Twin studies have been instrumental in determining the extent to which genes and environment influence individual differences for a wide array of diseases and disease-related traits, as well as for many normal behaviors and traits. Twin registers have been established to create a more long-term and stable framework for research (Hur & Craig, Reference Hur and Craig2013), aimed at elucidating genetic and environmental influences on health and development. In recognition of the need for collaboration, there has been increased networking of twin study groups, as illustrated by the International Network of Twin Registries (Buchwald et al., Reference Buchwald, Kaprio, Hopper, Sung, Goldberg, Fortier and Harris2014). Twin data are also useful in collaborations that involve non-twin samples. For example, twin data have been included in many consortia and genome-wide association (GWA) studies, often providing the largest numbers within the GWA consortia.
Recent advances in molecular and omic methodologies have greatly increased the utility of the twin design for uncovering mechanisms and pathways that underlie disease development (van Dongen et al., Reference van Dongen, Slagboom, Draisma, Martin and Boomsma2012). Comparing MZ twins who are discordant for a particular trait or disease provides a unique case-control study, where the cases and controls are matched perfectly for age, sex, and genetic background, as well as for the influence of in utero and early environmental factors known to affect health and developmental trajectories. Not only may these comparisons provide insight into the influence of, for example, differential exposures, behavioral choices and life events, but when DNA is available for the twins, the contribution of epigenetic factors may also be explored. Despite the full identity of the genomic sequence in twins from MZ pairs, MZ twins often differ in concordance for complexly determined health and behavioral outcomes, such as diabetes, cancer, cardiovascular disease, or mental health. Epigenetic factors are one possible mechanism that could explain these intra-pair differences. The addition of data from DZ DISCOTWIN pairs and other family members helps to elucidate the causal directions within those pathways (de Moor et al., Reference De Moor, Boomsma, Stubbe, Willemsen and de Geus2008; Kujala et al., Reference Kujala, Kaprio and Koskenvuo2002).
Scarcity of discordant pairs is the main hindrance to more prominent use of the co-twin control design. For any given disease or trait under study, the number of discordant MZ twin pairs is often limited as a function of heritability and with increasing prevalence of the disorder (Smith, Reference Smith1970). As in genome-wide association studies (GWAS) consortia, collaboration is the best way to increase numbers, such that fully powered epigenetic studies are possible. Many of twin registers already have strong connections, in particular the European and Australian twin registers, which as part of the fifth European Framework Program (Quality of Life and Management of the Living Resources, 1998–2002; European Commission, 2004) worked together within the GenomEUtwin consortium (Peltonen & GenomEUtwin, Reference Peltonen and GenomEUtwin2003). This collaboration led to the publication of comparative twin analyses for various traits, including body composition (Kettunen et al., Reference Kettunen, Perola, Martin, Cornes, Wilson and Montgomery2009; Perola et al., Reference Perola, Sammalisto, Hiekkalinna, Martin, Visscher and Montgomery2007; Schousboe et al., Reference Schousboe, Willemsen, Kyvik, Mortensen, Boomsma, Cornes and Harris2003; Silventoinen et al., Reference Silventoinen, Sammalisto, Perola, Boomsma, Cornes, Davis and Kaprio2003), exercise participation (Stubbe et al., Reference Stubbe, Boomsma, Vink, Cornes, Martin, Skytthe and de Geus2006; Vink et al., Reference Vink, Boomsma, Medland, de Moor, Stubbe, Cornes and de Geus2011), migraine (Mulder et al., Reference Mulder, Van Baal, Gaist, Kallela, Kaprio, Svensson and Palotie2003), subarachnoid hemorrhage (Korja et al., Reference Korja, Silventoinen, McCarron, Zdravkovic, Skytthe and Haapanen2010), and cholesterol (Surakka et al., Reference Surakka, Whitfield, Perola, Visscher, Montgomery and Falchi2012). With this past collaborative success in mind, a new twin consortium has now been created specially focusing on discordant twins: the DISCOTWIN consortium. In addition to extensive longitudinal data on health and lifestyle, information on biomarkers, and genotypic information is also available within these cohorts, enabling the study of the effect of unique environmental influences as well as epigenetic studies.
As a demonstration project, we examined twin discordance for T2D. T2D is a complex disease, caused by insufficient pancreatic beta-cell action and/or progressive insulin resistance, and related to increasing adiposity, inactivity, and age (McCarthy, Reference McCarthy2010). It leads to a significantly reduced life expectancy and, due to its chronic multi-systemic comorbidity, is a massive drain on healthcare resources (Zimmet et al., Reference Zimmet, Alberti and Shaw2001). In 2014, the World Health Organization (WHO, 2015) estimated the global prevalence of diabetes to be 9% among adults aged 18 years or over, with ~90% of these due to T2D (http://www.who.int/mediacentre/factsheets/fs312/en/). The dramatic increase in prevalence of T2D in the last 50 years is strongly driven by the rise of the modern ‘obesogenic’ environment, with the associated rapid upsurge in obesity due to the combined effects of reduced physical activity and increased access to high calorific foods (Friedman, Reference Friedman2009). These serious escalating T2D prevalence rates, while first appearing in developed Western nations, are now rapidly emerging within the developing world as they increasingly adopt modern urban lifestyles (Gluckman et al., Reference Gluckman, Hanson, Zimmet and Forrester2011). Heritability plays a role in T2D, with estimates from twin and family studies ranging from 25% up to 69%, depending upon age and glycemic cut-offs (Almgren et al., Reference Almgren, Lehtovirta, Isomaa, Sarelin, Taskinen and Lyssenko2011; Poulsen et al., Reference Poulsen, Kyvik, Vaag and Beck-Nielsen1999). GWAS have now confirmed 92 common single nucleotide polymorphism (SNP) T2D-risk associations, including genes potentially implicated in both insulin secretion and insulin resistance, as well as unknown mechanisms of pathogenicity (Bonnefond & Froguel, Reference Bonnefond and Froguel2015).
For adequate prevention and treatment protocols, more insight into the pathways from genes to T2D development is needed. The use of twin-pairs discordant for T2D presents a unique way to disentangle environmental, genetic, and epigenetic causes of the disease. Concordance rates for T2D have been estimated in numerous studies over the years, and these have varied for multiple possible reasons, including gene pool differences, regional variation, the influence of a changing prevalence over time, age at comparison, as well as different diagnostic criteria. In an early study to distinguish between T1D and T2D, as opposed to previous childhood- and adult-onset diabetes categories, Barnett et al. (Reference Barnett, Spiliopoulos, Pyke, Stubbs, Burrin and Alberti1981) observed 48 of 53 (90.6%) MZ twin pairs to be concordant for T2D in the United Kingdom. In 1987, an American study estimated an MZ concordance rate of 58% for the National Heart, Lung, and Blood Institute Twin Study, with a population-expected prevalence at that time of 10% (Newman et al., Reference Newman, Selby, King, Slemenda, Fabsitz and Friedman1987), while a Japanese study (Committee on Diabetic Twins, 1988) showed that for 83% of MZ twins with T2D, their co-twin could also be diagnosed, while this was only 40% in the DZ twins. Subsequently, in a Finnish study including 505 twins, Kaprio et al. (Reference Kaprio, Tuomilehto, Koskenvuo, Romanov, Reunanen, Eriksson and Kesäniemi1992) found a probandwise 34% and pairwise 20% MZ concordance, compared to 16% and 9% respectively in DZ twins. In the United Kingdom, MZ concordance rates for twin pairs who were initially ascertained as disease-discordant, were 17%, 33%, 57%, and 76% after 1, 5, 10, and 15 years of follow-up, respectively (Medici et al., Reference Medici, Hawa, Ianari, Pyke and Leslie1999), clearly demonstrating the effect of age and necessity for significant follow-up. The concordance rate was 96% when twins were classified for any glucose abnormality by 15 years. More recent data gathered in 2010 from a Finnish population-based study encompassing 1,332 twins (MZ and DZ) who developed T2D showed a similar pattern of concordance as for the earlier Finnish study, with 41% probandwise and 34% pairwise for MZ and 19% probandwise and 12% pairwise for DZ twins. Over the follow-up period of up to 28 years, ~50% of MZ co-twins subsequently became type 2 diabetic while the rate for DZ was approximately one third (Lehtovirta et al., Reference Lehtovirta, Pietiläinen, Levälahti, Heikkilä, Groop, Silventoinen and Koskenvuo2010). At present, few studies have applied the discordant design to T2D. Using a longitudinal discordant design, Kujala et al. (Reference Kujala, Kaprio and Koskenvuo2000) showed that lifestyle factors may influence a genetic pre-disposition for diabetes, as physically active twins developed diabetes less often than their sedentary co-twins. Ribel-Madsen et al. (Reference Ribel-Madsen, Fraga, Jacobsen, Bork-Jensen, Lara, Calvanese and Poulsen2012) observed small quantitative differences in DNA methylation in skeletal muscle and adipose tissue in T2D discordant MZ twins, while Bork-Jensen et al. (Reference Bork-Jensen, Scheele, Christophersen, Nilsson, Friedrichsen, Fernandez-Twinn and Vaag2015) found differences in T2D discordant MZ pairs in the regulation of several microRNAs. Furthermore, acquired obesity has been shown to significantly contribute to insulin resistance and pre-diabetes in weight-discordant MZ twin pairs (Naukkarinen et al., Reference Naukkarinen, Rissanen, Kaprio and Pietiläinen2012).
Here, we examine the T2D prevalence, concordance pattern, and heritability in same-sex twin pairs aged 45 years and older, in twin cohorts from seven European countries (Denmark, Finland, Norway, the Netherlands, Spain, Sweden, and the United Kingdom) and from Australia, and identify T2D discordant MZ twin pairs for future in-depth genotyping, omics, and phenotyping studies.
Materials and Methods
Participants and Cohorts
Table 1 provides an overview of the number of complete twin pairs available for each of the twin cohorts, as well as their average age. All cohorts that provided data for this paper have been described in detail elsewhere, but a brief description of each register is provided below.
TABLE 1 Overview of the Countries and Cohorts Involved, the National Diabetes Prevalence and the Cohort T2D Prevalence
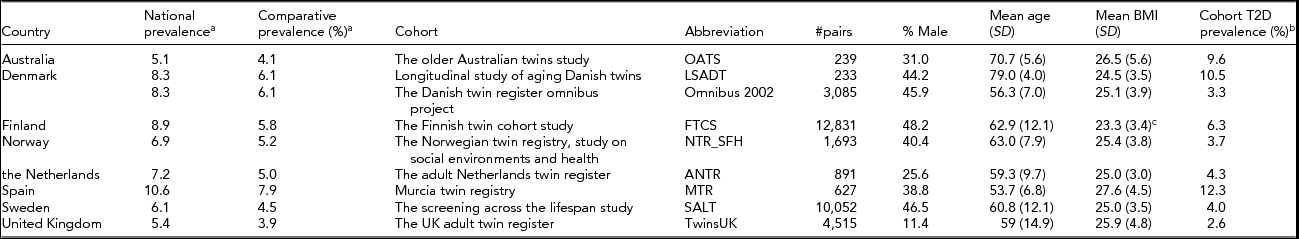
aPrevalence as reported by the International Diabetes Federation (2015). The national prevalence is the percentage adults with diabetes within the country. The comparative prevalence is the prevalence of diabetes corrected for the age profile of the population, using the age profile of the world population, to be used for cross-country comparison purposes only. bIn the Danish cohorts (LSADT and Omnibus 2000) this reflects overall diabetes prevalence. cBaseline BMI in 1975, not assessed at end of follow-up for cumulative prevalence in the FTCS.
The Older Australian Twins Study (OATS) is a multicenter, longitudinal study that commenced in 2007. Study participants are identical and non-identical twin pairs aged 65 years and older living in the three eastern states of Australia who completed a comprehensive face-to-face assessment, including data on demographic, psychiatric, neuropsychological, and medical measures. The initial cohort comprised 623 individuals (including 284 twin pairs), and 450 participants completed a follow-up assessment at two years. For the majority of participants, DNA was extracted from peripheral blood samples and/or saliva samples. For more details, see Sachdev et al. (Reference Sachdev, Lee, Wen, Ames, Batouli and Bowden2013).
The Longitudinal Study of Aging Danish Twins (LSADT) is a longitudinal survey among Danish twins aged 70 years and older, followed every second year for up to six times from 1995–2005. In 1995, the twins were aged 75 years or older, but intake age was progressively decreased to age 70 years in the 1999 survey. A total of 4,731 twins have completed an intake LSADT assessment comprising 1,152 pairs. Approximately 80% of the participants have provided blood spots and 689 twins, comprising 290 same-sexed pairs, provided full blood samples in 1997 (Skytthe et al., Reference Skytthe, Christiansen, Kyvik, Bødker, Hvidberg, Petersen and Christensen2013). The data in the present study were based on intact pairs who participated in survey 2005.
The Danish Twin Register Omnibus Project (‘Omnibus 2002’) is a longitudinal survey among the twin birth cohorts 1953–1982 from the population-based Danish Twin Registry. Participants received a nationwide questionnaire about physical health and health-related behavior in 1994 and a follow-up survey in 2002. As most of the participants reported no diabetes in the 1994 survey, the follow-up from 2002 was used to obtain the numbers of discordant pairs (Fejer et al., Reference Fejer, Hartvigsen and Kyvik2006).
The Finnish Twin Cohort Study (FTCS)
The older cohort of the Finnish Twin Cohort Study consists of same-sex twin pairs born before 1958 and initially recruited in 1975. Follow-up questionnaires have been sent in 1981, 1990, and 2011 (Kaprio, Reference Kaprio2013), and the twins have been linked to national medical registers (hospital discharge, medication, and cause-of-death data). Information from these registers, hospital charts, and questionnaire surveys were used to identify and classify twins with diabetes and the type of diabetes. Cases up to the end of 2004 were identified. For details, see Lehtovirta et al. (Reference Lehtovirta, Pietiläinen, Levälahti, Heikkilä, Groop, Silventoinen and Koskenvuo2010).
The Norwegian twin registry study on social factors and health (NTR_SFH)
The NTR was established in 2009 as a merger of three major population-based Norwegian Twin Panels (Nilsen et al., Reference Nilsen, Brandt, Magnus and Harris2012). It is housed at the Norwegian Institute of Public Health (NIPH) in Oslo. The NTR contains approximately 30,000 consenting twins, covering birth years 1896–1960 and 1967–1991, and has conducted studies in several areas including social factors, coronary heart disease, and its risk factors, mental health, dementia, cancer, and the epigenetics of autoimmune diseases (Nilsen et al., Reference Nilsen, Knudsen, Gervin, Brandt, Røysamb, Tambs and Harris2013). Among its strengths are linkage opportunities with nationwide health and socio-economic registries. The NTR data reported here are from a recent study conducted by Dr Harris on nearly 6,000 twins aged from 40–80 years, to study social factors and health.
The Adult Netherlands Twin Register (ANTR) is an ongoing twin-family study on health-related behavior that assesses families with adolescent and (young) adult twins since 1991 and includes data on more than 22,000 individuals. Participants are invited every two or three years to complete a survey that contains questions about health, lifestyle, personality, and psychopathology. In addition, participants have been invited to provide DNA. For more details, see Willemsen et al. (Reference Willemsen, Vink, Abdellaoui, den Braber, van Beek, Draisma and Boomsma2013) and http://www.tweelingenregister.org.
The Murcia Twin Registry (MTR) is a population-based register of adult twins in the region of Murcia, located in south-east Spain. To date, the MTR has identified 2,281 participants born between 1940 and 1966. Information is obtained longitudinally by face to face or telephone interviews and includes data on demographics, health, lifestyle, and related variables. In addition, biological samples (DNA) have been collected in a subset of the sample. For more details, see Ordoňana et al. (Reference Ordoñana, Rebollo-Mesa, Carrillo, Colodro-Conde, García-Palomo, González-Javier and Pérez-Riquelme2013).
The Screening Across the Lifespan of Twins Study (SALT)
Data were collected during a computer-assisted telephone interview between 1998–2002 on Swedish twins born 1958 or earlier. Introductory letters describing the study were sent to a random sample of approximately 1,000 twin pairs each month. A checklist of common illnesses, prescription, and non-prescription medication use was included in the study, and special emphasis was put on diagnostic items that could determine whether a twin was likely to have a disease (rather than simply asking the twin whether they have a disease). Items were presented in a branching format such that individuals were asked follow-up items (within item domain) if they responded positively to key introductory items. In total, 44,919 twin individuals participated in the SALT study (Lichtenstein et al., Reference Lichtenstein, deFaire, Floderus, Svartengren, Svedberg and Pedersen2002; Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlström and Pedersen2006).
The UK adult twin register (TwinsUK)
The TwinsUK Resource is an active, nationwide adult twin registry based within the Department of Twin Research & Genetic Epidemiology, at the St. Thomas’ Hospital campus, King's College London. The resource recruits voluntary MZ and DZ same-sex twins throughout the country via media drives, and the registry currently has ~13,000 registered twins. These twins are predominantly healthy, female (83%), with equal numbers of MZ (51%) and DZ (49%), and mostly middle-aged and older (range 18–103; mean 61 years). The database has an extensive range of phenotypes obtained via questionnaires, and over 7,000 have attended detailed clinical visits in the department over this period, enabling blood/urine/tissue sample collection, with many visiting at multiple time-points from 1992 onwards (~700 with four visits). These resources are used to study the genetic and environmental etiology of complex diseases, as well as the healthy aging process (see Moayyeri, Hammond, Hart et al., Reference Moayyeri, Hammond, Hart and Spector2013; Moayyeri, Hammond, Valdes et al., Reference Moayyeri, Hammond, Hart and Spector2013; and http://www.twin-research.ac.uk).
Type 2 Diabetes Phenotyping
Data were included if participants were aged 45 years or over and did not report Type 1 diabetes. T2D was established in most cohorts (OATS, ANTR, SALT, NTR_SFH, TwinsUK) by questions in a survey asking whether a doctor ever diagnosed diabetes and whether any diabetes-related medication was used. In some cohorts, additional information was available from national/regional health registers (FTC, MTR, TwinsUK) or fasting blood glucose and/or insulin levels (OATS, ANTR). The exceptions were the Danish cohorts (LSADT, Omnibus 2002) where participants were asked to indicated whether they had diabetes with no distinction of type.
Analyses
Descriptive and genetic analyses were based on summary data on prevalence and twin concordance and discordance, provided by the individual cohorts. Meta-analysis of the concordance data was carried out using structural equation modeling in Mx (Neale et al., Reference Neale, Boker, Xie and Maes2003). Data on the number of pairs concordant and discordant for T2D were the basis to estimate tetrachoric correlations for MZ and DZ twins, assuming an underlying normal liability distribution (Falconer & Mackay, Reference Falconer and Mackay1996), where the two categories are the result of a cut-off or threshold reflecting the prevalence of the trait (i.e., above the threshold T2D is indicated). Next, the contribution of additive genetic (A), common environmental (C), and unique environmental (E) influences to the variance in T2D liability was estimated by specifying a model in which A factors are correlated unity in MZ twin pairs and in DZ twins 0.50. Common environmental factors are correlated one in MZ and DZ twin pairs. The contribution of unique environment is obtained by constraining the sum of the squared path loadings due to A, C, and E to unity (Rijsdijk & Sham, Reference Rijsdijk and Sham2002). The number of estimated parameters within the model is three (one threshold and two path loadings). To test for heterogeneity, data from all cohorts were simultaneously analyzed. First, estimates for thresholds and path loadings were allowed to differ and next, path loadings were constrained to be equal across the cohorts. Within this model, 11 parameters (nine thresholds and two path loadings) were estimated. At a difference of 16 degrees of freedom (comparing the combined model with 11 parameters to the sum of the nine individual models with three parameters each), heterogeneity is implied if the difference in chi-square between the model and the total of the individual cohort models exceeds 26.296.
Results
Table 1 shows the distribution of the total number of twin pairs (34,166 pairs) across the participating cohorts. In addition, the table provides the national prevalence and the comparative prevalence (the prevalence adjusted for the age profile of the nation) for each of the countries involved, as obtained from the International Diabetes Federation (IDF, 2015), and the cohort prevalence, as calculated based on the occurrence of diabetes in the same-sex twin pairs. The T2D prevalence data show a clear increase with age and BMI for T2D, but also reveal country differences, with Spain showing the highest and the United Kingdom showing the lowest rates of diabetes. The twin cohort prevalence broadly reflects the pattern that is seen in the standardized figures from the IDF, though some cohorts may have increased or decreased rates, possibly due to the age range of the participants or the varying criteria used for diagnosis.
Table 2 provides information on the number of MZ and DZ pairs and the rates of T2D concordance and discordances by country. Overall, the majority of the twin pairs (87.6–97.2%) are concordant for not having T2D, and the concordance rates for having T2D are lower than for being T2D discordant. The T2D discordance rates differ across the cohorts, ranging from 2.9–11.2% for the MZ pairs and from 4.9–13.5% in same-sex DZ pairs, while discordance rates are always lower in MZ than in DZ twin pairs in all countries. Collectively, there are 716 (5.1%) discordant MZ twin and 1,619 (8.0%) discordant same-sex DZ twin pairs. Cohorts with more old age individuals and a higher T2D prevalence contribute relatively more to the number of T2D discordant pairs.
TABLE 2 The Number and Percentage of Twin Pairs Concordant and Discordant for Having T2D Diabetes for Each of the Cohorts
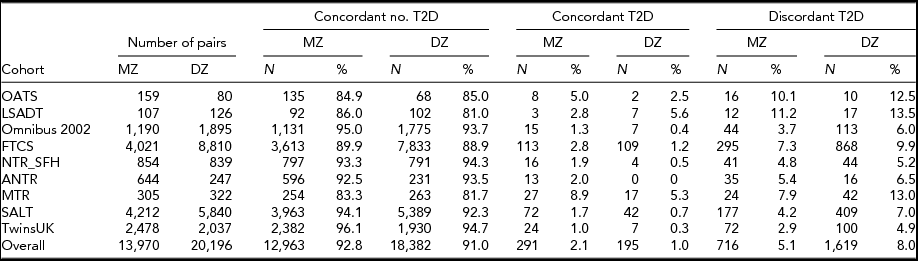
Collectively, there are 716 (5.1%) discordant MZ twin and 1,619 (8.0%) discordant same-sex DZ twin pairs. Cohorts with more old age individuals and a higher T2D prevalence contribute relatively more to the number of T2D discordant pairs.
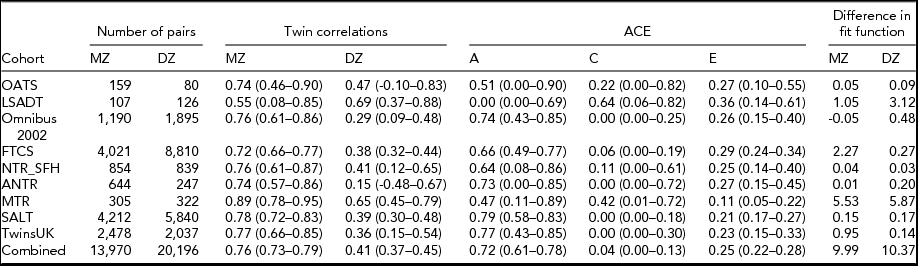
Table 3 presents the results of genetic analyses. The MZ correlations are remarkably similar across the cohorts, with six of the eight correlations ranging within 0.72–0.78, and while the DZ correlations show more variation, five of the eight are within the range of 0.36–0.47. With respect to the ACE modeling, again a consistent pattern emerges. In all cohorts but LSADT, there is moderate to high heritability in the liability to T2D, with estimations for the larger cohorts ranging from 64% to 79%. The influence of common environment is only suggested in the smaller cohorts, where the large confidence intervals indicate the uncertainty about these estimates. When analyzing all cohort data simultaneously and equating the path loadings from the ACE factors, the chi-square for the model was 31.31. The chi-square estimates for the individual models added up to 10.95, resulting in a chi-square difference of 20.18 which, at a difference of 16 degrees of freedom, indicates there is no significant evidence for heterogeneity. To explore the difference in chi-square more closely, the difference in fit function between the individual models and the combined model was examined (see Table 3): more than 50% of the difference in chi-square is accounted for by the MTR. Overall, the results show that additive genetic effects account for the majority (72%) of the variance in liability to T2D.
TABLE 3 Twin Correlations, the Percentage Explained Variance by A, C, and E for the Full Model and the Difference in Fit Function Between the Individual Models and the Combined Model in Which the ACE Estimates Were Equated for the Cohorts
Discussion
Although the discordance for T2D within MZ twin pairs is relatively low, the DISCOTWIN consortium is able, by combining the resources of twin cohorts, to identify a substantial number of T2D discordant MZ pairs. The collaborative effort of DISCOTWIN also allows for good estimates of T2D heritability and MZ concordance, which are needed as the previous literature has been inconsistent. The data collected from different countries were surprisingly consistent, with all cohorts reporting over 87% concordance in MZ pairs, due mostly to both twins reporting no T2D. When one of the twins reported T2D, in 20–53% of the MZ pairs the co-twin also reported T2D, while this was much lower (0–29%) for DZ twin pairs. The increasing prevalence of obesity as the main risk factor for T2D may be responsible for further increases in rates of concordance. In a way, the concordance rate is perhaps the upper limit for the potential of predictive strategies for personalized medicine, and high concordance rates for T2D are therefore encouraging for future efforts at early prediction using omics.
The numbers observed in the EURODISCOTWIN consortium will be sufficient to look at more complex interactions and follow-up on findings by replication and in-depth analyses. The current overview is pre-dominantly based on survey data and thus reflects the starting point for twin pair selection. Most twin registers are able to contact their twin participants for additional phenotyping or can link to national health register or population databases to obtain more refined diagnoses. For selected twin pairs, T2D status can therefore be verified, for instance, by fasting glucose or glucose tolerance tests, or via doctor diagnosis in patient records. A thorough verification of the type of diagnoses may still be necessary, even when excluding Type 1 diabetes cases and restricting the sample to age 45 years and over, as late-onset autoimmune diabetes of adulthood (sometimes called LADA, slow onset diabetes or diabetes type 1.5) may occur frequently and is often mistakenly taken for T2D (Landin-Olsson, Reference Landin-Olsson2002). It should be noted that the recruitment method and type of data available may also influence the discordance rates. For instance, register data may not cover all twins in the population, while those relying on hospital records will have an under-representation of cases. When comprehensive medication data are available (as in the Nordic countries), all medicated cases will be identified, but unless survey data are available, non-medicated individuals will be missed. Many non-medicated cases will become registered in the healthcare system and receive medication over time. Hence, models that take into account the lifecourse and development of T2D as well as censoring and competing causes of death will provide more sophisticated estimates of the overall contribution of genetic factors to the disease. Within the DISCOTWIN consortium, a wide range of such selection strategies across the countries are available, with sometimes the possibility of combining strategies. In addition to the possibility of follow-up on diagnoses and the acquisition of new genotypic and phenotypic data in identified pairs, twin registers generally have already followed their twin participants for a number of years, thereby allowing investigations into lifestyle and other behavioral differences in the T2D discordant pairs, using earlier (longitudinal) data. Establishing a format for information collection in discordant twin pairs, once they are identified, will result in an ever-growing informative database, as more twins reach the age at which T2D may be expected to develop, with data preceding and following T2D development. The follow-up of discordant T2D pairs may be particularly important, since the non-T2D twin is at increased risk of developing T2D, as may be evident from the higher T2D concordance in the older twin cohorts and as documented by Lehtovirta et al. (Reference Lehtovirta, Pietiläinen, Levälahti, Heikkilä, Groop, Silventoinen and Koskenvuo2010).
The contingency tables allowed for an estimation of the heritability that provided a consistent pattern across the cohorts. Heterogeneity was not implied, and using the combined data the heritability for T2D was estimated at 72%. This is in line with the upper limit (69%) reported by Almgren et al. (Reference Almgren, Lehtovirta, Isomaa, Sarelin, Taskinen and Lyssenko2011) when the age range was restricted to 35–60 years. In the present study, data were restricted to twin pairs who were at least 45 years of age at the time the T2D assessment was obtained, to ensure both twins had a chance to develop T2D. It is likely that age differences contribute to the differences in heritability estimates for T2D across studies. The high heritability seen here for our twins further reduces the chances of identifying T2D discordant MZ pairs, thereby providing additional support for the establishment of the DISCOTWIN consortium to obtain sufficient numbers for more in-depth study.
The combination of phenotypic and genotypic information, especially when collected longitudinally, in these T2D discordant pairs can lead to important new insights into epigenetic processes. With regards to epigenome-wide association studies (EWAS), a population-based case-control methodology encounters significant issues, particularly due to the inherent power reduction because of the influence of genetic heterogeneity on the epigenome (Mill & Heijmans, Reference Mill and Heijmans2013). This genetic confounding effect may be direct, in cis or trans adding huge complexity, especially if size effects are small. Leveraging the virtually identical genetic backgrounds of MZ twins in a disease-discordant model has thus immense advantages in the hunt for disease-associated non-genetic factors. Accounting for these confounding genetic effects and additionally many shared environmental and cohort effects provides significantly greater power than other study designs (Rakyan et al., Reference Rakyan, Down, Balding and Beck2011), and these combined numbers across the consortium will enable rigorous replication of small scale positive findings, as well as novel discoveries to be made. Many other omics technologies may be applied to disease discordant twins (metabolomics, transcriptomics, glycomics, metagenomics, proteomics), providing a complete picture of the processes involved in disease development and progression (Pallister et al., Reference Pallister, Spector and Menni2014).
In conclusion, through collaboration, the twin registers can generate the numbers and data to conduct in-depth analyses of the (epi)genetic and environmental pathways that lead to twin differences in disease. With the discordant twin design as a powerful base, the DISCOTWIN consortium can provide new insights that may lead to prognostic, predictive, and therapeutic targets for the prevention and treatment of a wide range of diseases, such as T2D.
Acknowledgments
OATS would like to acknowledge access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the Australian National Health & Medical Research Council (NHMRC). OATS is supported by a NHMRC/ARC Strategic Award Grant (ID 401162), and a NHMRC Project Grant (ID 1045325) and Program Grant (ID 350833). Karen Mather is supported by an Alzheimer's Australia Dementia Research Foundation Postdoctoral Fellowship. We gratefully thank all our participants, and wish to acknowledge the OATS research team and Chief Investigators, in particular Julian Trollor, Henry Brodaty, David Ames, Nick Martin, Margie Wright, Katherine Samaras, Teresa Lee, and Wei Wen. LSADT was supported by the National Institute on Aging grant P01 AG08761. The Danish Omnibus survey was supported by The Danish Medical Research Council and the Novo Nordic Foundation. The FTCS is funded by Academy of Finland grants, 265240, 263278 to JK and 272376 and 266286 to KP, and Novo Nordisk Foundation (KP). Data from the Norwegian Twin Registry is supported, in part, from the European Union's Seventh Framework Programs ENGAGE Consortium (grant agreement HEALTH-F4-2007-201413, and BioSHaRE EU (grant agreement HEALTH-F4-2010-261433). ANTR was supported by the Netherlands Organization for Scientific Research (900-562-137, 904-61-090, 985-10-002, 904-61-193, 56-464-14192, 400-03-330, 480-04-004, 400-07-080, 911-09-032, 451-06-004, 451-08-026, 451-10-005), the Netherlands Organization for Health Research and Development (ZonMW 3100.0038, 940-37-024, 31160008), EMGO+ Institute for Health and Care Research, Neuroscience Campus Amsterdam, Center for Medical Systems Biology, BBMRI – NL (184.021.007: Biobanking and Biomolecular Resources Research Infrastructure), National Institutes of Health (NIH 5R37DA018673-03, R01 MH059160, 1RC2 MH089951-01, 4R37DA018673-06, 1R01 MH087646-01A1), National Institute of Mental Health (RFA MH08120), FP7 ENGAGE (FP7-HEALTHF4-2007-201413), European Research Council (230374-GMI, 284167). The MTR is funded by Fundación Séneca, Murcia, Spain (15302/PHCS/10) and grateful to the Murcia Health Council for data validation accessibility (CMBD and OMI-AP). SALT was supported by grants from the NIH (AG08724), The Swedish Council for Working Life and Social Research, and the Swedish Research Council. TwinsUK is funded by the Wellcome Trust and European Community's Seventh Framework Program (FP7/2007–2013). They also receive support from the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust in partnership with King's College London. Tim Spector is holder of an ERC Advanced Principal Investigator award. SNP Genotyping was performed by The Wellcome Trust Sanger Institute and National Eye Institute via NIH/CIDR.





