INTRODUCTION
Acute diarrhoeal illness in early childhood is a major public health problem, responsible for a significant burden of morbidity and mortality. Three quarters of the estimated 3–4 billion annual cases in developing countries occur in children aged <5 years, with over 1·5 million deaths. This is despite improvements in hygiene and sanitary conditions, improved child nutrition and care, and progress in case management over recent decades [Reference Guerrant, Steiner, Mandell, Bennett and Dolin1–Reference Bryce4]. Israel is considered a developed country, with Western public health standards and minimal mortality from infectious disease; nevertheless, the rate of enteric infection, paradoxically, is high [5, 6].
Incidence rates and aetiological agents of acute childhood diarrhoeal disease differ between developing and developed countries [Reference Thapar and Sanderson7–Reference Steiner, Samie and Guerrant10]. Rotaviruses are the leading cause of severe diarrhoea in children worldwide; with the increasing use of the novel rotavirus vaccines, bacterial pathogens will presumably play a more significant role in future [11]. The most common bacteria causing gastroenteritis in children in developed countries are Shigella, Salmonella and Campylobacter [Reference Dennehy8, Reference Koehler9, Reference Kotloff12]. Many cases result from transmission in the household or in child-care facilities [13], and from outbreaks in deprived socioeconomic communities. No vaccines against these bacterial infections are commercially available, although various vaccine candidates are at present under development. Hence, preventive public health strategies should focus on the traditional approach of promoting appropriate personal and environmental hygiene measures [14].
The aim of our study was to investigate population-based, age-specific epidemiological trends of enteric bacterial infections of Shigella, Salmonella and Campylobacter in the Jerusalem district, with a special focus on disease patterns in young children aged <5 years.
METHODS
Infections caused by Shigella, Salmonella and Campylobacter are legally notifiable in Israel and apply both to physicians and microbiological laboratories [5]. The District Health Office communicable diseases database is compiled from these reports. Since Escherichia coli O157:H7 is rarely reported (<1 case/year), and Yersinia enterocolitica is not notifiable, these two infections were not included in the study. Data on all reported cases of these three enteric bacterial infections in Jerusalem district from 1990 to 2008 were collected, including epidemiological investigations, hospital files and laboratory reports. The variables recorded were: age, gender and ethnicity, date of disease onset, hospitalization, bacteria isolation, serogrouping and biotyping. Isolates of bacteria were sent to the national public health laboratories for determination of serogroup or biotype. Serogrouping was performed according to standard laboratory methods [Reference Bopp, Murray, Baron, Tenover and Yolken15, Reference Nachamkin, Murray, Baron, Tenover and Yolken16].
Cases were classified according to the standard definitions, i.e. a confirmed case was a clinical case of diarrhoea with the isolation of Shigella/Salmonella/Campylobacter from stool [17]. We defined household transmission of disease as the occurrence of more than a single case of the same enteric pathogen in a given household within a period of 14 days.
District population and incidence rates
The district population data are provided annually by the National Population Register of the Ministry of Interior Affairs in Israel. From 1990 to 2008, Jerusalem's population increased from 578 400 to 879 700. The population is comprised essentially of two ethnic groups: Jews (70·1%) and Arabs (29·9%). Children aged <15 years comprised 35%, children aged <5 years 12·9%, and infants <1 year 2·7% of the district's population [Reference Choshen18]. The annual incidence rates in Jerusalem district were compared to the national rates provided by the Ministry of Health [5, 6].
Statistical analysis
The demographic, laboratory and clinical data were analysed using SPSS software [19]. Incidence rates per 100 000 population and seasonal trends were analysed with WINPEPI software [20]. Rates of the different bacteria were compared using Tukey's analysis for multiple pairwise comparisons. The Kruskal–Wallis test was used to compare medians. The rate ratio (RR), odds ratio (OR) and 95% confidence intervals (CI) are reported. Variability in seasonality was evaluated by the Ratchet circular scan test for a 3-month peak. Continuous variables were compared by Student's t test; dichotomous variables were analysed by Pearson's χ2 test. A P value ⩽0·05 was considered significant for all comparisons.
RESULTS
Between 1990 and 2008, 32 408 cases of enteric bacterial infection were reported in the Jerusalem district; 15 374 were Shigella infections (47·4%), 11 143 Salmonella infections (34·4%) and 5891 Campylobacter infections (18·2%). The overall average annual incidence of enteric infection was 232·1/100 000, which was higher than the national average of 169·9/100 000 (RR 1·38, 95% CI 1·31–1·45, P=0·0001). The difference in enteric infection rates between Jerusalem and the rest of the country, which amounted to 62·2/100 000, was attributable entirely to Shigella (61% of the difference) and Salmonella (39%) infections; there was no difference in Campylobacter infection rates. Disease patterns in Jerusalem changed considerably over the years 1990–2008, with annual overall incidence rates ranging from 111·4 to 412·9/100 000 (Fig. 1). The incidence of Campylobacter infection increased steadily from 15 to 110·8/100 000 (RR 7·36, 95% CI 5·92–9·18, P=0·0001). The Salmonella incidence rate per 100 000 in 1990 was 74·2, which then rose threefold, peaking in 1995 at 199·6; following this it fell gradually to 39·4 in 2008 (RR 0·53, 95% CI 0·46–0·61, P=0·0001). In contrast, Shigella infections presented a sinusoidal pattern, with alternating endemic and epidemic years varying from 19·7/100 000 in 1996 to a peak of 252·8 in 2002 (2002 vs. 1996; RR 12·8, 95% CI 10·8–15·3, P=0·0001).

Fig. 1. Annual reported incidence rates (per 100 000) of infection with bacterial enteric pathogens [Shigella (····•····), Salmonella (—▴—), Campylobacter (- -▪- -)] in the Jerusalem district, Israel, 1990–2008.
The seasonality of the three pathogens varied considerably. While Shigella infections prevailed in late winter (February–April, 38·7% of cases, P<0·005); Campylobacter prevailed in spring (May–July, 29·8% of cases, P<0·005), and Salmonella in summer (June–August, 35·7% of cases, P<0·005).
Most infections occurred in children: 75% were aged <15 years, 56·4% <5 years and 7·1% were infants aged <1 year. The overall average age was 12·3±17·3 with median age of 4 years. Children <5 years had a markedly higher overall incidence (930·6 vs. 107·2; RR 8·7, 95% CI 7·9–9·5, P=0·0001), as well as higher pathogen-specific incidence rates (RR 13·7 for Shigella, 8·3 for Salmonella and 4·6 for Campylobacter, all P=0·0001) compared to persons aged ⩾5 years (Fig. 2). Peak incidences for all three pathogens were observed in the second year of life, with an overall rate of 1272·2/100 000.
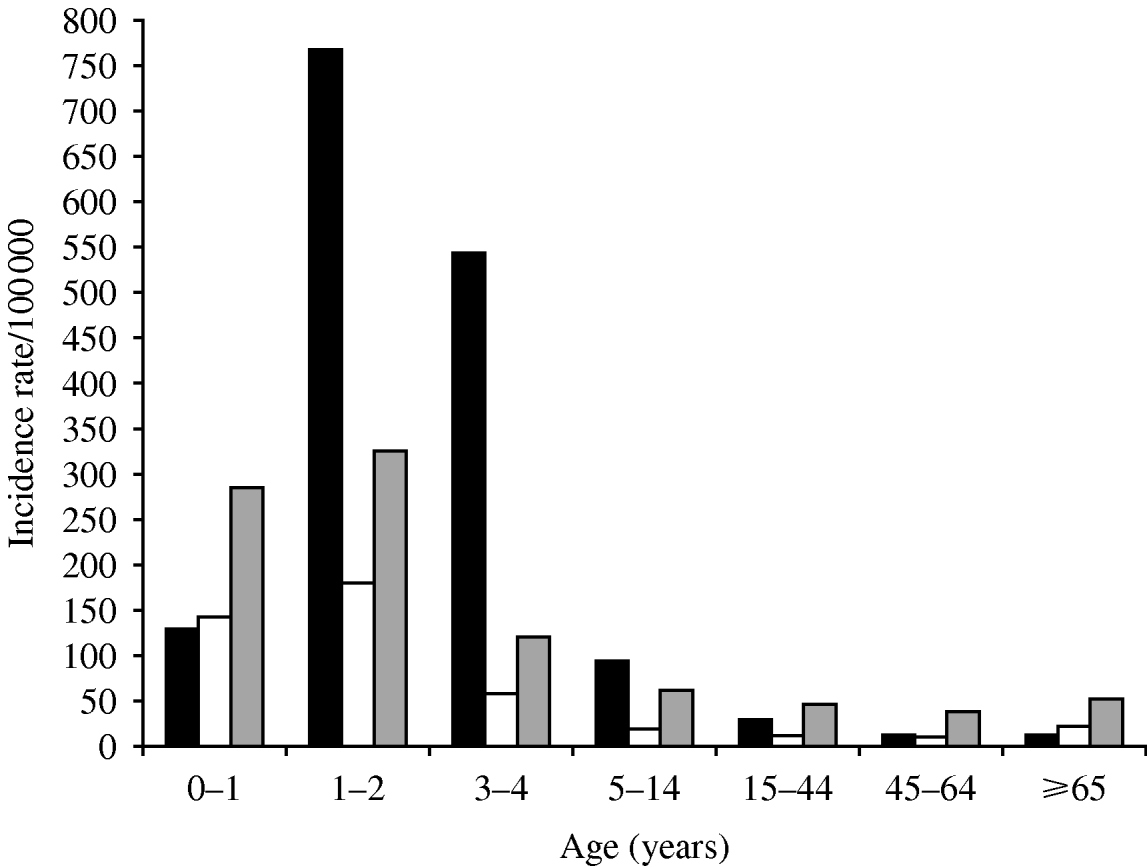
Fig 2. Age-specific reported incidence rates (per 100 000) of infection with bacterial enteric pathogens [Shigella (▪), Salmonella (□), Campylobacter (![]() )] in the Jerusalem district, Israel, 1990–2007.
)] in the Jerusalem district, Israel, 1990–2007.
The age distribution differed considerably between the three pathogens (Fig. 2), with Shigella being the predominant pathogen for those aged 1–14 years (62·2%) and Campylobacter the predominant pathogen in those aged <1 year (51·4%) and >15 years (55·3%). Shigella predominated in the 1–4 years age group (65·7%) followed by Campylobacter (22·4%) and Salmonella (1·9%). In infants aged <1 year Campylobacter (51·4%) was the most common, followed by Salmonella (25·4%) and Shigella (23·2%). In infants aged <6 months Campylobacter predominated (58·4% of cases). However, the median age of Campylobacter cases (9·1 years) was significantly higher than the median ages of Salmonella and Shigella cases which were 3·7 and 3·5 years, respectively (P=0·0001).
On the whole, bacterial enteric infections were more common in males: 53·4%, compared to 49·9% of males in the Jerusalem population (RR 1·07, 95% CI 1·05–1·09, P=0·0001). This was particularly evident with regard to Campylobacter (57·3%), less so for Salmonella (54·1%) and least for Shigella (50·4%). Infants aged <1 year had a higher male/female ratio than persons aged ⩾1 year (1·4:1 vs. 1·1:1; OR 1·2, 95% CI 1·03–1·5, P=0·02).
Overall, 11·7% (n=3540) of the cases were Arab, which was significantly lower than expected based on their proportion in the district's population (29·9%) (OR 3·2, 95% CI 2·5–4·1, P=0·0001). The proportion of Arab cases was also significantly lower than expected for each of the three pathogens: 17·7% of Salmonella cases, 18·8% of Campylobacter cases, and 4·5% of Shigella cases (P<0·0001 for all the above comparisons). However, when evaluated by age, 33·2% of infant cases aged <1 year were Arab, against 10% of children aged 1–14 years (OR 4·4, 95% CI 3·6–5·5, P=0·0001).
Hospitalization
The proportions hospitalized differed considerably between pathogens: 1·8% for Shigella, 2·3% for Campylobacter, and 6·9% for Salmonella cases (P=0·001). The average age of hospitalized patients was 15·7±21·8 years, and the median age 4·8 years. The overall proportion hospitalized decreased with age: <1 year (4·6%), 1–4 years (2·1%) and 5–14 years (2·3%). Infants aged <1 year were more likely to be hospitalized than children aged 1–14 years (OR 2·2, 95% CI 1·4–3·6, P=0·001). Additionally, infants aged <3 months had an increased rate compared to those aged 3–12 months (12·2% vs. 3·9%; OR 3·4, 95% CI 1·03–10·4, P=0·016). The most common indications for hospitalization of children were dehydration and electrolyte disturbances.
Household transmission
The proportion of secondary infections in the households also differed noticeably. A higher proportion of secondary cases was observed among Shigella cases, 21·2% against 5·45% and 5·04% for Salmonella and Campylobacter, respectively (P<0·001).
Laboratory results
Most Shigella isolates were S. sonnei (95·3%); the rest were S. flexneri (3·7%), S. boydei and others (1%). S. flexneri was more prevalent in Arab (9%) compared to Jewish (3·4%) patients (RR 2·6, 95% CI 1·6–4·3, P=0·0001). S. Enteritidis (19·9%), S. Typhimurium (11·5%), S. Virchow (11·2%) and S. Hadar (7·4%) were the commonest serotypes isolated. The hospitalization rate in S. Typhimurium cases (44·1%) was higher than any other Salmonella serotype (average 2·4%) (OR 32·1, 95% CI 20·7–50·2, P=0·0001). Most Campylobacter isolates were classified as C. jejuni (97%).
DISCUSSION
Acute diarrhoeal disease is a leading cause of death in children aged <5 years worldwide, second only to respiratory tract infections. The global burden of acute bacterial diarrhoea is estimated at millions of children with devastating human and economic consequences [Reference Guerrant, Steiner, Mandell, Bennett and Dolin1–Reference Bryce4, Reference Guerrant21, Reference Flint22].
We demonstrated a substantial burden of acute bacterial enteric infections in young children in Jerusalem. Children aged <1, <5 and <15 years constituted 2·7%, 12·9% and 35% of the district's population, respectively, yet they were overrepresented by contributing 7·1%, 56·4% and 75% of the cases. Children aged <5 years had a significantly higher probability of infection with Shigella (RR 13·7), Salmonella (RR 8·3) and Campylobacter (RR 4·6) compared to persons aged >5 years. This distribution of enteric pathogens is rather different from that in developed countries, which generally report the highest incidence rates for Campylobacter, followed by Salmonella and Shigella infection, respectively [Reference Koehler9, Reference Musher and Musher23, 24].
For children aged 1–4 years, Shigella (mainly S. sonnei) was the leading pathogen, accounting for 68% of bacterial enteric infections, followed by Campylobacter (21%) and Salmonella (11%). In infants, Campylobacter was the leading pathogen, accounting for half the cases. Shigella and Salmonella each accounted for around a quarter of the cases. Campylobacter was particularly predominant in infants aged <6 months, accounting for about two thirds of cases.
Bacterial enteric infections were more prevalent in males. This applied particularly to Campylobacter infections, to a lesser extent in Salmonella and negligibly in Shigella. The male predominance in Campylobacter infection in young children in the USA has been reported previously [Reference Koehler9], and has recently been shown in a UK population to extend from birth to the late teens [Reference Gillespie25]. The reason for this tendency is not clear.
The Jerusalem district population consists mainly of two ethnic groups – Jews and Arabs. The ethnicity distribution differed between pathogens and with the patient's age, with a higher percentage of Arab patients among infants aged <1 year. These findings may be attributed to differences in provision and utilization of health services and to the under-diagnosis and under-reporting of cases in the Arab population, requiring further surveillance and evaluation in addition to interventional programmes. The higher proportion of S. flexneri in Arab patients may reflect socioeconomic differences. It has been reported that S. sonnei predominates in developed countries while S. flexneri and S. dysenteriae prevail in developing countries [Reference Amieva26]. Evidence of previous exposure to Shigella infection was significantly higher in adolescents from a low socioeconomic background than a higher one in Israel [Reference Hasin27].
There was notable variability with regard to seasonality of the pathogens. Shigella infections peaked in late winter, Campylobacter in spring and Salmonella in summer. The summer and early autumn seasonality of Salmonella and other enteric infections has been reported previously [Reference Koehler9]. Campylobacter enteritis has a variable seasonality in developed and developing countries [Reference Coker28]: in developed countries with a temperate climate it has been demonstrated that Campylobacter infections generally peak in spring [Reference Nylen29].
Overall, during the study period Shigella was the leading enteric bacterial pathogen, especially in the <5 years age group. Shigella infections were characterized by a see-saw pattern, alternating between endemic and epidemic years (Fig. 1). In endemic years, most cases were sporadic, whereas in epidemic years outbreaks occurred in young children, largely in childcare facilities. Periodic Shigella outbreaks have been reported in Israel, with a predominance of S. sonnei and a characteristic peak in wintertime [5, 6]. Outbreaks such as these, arising in distinct communities such as ultra-orthodox Jewish communities in the USA or in public settings (e.g. child day-care facilities), have also been described elsewhere [14, Reference Sobel30–Reference Garrett32]. The apparent biennial–triennial pattern of S. sonnei outbreaks suggest that a pool of susceptibles must accumulate before an outbreak occurs. The peak incidence rates were observed in children aged 1–4 years, especially in those in their second year of life which is the regular age of entry to day-care centres in Israel. Infection before age 6 months was rare, possibly indicating passively acquired immunity. The higher incidence in young children has been attributed to poor hygiene practices in addition to the low infectious dose for Shigella [Reference Guerrant, Steiner, Mandell, Bennett and Dolin1, Reference Dennehy8, Reference Koehler9, Reference Musher and Musher23]. Indeed, household secondary infection was four times higher with Shigella infections than with Salmonella or Campylobacter.
The incidence of Salmonella infection increased in the early 1990s and then declined after 2000. The epidemiology of salmonellosis in Israel resembles the global trends, and after three decades of rising, the incidence began to decline [Reference Weinberger and Keller33]. The recent decrease may be a result of improved food safety practices. However, the decline may be temporary, as changes in the incidence of Salmonella infections tend to evolve rapidly [Reference Weinberger and Keller33–Reference Bar-Meir35]. Salmonella incidence (particularly S. Typhimurium) has decreased over the last decade in the USA [24], and of all salmonellosis in the European Union by an average of 27% from the peak year of 1997 to 2001, and watchful surveillance is essential [Reference O'Brien and de Valk34]. We found a significantly higher rate of hospitalization in Salmonella patients, especially those with S. Typhimurium, probably due to the severity of clinical symptoms. The rising rates of antimicrobial resistance worldwide are of major concern in non-typhoid Salmonella [Reference Weinberger and Keller33].
Campylobacter enteritis rates increased steadily from 1990 in Jerusalem, stabilized for several years, and then rose significantly, so that by 2008 it became the leading cause of diagnosed bacterial enteric infection. Campylobacter infects all age groups; in developing countries early childhood infection is common, reflecting exposure to contaminated water, food or direct animal contact with farm animals [Reference Amieva26]. Campylobacter jejuni is the leading known bacterial cause of diarrhoea in developed countries such as Canada [Reference Galanis36] and the USA [24] and is the second commonest known cause of travellers' diarrhoea after E. coli.
The communicable diseases surveillance system of the Jerusalem District Health Office enabled us to assess temporal trends of specific pathogens and to characterize cases according to demographic variables. However, the number of reported cases is probably an underestimate because of asymptomatic infections, under-diagnosis and under-reporting, mainly of mild disease cases. The gap between reported and expected cases among the Arab population supports this conjecture. The actual disease incidence is probably several times higher. The estimated ratio of reported to actual cases of bacterial enteric infection has been studied in several community settings. They reportedly can range from 1:8 (for Salmonella and Campylobacter) in a UK survey of GP reporting practices [Reference Day and Sutton37] to as much as 1:50 in Canada [Reference Thomas38]. The bacterial enteric infection rate in the Jerusalem district was 36% higher than the national average [5] and about six times higher than that reported in the USA [24, Reference Mead39]. The difference between sub-populations had been described previously [Reference Hasin27] and requires additional evaluation.
Israel's public health achievements rank with those of other developed countries, yet the burden of enteric disease, particularly in children, is more reminiscent of less advanced societies. The explanation probably lies largely in Israel's socioeconomic structure. A very high percentage of women are in the workforce, and maternity leave following birth is only 12 weeks. Consequently, children are placed in various child-care facilities from a young age – a situation that is known to be conducive to transmission of communicable diseases, particularly respiratory and enteric. This population-based study verified a high disease burden, particularly in young children. In the absence of a reliable vaccine, prevention depends primarily on appropriate personal and environmental hygiene measures taken by children and their caretakers.
The increasing use of effective rotavirus vaccines may induce a shift in the aetiology of childhood diarrhoea towards enteric bacteria, hence it seems prudent to collect surveillance-based data on bacterial pathogens. Our study indeed revealed significant changes in the patterns of bacterial enteric disease, which are important in our understanding of these diseases.
DECLARATION OF INTEREST
None.




