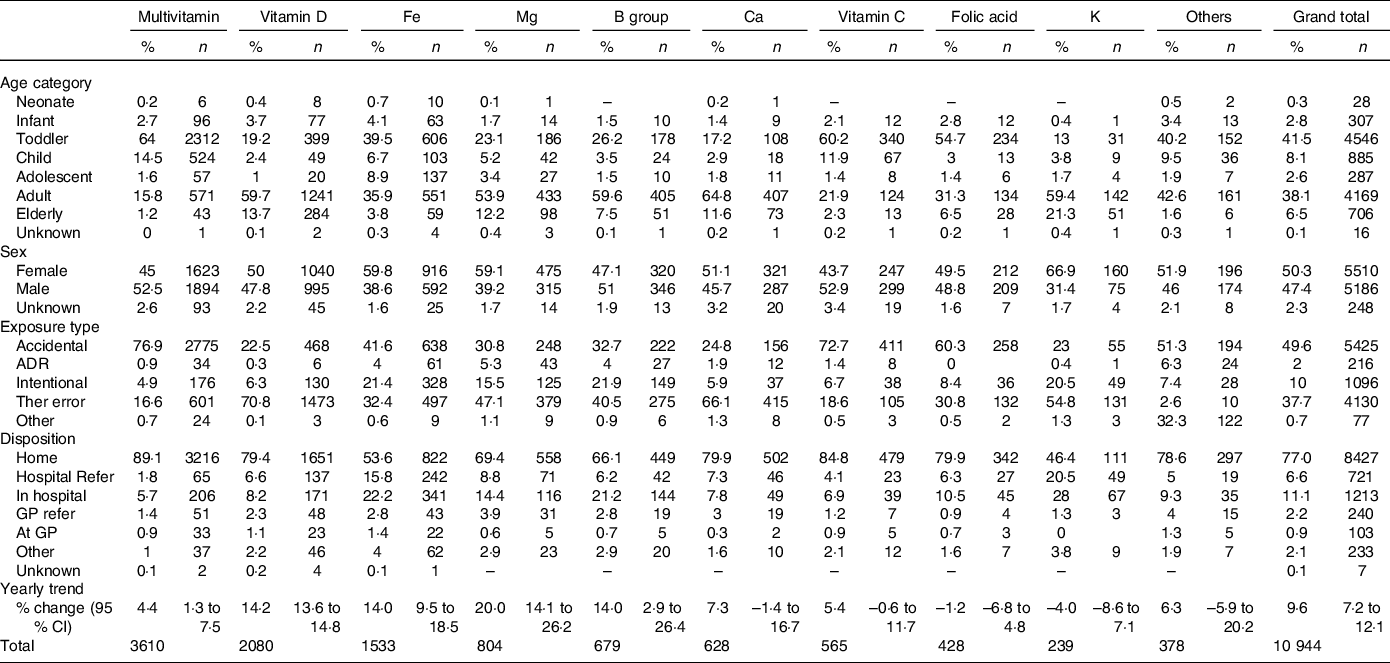
ADR, adverse drug reaction; Ther error, therapeutic error; GP, general practitioner.
Trends differed between different VMS types (Table 1). Hospitalisation rate was the highest for Fe supplements and K supplements. Multivitamins had higher rates of toddlers, whereas Ca and vitamin D were more likely to be ingested by adults (20–74 years) and older adults (75 + years). Trends over time also differed, and the largest increases seen with Mg, vitamin D, Fe and B-group vitamins (Table 1, Fig. 1).
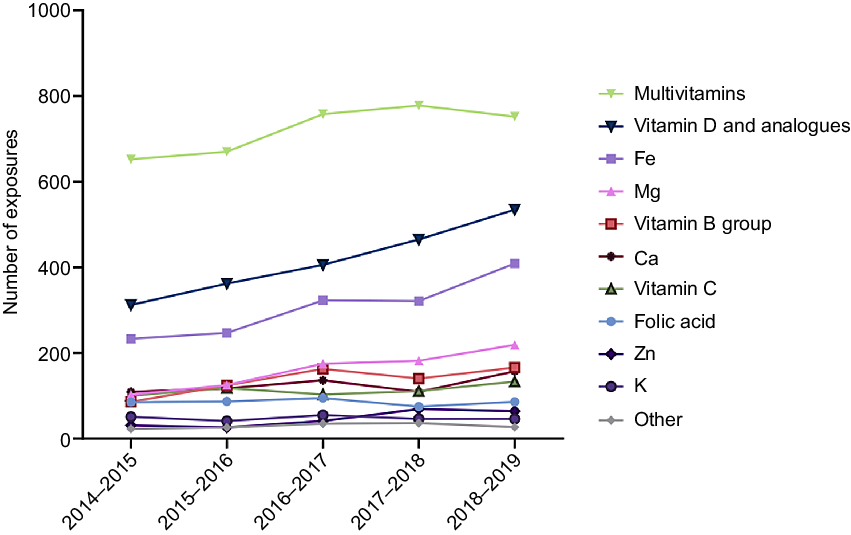
Fig. 1. Time trends in vitamin and mineral supplement exposures reported to NSWPIC. NSWPIC, New South Wales Poisons Information Centre.
Due to the high rates of use and high risk posed by Fe supplements, we examined these exposures in more detail. From 2014–2015 to 2018–2019, NSWPIC received 1036 high-strength Fe supplement calls (> 45 mg elemental Fe per unit dose), increasing by 13·6 % each year (95 % CI, 8·4, 19·7 %), compared with the 306 low-strength calls which increased by 22·1 % per year (95 % CI, 8·1, 38·2 %), Fig. 2. There were 191 Fe exposures with unknown/unspecified tablet strength. Intentional exposure was more common in high-strength calls (21·7 %) compared with low-strength calls (9·5 %). The proportion of accidental exposures and therapeutic errors were similar between the high Fe strength calls (44·1 % and 31·5 %, respectively) and low-strength calls (45·4 % and 39·2 %, respectively). When sufficient detail on product and patient weight was recorded, we conducted a risk assessment. Of the 592 eligible cases, there were 101 high-risk and 491 low-risk exposures. Of the low-risk patient category, 60·49 % were recommended to stay at home and 31·57 % were referred to hospital, whereas 92·08 % of high-risk patients were referred to hospital. High-risk patients were more likely to be symptomatic at the time of call to PIC (32·67 %, v. low risk 19·76 %).
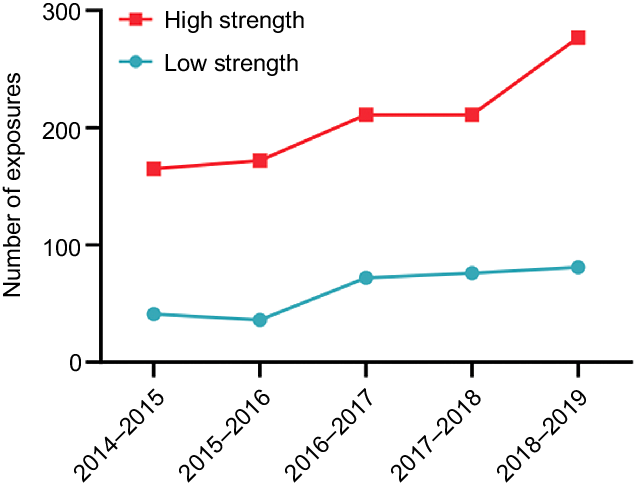
Fig. 2. NSWPIC calls regarding acute iron exposure from 2014–2015 to 2018–2019, by iron supplement strength (where known). NSWPIC, New South Wales Poisons Information Centre.
Discussion
Our study is the first to comprehensively describe Australian poisoning exposures to VMS. We show an increasing trend with VMS exposures, 2014–2015 to 2018–2019. This is consistent with literature from the USA, showing an increase in VMS exposures(Reference Rao, Spiller and Hodges20), particularly with vitamin D(Reference Spiller, Good and Spiller21). While the majority of cases can be managed at home, there is a high rate of hospital referral for some products, particularly high-strength Fe products and K salts. This is consistent with the potential for toxicity from these products(Reference Chang and Rangan18,Reference Bosse, Platt and Anderson26) . This signals the need for poisoning prevention strategies.
Several themes were identified. There were many accidental exposures in young children, reflecting normal exploratory behaviours in toddlers. This is similar to the experience in the USA, where 21 % of emergency department visits for VMS adverse events involved unsupervised children(Reference Geller, Shehab and Weidle22). In our study, over half of exposures were in children in the age of 14 years and under. The high percentage in comparison with the US study is likely attributable to the fact that the majority of these cases can be managed at home following the call to PIC. The high number of exposures in this age group could also be explained by the recent advent of palatable, multivitamin ‘gummies’ or chews, marketed for young children. These are not in child-resistant packs and are often consumed in large quantities by children who think they are confectionery. Fortunately, the vast majority of these cases can be managed at home due to the relatively low concentration of vitamins and minerals in these products. Conversely, vitamin D and Ca exposures were commonly in adults and the elderly, and often therapeutic errors, likely reflecting rates of use of these supplements in the older population. Interestingly, in contrast to our study, the US poisons centre study indicated that the majority of vitamin D exposures were in children under 6 years of age(Reference Spiller, Good and Spiller21). We speculate that this may reflect different use patterns within the community in Australia and the USA, or perhaps different packaging of products. Unsurprisingly, the VMS with a high potential for acute toxicity, Fe and K, had the highest rates of hospitalisation.
We had a particular focus on Fe supplements, due to their widespread usage and high potential for acute toxicity. Concerningly, poisoning exposures to Fe supplements are increasing. This is likely to be due to increased use of these supplements by the community. There was a 10 % annual increase in the sales of these products (personal communication, manufacturer of Ferrograd®, January 2020). These high-strength products should only be used in people with diagnosed Fe-deficiency anaemia (inappropriate chronic use can also lead to toxicity; however, this was not the focus of our study). Currently, high-strength Fe products are Schedule 2 (Pharmacy Medicine), meaning they can only be sold in pharmacies; however, they are not behind the pharmacist’s counter, and a pharmacist does not have to be involved in the sale. These products are advertised to the community. It is possible that people are self-selecting high-strength Fe without diagnosed deficiency. Increased pharmacist oversight may be necessary to reduce inappropriate use of these products and poisoning exposures. Pharmacists also have a role in providing safe storage advice for Fe, to minimise accidental paediatric exposures. Other possible preventative measures include blister packing (as opposed to bottles), which has been shown to reduce the number of tablets taken in poisonings with other substances(Reference Buckley, Newby and Dawson27). Tenenbien demonstrated that in the USA, introducing multiple preventative strategies such as limiting high-strength supplements to unit-dose packaging, enforcing child-resistant seals and new labelling regulations of products containing elemental Fe doses over 30 mg, resulted in a significant reduction in the number of accidental and intentional Fe poisoning(Reference Tenenbein28).
These results should be considered in light of the strengths and limitations of the present study. We present a large sample of Australian data, representing 50 % of Australia’s PIC calls (with 35 % of cases from non-NSW jurisdictions). We report on individual substances involved, and re-coded Fe data to report on strength. This level of detail is not available in other data sources (e.g. hospital admitted patient data collections). However, the present study also has limitations. Firstly, calls to NSWPIC are based on spontaneous self-reporting, and thus we expect this is a significant underestimate of the true incidence of these exposures in the Australian community, especially since VMS are often considered safe, meaning the public may be less likely to call. Australian PIC do not routinely conduct follow-up calls, and thus we are unable to report on outcomes of cases. Australian PIC are primarily acute poisoning reporting services, and thus we are unable to comprehensively describe chronic adverse reactions resulting from VMS. Finally, the present study is limited in its retrospective design.
Conclusion
We report an increase in VMS poisoning exposures in Australia over a 5-year period. Most exposures were managed at home; however, some products are of particular concern, including high-strength Fe and K supplements. VMS are often considered safe and without the potential for adverse effects, highlighting the importance of public education into the potential risks of misuse of VMS. Altering access, packaging and labelling of high-risk products is another option to reduce the burden of VMS harms in the Australian community.
Acknowledgements
The authors would like to thank staff at the New South Wales Poisons Information Centre for collecting the data.
U. L. was supported by a University of Sydney Summer Research Scholarship. The funder had no role in the design, analysis or writing of this article.
U. L. carried out initial analysis and drafted the original manuscript. J. H. conceived the study and provided guidance for analysis and interpretation. R. C. extracted the data and performed additional analyses and provided guidance for analysis and interpretation.
The authors declare that there are no conflicts of interest.






