Late-life depression is a multifactorial illness that is associated with neurobiological, psychological and social factors. With regard to the neurobiological factors, it is unlikely that depression is simply associated with abnormalities of a single brain region. Rather, it is postulated that depression is associated with disruption of the neural networks that regulate mood and behaviour. In particular, disruption of frontal-subcortical and limbic circuitry is hypothesised to play a key role in late-life depression. Reference Alexopoulos1 Magnetic resonance imaging (MRI) studies have made an important contribution to the hypothesis that abnormalities in both grey and white matter contribute to network disruption in late-life depression. With regard to grey matter, a recent meta-analysis reported significant volume reductions in late-life depression in the hippocampus, orbito-frontal cortex, putamen and thalamus. Reference Sexton, Mackay and Ebmeier2 With regard to white matter, reduced integrity within frontal and temporal lobes has been frequently reported in late-life depression, Reference Sexton, Mackay and Ebmeier3 as well as a more widespread pattern of white matter abnormalities. Reference Sexton, Allan, Le Masurier, McDermott, Kalu and Herrmann4,Reference Shimony, Sheline, D'Angelo, Epstein, Benzinger and Mintun5 However, it is important to note that there is great heterogeneity in both grey and white matter findings to date. One possible explanation for the variation in the results of case–control studies is the range in age at onset within and between late-life depression groups studied. The nature and aetiology of such structural abnormalities in late-life depression is hypothesised to vary with age at onset. For this purpose, people with late-life depression are commonly divided into those with early- and those with late-onset depression, with various age cut-offs ranging from 50 Reference Pantel, Schroder, Essig, Popp, Dech and Knopp6,Reference Taylor, Steffens, Payne, MacFall, Marchuk and Svenson7 to 65 Reference Avila, Ribeiz, Duran, Arrais, Moscoso and Bezerra8 employed.
The vascular depression hypothesis proposes that vascular factors (such as cardiovascular disease, diabetes, hypertension) may ‘predispose, precipitate, or perpetuate’ late-onset depression. Reference Alexopoulos, Meyers, Young, Campbell, Silbersweig and Charlson9 Specifically, vascular factors are hypothesised to cause white matter damage within frontal-subcortical circuits. Reference Alexopoulos1,Reference Alexopoulos, Meyers, Young, Campbell, Silbersweig and Charlson9 In support of this hypothesis, late-onset depression is associated with more frequent and severe white matter hyperintensities compared with early-onset depression, Reference Herrmann, Lemasurier and Ebmeier10 and there is increasing evidence supporting a vascular basis for white matter hyperintensities in depression. Reference Chen, Chen, Kuo, Chiang, Ko and Lin11–Reference Thomas, Perry, Kalaria, Oakley, McMeekin and O'Brien14 However, few studies have examined the influence of age at onset on white matter integrity in late-life depression using diffusion tensor imaging (DTI). Bae et al Reference Bae, MacFall, Krishnan, Payne, Steffens and Taylor15 compared fractional anisotropy between early-onset, late-onset depression and control groups. Although there were no significant differences in fractional anisotropy values between the early- and late-onset depression groups, the participants with early-onset depression displayed greater fractional anisotropy differences compared with control participants than did the participants with late-onset depression in several regions of interest.
Associations between age at onset and structural abnormalities will also be influenced by those with earlier age at onset, who have a longer duration of illness. The glucocorticoid cascade hypothesis was developed from animal models, in which stress-induced increases in glucocorticoid levels lead to regression of dendritic processes, inhibition of neurogenesis and neurotoxic effects in the hippocampus. Reference Sapolsky16 Furthermore, as the hippocampus is a major site in the glucocorticoid negative feedback circuit on the hypothalamic–pituitary–adrenal (HPA) axis such effects will, in turn, result in further increases in glucocorticoid levels. Reference Sapolsky, Krey and McEwen17 In line with this theory, it follows that greater duration of illness will result in greater hippocampal damage because of repeated exposure to elevated glucocorticoid levels. Two studies of late-life depression have found negative associations between the duration of illness and hippocampal volume. Reference Bell-McGinty, Butters, Meltzer, Greer, Reynolds and Becker18,Reference Sheline, Wang, Gado, Csernansky and Vannier19 However, several studies have failed to replicate this finding. Reference Andreescu, Butters, Begley, Rajji, Wu and Meltzer20–Reference Weber, Giannakopoulos, Delaloye, de Bilbao, Moy and Moussa23 Similarly, hippocampal volume has not been significantly associated with age at onset in several studies of late-life depression, Reference Andreescu, Butters, Begley, Rajji, Wu and Meltzer20,Reference Ashtari, Greenwald, Kramer-Ginsberg, Hu, Wu and Patel24,Reference Steffens, Byrum, McQuoid, Greenberg, Payne and Blitchington25 and has even been found to be negatively associated with age at onset in one. Reference O'Brien, Lloyd, McKeith, Gholkar and Ferrier22
It is important to note that the vascular and glucocorticoid hypotheses are neither mutually exclusive nor incompatible theories of late-life depression. Rather, the heterogeneity of depression indicates that multiple neural mechanisms may be implicated. For example, abnormalities in grey matter and white matter may also be related to Alzheimer's pathology. Reference Butters, Young, Lopez, Aizenstein, Mulsant and Reynolds26,Reference Caraci, Copani, Nicoletti and Drago27 However, the relationship between onset of late-life depression and Alzheimer's disease is not yet clear, with both early- and late-onset depression associated with increased risk for dementia. Reference Byers and Yaffe28 Therefore, in this paper, we focus on the relationship between age at onset and MRI measures in late-life depression with reference to the vascular and glucocorticoid cascade hypotheses. We expected that both the vascular and glucocorticoid hypotheses would have an impact on the results and hypothesised that (a) hippocampal volume would be positively associated with age at onset, in line with the glucocorticoid cascade hypothesis and (b) white matter integrity in frontal tracts would be negatively associated with age at onset, in line with the vascular hypothesis. We have separately reported comparison of this late-life depression group with a control group: white matter integrity was widely reduced in late-life depression, without significant group differences in grey matter measures. Reference Sexton, Allan, Le Masurier, McDermott, Kalu and Herrmann4
Method
Participants
The study was conducted with approval from the local research ethics committee (License 06/Q160/90). Informed written consent was obtained from all participants. Participants with late-life depression were identified from the general adult and old age psychiatric services of Oxford Health National Health Service Foundation Trust and also directly from the community by word of mouth and advertisements. Eligible participants were over the age of 60, with no potentially confounding comorbid medical, psychiatric or neurological conditions (including diagnoses of Alzheimer's disease or other dementias, bipolar disorder, mild cognitive impairment, Parkinson's disease, stroke, schizophrenia), and no implanted metallic devices, as required by standard MRI protocols. Participants with comorbid anxiety, controlled hypertension or diabetes were not excluded. Participants met the DSM-IV 29 criteria for major depression in the past, as assessed by an experienced psychiatrist, but were not necessarily currently depressed.
Clinical assessment
All participants underwent a clinical and neuropsychological assessment at the University of Oxford Centre for Clinical Magnetic Resonance Imaging (OCMR) or the University of Oxford Department of Psychiatry. A clinical assessment was performed by an experienced psychiatrist to verify inclusion and exclusion criteria, and determine age at onset, current symptom severity, current medication status, medical history and Framingham Stroke Risk. Reference D'Agostino, Wolf, Belanger and Kannel30 Blood pressure and pulse were recorded for all participants and a more detailed physical examination was also performed if indicated by the history. Age at onset was defined as the age at which an individual experienced their first episode of major depression and was determined from personal testimony and hospital notes. Duration of illness was estimated by subtracting age at onset from current age. Current symptom severity was assessed using the 17-item Hamilton Rating Scale for Depression (HRSD) Reference Hamilton31 and the 15-item Geriatric Depression Scale. Reference Yesavage, Brink, Rose, Lum, Huang and Adey32 Psychiatric medication was classified into the following categories: anticonvulsant, antidepressant, antipsychotic, anxiolytic and lithium salts. Framingham Stroke Risk was calculated based upon the following predictors: age, systolic blood pressure, diabetes mellitus, cigarette smoking, prior cardiovascular disease, atrial fibrillation, left ventricular hypertrophy (ascertained from personal testimony and hospital notes) and use of hypertensive medication. Electrocardiograms and glucose levels were not included as part of the clinical assessment.
A neuropsychological assessment was performed by trained graduate psychologists/neuroscientists and included the Addenbrooke's Cognitive Examination – Revised (ACE-R) Reference Mioshi, Dawson, Mitchell, Arnold and Hodges33 and the Mini-Mental State Examination (MMSE), Reference Folstein, Folstein and McHugh34 as measures of cognitive impairment. Tests included: Cambridge Neuropsychological Test Automated Battery (CANTAB) reaction time, Reference Sahakian, Owen, Morant, Eagger, Boddington and Crayton35 category fluency, Reference Mioshi, Dawson, Mitchell, Arnold and Hodges33 copied drawings, Reference Mioshi, Dawson, Mitchell, Arnold and Hodges33 clock drawing, Reference Mioshi, Dawson, Mitchell, Arnold and Hodges33 digit span, Reference Wechsler36 digit symbol, Reference Wechsler36 graded naming test, Reference McKenna and Warrington37 Hopkins Verbal Learning Task – Revised (HVLT-R), Reference Brandt38 letter fluency, Reference Mioshi, Dawson, Mitchell, Arnold and Hodges33 Rey–Osterrieth complex figure (RCF) Reference Osterrieth39 and Trail Making Test (TMT) A and B. Reference Reitan40 Full results of the neuropsychological assessment performed are detailed elsewhere. Reference Sexton, McDermott, Kalu, Herrmann, Bradley and Allan41
Age at onset can either be treated as a continuous variable or divided into early- and late-onset depression. As division into early- and late-onset depression is not typically based upon reliable clinical or biological data, and as a result various age cut-offs have been used, we decided to treat age at onset as a continuous variable, as recommended by the MacArthur Foundation's workshop on late-life depression. Reference Lyness, Pearson, Lebowitz and Kupfer42
Statistical analysis was performed using PASW Statistics version 18 for Windows. Pearson's correlation was performed between age at onset and age, education, Framingham Stroke Risk, duration of illness, symptom severity and number of medications. An independent-samples t-test was performed to test whether age at onset differed between male and female participants.
MRI acquisition
All participants underwent an MRI scan at OCMR using a 3.0 Tesla Trio Siemens scanner with a 12-channel head-coil. High-resolution 3D T 1-weighted MRI scans were acquired using a magnetisation-prepared rapid gradient echo (MPRAGE) sequence (repetition time (TR) = 2040 ms, echo time (TE) = 4.7 ms, flip angle: 8, field of view (FOV) = 192 mm, voxel dimension: 1 mm isotropic). Whole-brain DTI was acquired using an echoplanar imaging (EPI) sequence (TR = 7900/7800 ms, TE = 98/82 ms, FOV = 240 mm, voxel size: 2.5 mm isotropic, b value: 1000, number of directions: 60, number of acquisitions: 2).
MRI analysis
Image analysis was performed using tools from the FMRIB (Functional MRI of the Brain) software library (FSL version 4.1, www.fmrib.ox.ac.uk/fsl/) on a UNIX-based GNU/Linux operating system. Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens and Johansen-Berg43
Whole brain volume
T 1-weighted images were brain-extracted using the brain extraction tool (BET version 2.1). Reference Smith44 Whole brain volumes were calculated and correlated with age at onset using partial correlation analysis, with age and gender as covariates.
Hippocampal volume and shape
Segmentation and vertex analysis of the hippocampus was performed using an automated model-based segmentation tool (FMRIB's integrated registration and segmentation tool, FIRST version 1.2). Reference Patenaude, Smith, Kennedy and Jenkinson45 First, T 1-weighted images were aligned to Montreal Neurological Institute (MNI) 152 space at 1 mm resolution using three-stage affine registration. Second, based on the learned models of manually labelled images, linear combinations of shape modes of variation were searched through for the most probable shape instance given the observed intensities in the T 1-weighted image. Third, boundary correction classified boundary voxels in the volumetric output. Both the registrations and subsequent segmentations were manually checked for errors and none was found.
Hippocampal volume was extracted and expressed as a percentage of whole brain volume. Normalised hippocampal volume was correlated with age at onset using partial correlation analysis with age and gender as covariates.
Vertex-wise statistics examining hippocampal shape were performed in MNI space using FIRST, with age and gender included as confound regressors. This tool, FIRST, employs a multivariate general linear model to test for correlations between age at onset and mean vertex location, using Pillai's trace to derive F-statistic values. False discovery rate correction for multiple comparisons was then applied to obtain thresholded F statistics.
Voxel-based morphometry
Voxel-based morphometry analysis was performed using FSL-VBM version 1.1. Reference Ashburner and Friston46,Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak47 Grey matter partial volume images were aligned to MNI 152 standard space using the affine registration tool FMRIB's linear image registration tool (FLIRT version 5.5), Reference Jenkinson, Bannister, Brady and Smith48,Reference Jenkinson and Smith49 followed by non-linear registration using FMRIB's non-linear image registration tool (FNIRT version 1.0), Reference Andersson, Jenkinson and Smith50,Reference Andersson, Jenkinson and Smith51 which uses a b-spline representation of the registration warp field. Reference Rueckert, Sonoda, Hayes, Hill, Leach and Hawkes52 The resulting images were averaged to create a study-specific template, to which the native grey matter images were then non-linearly re-registered. The registered partial volume images were then modulated by dividing by the Jacobian of the warp field. The modulated segmented images were smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. Voxel-wise statistics were performed using Randomise version 2.1, a permutation-based inference tool for non-parametric statistical thresholding that corrects for multiple comparisons across space. Reference Nichols and Holmes53 The significance threshold for correlations with age at onset was set at P<0.05, using the threshold-free cluster enhancement (TFCE) option, with age and gender included as confound regressors. Reference Smith and Nichols54 As modulation in FSL-VBM is based only on the non-linear registration stage, rather than both the affine and non-linear stages of registration, it was not necessary to also include whole brain volume as a confound regressor.
TABLE 1 Demographic data

| Late-life depression | r | P | |
|---|---|---|---|
| Participants, n | 36 | N/A | N/A |
| Gender, female:male, n | 24:12 | N/A | N/A |
| Age at onset, mean (s.d.) | 45.39 (18.97) | N/A | N/A |
| Age, mean (s.d.) | 71.83 (7.71) | 0.238 | 0.163 |
| Cognitive impairment | |||
| Addenbrooke's Cognitive Examination – Revised | 91.50 (6.26) | –0.369 | 0.027 |
| Mini-Mental State Examination | 28.97 (1.36) | –0.348 | 0.038 |
| Years of education, mean (s.d.) | 13.94 (3.74) | –0.279 | 0.099 |
| Framingham Stroke Risk, mean (s.d.) | 10.64 (4.11) | 0.056 | 0.745 |
| Duration of illness, mean (s.d.) | 26.44 (18.70) | –0.916 | <0.001 |
| Severity | |||
| Hamilton Rating Scale for Depression | 4.19 (4.77) | 0.067 | 0.699 |
| Geriatric Depression Scale | 3.83 (3.47) | –0.119 | 0.496 |
| Number of current medications, mean (s.d.) | 1.44 (0.77) | 0.099 | 0.566 |
N/A, not applicable.
a Data shown in bold indicate correlation with age at onset is statistically significant (P<0.05).
White matter integrity
The DTI data were manually checked and artefacts removed before eddy-current correction was run to correct for eddy-current induced distortions and head motion.
Tract-based spatial statistics
Analysis of the DTI data was carried out using Tract-Based Spatial Statistics (TBSS version 1.2). Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay55 Fractional anisotropy images were created by fitting a tensor model to the raw diffusion data using FMRIB's diffusion toolbox, and then brain-extracted using BET. Reference Smith44 All participants’ fractional anisotropy data were then aligned into a common space using FNIRT. Reference Andersson, Jenkinson and Smith50,Reference Andersson, Jenkinson and Smith51 Next, the mean fractional anisotropy image was created and thinned to create a mean fractional anisotropy skeleton that represents the centres of all tracts common to the group. Each participant's aligned fractional anisotropy data were then projected onto this skeleton. Voxel-wise statistics were performed using Randomise, Reference Nichols and Holmes53 with age and gender included as confound regressors. The significance threshold for correlations with age at onset was set at P<0.05, using the TFCE option. Reference Smith and Nichols54
To aid in the localisation of significant differences in fractional anisotropy, skeleton masks (tracts of interest, TOI) were created for the anterior thalamic radiation; genu, body, and splenium of the corpus callosum; cingulum; corticospinal tract; fornix; inferior longitudinal fasciculus; superior longitudinal fasciculus; and uncinate fasciculus. The TOI were based on the International Consortium for Brain Mapping ICBM-DTI-81 White-Matter Labels Atlas and the JHU (Johns Hopkins University) White-Matter Tractography Atlas within the FSL atlas tool, as well as the MRI Atlas of Human White Matter. Reference Mori, Wakana, Nagae-Poetscher and van Zijl56 The percentage of mask voxels that were significantly different in fractional anisotropy was calculated for each TOI.
Results
Demographic data are presented in Table 1. Age at onset ranged from 10 to 78 years; distribution of age at onset is illustrated in online Fig. DS1. Age at onset did not differ between males and females (t 34 = 1.404, P = 0.169) and was not correlated with age, gender, education, Framingham Stroke Risk or number of medications (Table 1). Age at onset was strongly associated with duration of illness and moderately associated with cognitive impairment (Table 1).
The HRSD scores ranged from 0 to 18. Twenty-seven participants had HRSD scores indicative of remission (HRSD⩽7). In total 33 participants were currently taking antidepressants, 7 antipsychotics, 5 anxiolytics, 4 lithium salts and 1 an anticonvulsant. Two participants were not currently receiving any psychotropic medication. Participants received the following medication for other medical complaints: analgesics (pizotifen, carbamazepine, paracetamol; three participants); antacids and proton pump inhibitors (three participants); anti-arthritic drugs (glucosamine sulphate; one participant); anticancer adjuvant drugs (anastrozole, Iscador; two participants); antidiarrheal drugs (one participant); antihypertensives (four participants angiotensin-converting-enzyme (ACE) inhibitors, three participants angiotensin II receptor antagonists, one participant beta-blockers, four participants calcium-channel blockers, five participants diuretics); aspirin (two participants); calcium (two participants); cholesterol lowering drugs (ciprofibrate, statins; eight participants); hypnotics (two participants); inhalers (salbutamol; three participants); non-steroidal anti-inflammatories (NSAIDs; two participants); oral antidiabetics (four participants); steroids (two participants); and thyroxine (one participant).
Grey matter
There was a moderate negative correlation between age at onset and whole brain volume (r = –0.372, P = 0.03; online Fig. DS2), and a moderate-to-strong positive correlation with normalised hippocampal volume on both sides (left: r = 0.504, P = 0.002; right: r = 0.411, P = 0.016; online Fig. DS3). There were no significant correlations between age at onset and hippocampal shape. Also, no significant correlations between age at onset and grey matter were detected using FSL-VBM.
White matter
There were widespread negative correlations between age at onset and fractional anisotropy, with 20% of skeleton voxels significant at P<0.05. The spatial distribution of significant negative correlations is summarised in Fig. 1 and Table 2 (see online Fig. DS4 for a colour version of Fig.1). The anterior thalamic radiation and superior longitudinal fasciculus were particularly affected, with 63% and 54% of voxels significant respectively (Table 2, online Fig. DS5). There were no regions where age at onset was positively correlated with fractional anisotropy.
Discussion
In this paper, we investigated the relationships between age at onset and MRI measures of grey and white matter. Age at onset was negatively correlated with fractional anisotropy in widespread regions. In particular, later age at onset was associated with reduced fractional anisotropy within the anterior thalamic radiation and superior longitudinal fasciculus, both of which project to the frontal lobe. This result is in line with a greater prevalence and severity of white matter hyperintensities in late-compared with early-onset depression, Reference Herrmann, Lemasurier and Ebmeier10 and is compatible with the vascular depression hypothesis, which proposes that a later age at onset is associated with a greater degree of white matter abnormalities in frontal-subcortical and limbic tracts. Reference Alexopoulos, Meyers, Young, Campbell, Silbersweig and Charlson9 A positive correlation between age at onset and Framingham Stroke Risk Score would have added further support to the vascular depression hypothesis, but was not detected. However, it has been proposed that stroke risk factors may not be predictive of more subtle cerebrovascular disease. Reference Thomas, Kalaria and O'Brien57 For example, stroke risk scores do not include total cholesterol and high-density lipoprotein cholesterol as predictors, both of which can contribute to risk of cerebrovascular disease. In order to assess the vascular depression hypothesis more directly, future studies should include markers of vascular disease, for example measures of carotid intima-media thickness, pulse wave velocity, pulse wave analysis, orthostatic hypotension, heart rate variability or baroreflex sensitivity. Reference Chen, Chen, Kuo, Chiang, Ko and Lin11,Reference Smith, Blumenthal, Babyak, Doraiswamy, Hinderliter and Hoffman12,Reference Paranthaman, Greenstein, Burns, Cruickshank, Heagerty and Jackson58–Reference Vasudev, O'Brien, Tan, Parry and Thomas60
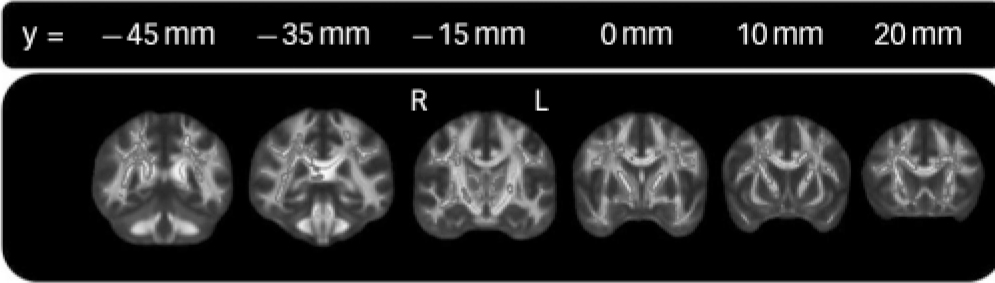
FIG. 1 Localisation of correlations between fractional anisotropy and age at onset.
Regions significantly correlated (P<0.05) between age at onset and fractional anisotropy in late-life depression are shown dilated for illustrative purposes and overlaid on a thinner skeleton. Age and gender were included as confound regressors. See online Fig. DS1 for a colour version of this figure.
Earlier age at onset was associated with reduced normalised bilateral hippocampal volumes. As duration of illness was calculated by subtracting age at onset from current age, and all analyses included age as a covariate, this finding is in line with studies that found greater duration of illness to be associated with reduced hippocampal volume. Reference Bell-McGinty, Butters, Meltzer, Greer, Reynolds and Becker18,Reference Sheline, Wang, Gado, Csernansky and Vannier19 Furthermore, this result is compatible with the glucocorticoid cascade hypothesis, which proposes that hippocampal damage results from repeated exposure to elevated cortisol levels. Reference Sapolsky16 However, a study directly examining the relationship between hippocampal volume and cortisol levels in late-life depression found that hippocampal volume reduction was not associated with increased cortisol levels. Reference O'Brien, Lloyd, McKeith, Gholkar and Ferrier22 Similarly, Gerritsen et al Reference Gerritsen, Comijs, van der Graaf, Knoops, Penninx and Geerlings61 found that although early-onset depression was associated with reduced hippocampal volumes compared with control participants, it was not associated with alterations in basal HPA axis regulation. Also, in contrast to the pattern of selective volume loss in the dentate gyrus and CA3 subfields, which is associated with stress-related damage in animal studies Reference McEwen62 and post-traumatic stress disorder, Reference Wang, Neylan, Mueller, Lenoci, Truran and Marmar63 there was an absence of localised hippocampal changes in late-life depression. An alternative explanation offered is that reduced hippocampal volume in early-onset depression pre-dates and predisposes to depression onset. In support of this explanation, reduced hippocampal volume has been detected in ‘at risk’ groups. Reference Chen, Hamilton and Gotlib64,Reference de Geus, van't Ent, Wolfensberger, Heutink, Hoogendijk and Boomsma65 Longitudinal studies examining hippocampal volume are needed, as ‘at risk’ groups may also include participants who are resilient to developing depression.
TABLE 2 Localisation of correlations between fractional anisotropy and age at onset

| Tract of interest | %Footnote a |
|---|---|
| Whole skeleton | 20 |
| Anterior thalamic radiation | 63 |
| Corticospinal tract | 8 |
| Cingulum | 6 |
| Corpus callosum | |
| Body | 17 |
| Genu | 25 |
| Splenium | 21 |
| Fornix | 12 |
| Inferior longitudinal fasciculus | 10 |
| Superior longitudinal fasciculus | 54 |
| Uncinate fasciculus | 6 |
a Percentage of voxels displaying a significant correlation between fractional anisotropy and age (P<0.05) in each tract of interest.
Methodological considerations
As with the majority of MRI studies of late-life depression, sample size is the main limitation of this study. A key strength was the wide range of age at onset within the late-life depression group, which spanned from 10 to 78 years of age. However, although patient testimony was combined with use of hospital notes, estimation of age at onset may have been susceptible to recall bias where hospital notes were limited. Reference Prusoff, Merikangas and Weissman66,Reference Wiener, Alexopoulos, Kakuma, Meyers, Rosenthal and Chester67 The definition of age at onset as the age at which an individual experiences their first episode of major depression did not take into account prior episodes of subsyndromal depression, which may have resulted from the same pathophysiology as later episodes of major depression. However, consistent and accurate identification of the onset of subsyndromal depression is arguably more difficult to obtain compared with the onset of major depression. Reference Lyness, Pearson, Lebowitz and Kupfer42 The possibility of recall bias also contributed to our decision to calculate illness duration by subtracting age at onset from current age. Calculation of the total number of days depressed using the total number of episodes and the length of each episode may represent a more accurate marker of illness duration, but is extremely difficult to accurately assess in a retrospective study of late-life depression. Calculation of Framingham Risk Score was limited by electrocardiograms not being performed and diabetes not being tested for at the time of the assessment.
We have previously reported differences in grey and white matter between the participants with late-life depression reported in this paper and an age-matched control group. Reference Sexton, Allan, Le Masurier, McDermott, Kalu and Herrmann4 Whereas late-life depression was associated with widespread reductions in white matter integrity, hippocampal volume was not significantly different between groups. Our finding that earlier age at onset is associated with reduced hippocampal volume within late-life depression in this paper offers a possible explanation as to why our sample of participants with late-life depression, when considered as a whole, did not differ from a control group, in contrast to some of the literature. Reference Sexton, Mackay and Ebmeier2,Reference Koolschijn, van Haren, Lensvelt-Mulders, Hulshoff Pol and Kahn68
Implications
Overall, older age at onset was associated with reduced fractional anisotropy in frontal tracts, supportive of the vascular hypothesis. Younger age at onset was associated with greater duration of illness and reduced hippocampal volume within late-life depression, compatible with the glucocorticoid cascade hypothesis. Future studies should use direct markers in order to explore these hypotheses in greater depth.
Funding
C.E.S., L.L.H., U.G.K., L.M. were supported by the Gordon Edward Small’s Charitable Trust (Scottish Charity Register: SC008962). C.L.A. had support from Oxford University Clinical Academic Graduate School.
Acknowledgements
We thank all participants who volunteered for this study, Dr. Philip Wilkinson and other colleagues for referring participants, and Steven Knight for operating the MRI scanner.






eLetters
No eLetters have been published for this article.