Introduction
Diagnosis with major depressive disorder (MDD) primarily involves either a depressed mood or a loss of interest or pleasure in most usual activities most of the day, nearly every day, according to the DSM-5.1 Reward-related symptoms such as a lack of motivation and anhedonia represent frequent and pervasive symptoms in depression, as in other neuropsychiatric diseases. Historically described as a “response bias” (bias that influences the response of participants to a stimulus away from an accurate response, irrespective of stimulus presentation), “negative cognitive set,” or “disruptions in arousal-activation,” reward-related impairments in depression interfere with cognitive performance,Reference Austin, Mitchell and Goodwin2 contribute to difficulties encountered in performing cognitive effortful tasksReference Weingartner, Cohen, Murphy, Martello and Gerdt3 and decision-making, and, thus, may account for cognitive impairments. Other more specific cognitive symptoms are equally integral to the diagnosis of MDD, such as a diminished ability to think or concentrate, indecisiveness, or psychomotor retardation.1 Depression-related impairments in attention, memory, psychomotor speed, and executive functioning that were assessed by laboratory tasks are well described at the acute phase of a major depressive episode (MDE), might persist beyond clinical recovery, are related to neuroimaging abnormalities, and could have trait-like characteristics.Reference Cléry-Melin and Gorwood4 Such impaired cognitive processes are likely to contribute to reward-related impairments assessed in depression.
However, there is a growing body of evidence suggesting that impaired ability to use information (ie, the cost and benefits or the magnitude and probability of rewards) to expend an effort and to guide or adapt their choice behaviors are crucial cognitive aspects of reward-related symptoms in depressed patients.Reference Treadway, Bossaller, Shelton and Zald5 Research is necessitated to obtain a comprehensive understanding of the underlying neuropsychological and neurobiological mechanisms of both reward-related and cognitive impairments, as they seem to coexist in depressed patients, even after remission, and lead to a higher risk of poorer treatment response, functional outcome, and relapse or recurrence.Reference Chen, Takahashi, Nakagawa, Inoue and Kusumi6–Reference Uher, Perlis and Henigsberg9
The present review aims to analyze reward processes and cognitions impaired in MDD and their characteristics and neurobiological substrates to provide better assessments, prevention, and treatment strategies. A literature search was initially conducted in the PubMed (Medline) database, to identify articles with the following key words: “reward” or “reward system” and “cognitive” or “cognition” and “depressive disorder” or “depression” not “bipolar” not “addiction” and not “schizophrenia”). Some articles that did not correspond to the topic of interest were excluded after screening the abstracts, and some were included after referencing retrospectively from the initially selected articles and additional ad hoc searches.
We focused on reward-related processes that are considered by many behavioral studies as cognitive processes and equally impaired in depression, as in suicidal behavior. Reward-related impairment may constitute possible endophenotypes of depression, ie, simpler phenotypes used to aid in characterizing the disorder and its underlying substrates.Reference Gottesman and Gould10 A better understanding of the neural network subserving the reward-related impairments in depression would help to characterize the disease better and potentially provide better assessments and preventive and therapeutic strategies to reduce reward-related impairments.
Cognitive Behavioral Studies of Reward-Related Processes in MDD
Focus on reward-related processes as cognitive processes
Reward processing is a broad psychological construct that can be parsed into 3 distinct neuropsychological sub-components known as “reward-related learning” or “reinforcement learning,” “hedonic capacity,” and “motivation to obtain a reward.” These reward-related processes play key roles in optimizing the allocation of brain resources necessary for evolutionary survival and for maintaining spontaneous activity, by helping to drive behavior adaptively among different options. They may be supported by partially dissociable brain systems according to neuroimaging findings and underlie dysfunctional reward-related behaviors in depression.Reference Admon and Pizzagalli11–Reference Berridge and Kringelbach13
Reward-related learning (or reinforcement learning) consists of the ability to represent the rewarding (or punishing) value of a stimulus and, then, to establish and update predictions of future reward to guide and modify behavior (with the highest and well-estimated overall predicted reward value) to maximize reward and/or minimize punishment.Reference Chase, Kumar, Eickhoff and Dombrovski14 The subject may either exploit a previously learned model (model-based learning) or rely on some trial and error experience (model-free learning) for action selection.Reference Chen, Takahashi, Nakagawa, Inoue and Kusumi6 Value prediction is usually assessed in classical conditioning involving signal detection (eg, probabilistic reward task [PRT]Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava15) or decision-making paradigms (eg, Iowa Gambling Task [IGT]Reference Must, Horvath, Nemeth and Janka8). They measure the ability to learn from reward feedback and involve a large cerebral network underlying cognitive and reward processing functions (striatum and frontocingulate regions).Reference Chen, Takahashi, Nakagawa, Inoue and Kusumi6, Reference O’Doherty16
Hedonic capacity (or reward sensitivity, reward responsiveness) is another reward-related process explored by examining “liking” reactions (eg, ratings or behavioral reactions toward pleasantnessReference Berridge and Kringelbach13) that capture some aspect of pleasure and mainly involve limbic structures (the nucleus accumbens and ventral pallidum).Reference Berridge, Robinson and Aldridge12 The degree of response bias (propensity to select one or the other response irrespective of stimulus presentation), for example, in signal detection tasks, can also be used to objectively assess reward responsiveness (such as a preference for the response paired with a more frequent reward).Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava15
Finally, motivation to obtain a reward has been linked to the concept of “wanting” and is also called incentive salience, a type of incentive motivation that does not always require elaborate explicit expectations of future outcomes. Motivation is focused more directly on reward-related (innate or learned) stimuli and involves subcortical neural systems that include mesolimbic dopamine projections. Research has established that “wanting” and “liking” rewards, as well as “wanting” and reward learning (association and prediction), are dissociable psychologically and neurobiologically.Reference Berridge, Robinson and Aldridge12 In studies recently performed and reviewed by Pessiglione et al,Reference Pessiglione, Vinckier, Bouret, Daunizeau and Le Bouc17 motivation is defined as the function that orients and activates the behavior to achieve a goal and assessed by investigating the trade-off between the expected cost entailed by potential actions (mostly focusing on the amount of effort the subject is willing to expend) and the expected benefit associated with potential rewards. Different types of tasks have been developed over the past decade, commonly known as effort-cost decision-making (ECDM) tasks. Effort-based decision tasks (eg, binary choice such as the button-pressing effort expenditure for rewards task [EEfRT]Reference Treadway, Bossaller, Shelton and Zald5, Reference Yang, Huang and Zhu18, Reference Yang, Huang and Lan19), incentive motivation tasks (eg, willingness to accept or decline performing a single actionReference Bonnelle, Veromann, Burnett Heyes, Lo Sterzo, Manohar and Husain20) and free-operant tasks (eg, selection within a continuous range such as a handgrip force measurement squeezing taskReference Schmidt, d’Arc and Lafargue21) are specific behavioral tasks quantifying motivation as an effort/reward trade-off. The underlying effort and reward networks include the dorsal anterior cingulate cortex (dACC) and its connections to the anterior insula (aI) and premotor and motor areas and ventral frontostriatal circuits, as well as the dopaminergic nuclei, mainly involving serotoninergic, dopaminergic, and noradrenergic modulationReference Pessiglione, Vinckier, Bouret, Daunizeau and Le Bouc17 (Figure 1).
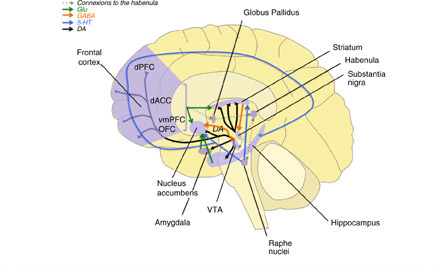
Figure 1 Reward-related processes: main regions and neurotransmitters 5-HT, serotonin; DA, dopamine; dACC, dorsal anterior prefrontal cortex; dPFC, dorsal prefrontal cortex; Glu, glutamate; GABA, gamma amino butyric acid; OFC, orbitofrontal cortex; vmPC, ventromedial prefrontal cortex; VTA, ventral tegmental area.
In a recent review by Hélie et al, the authors assumed that reward processing and valuation (a process of assigning values to states and actions based on the subject’s current representation of the environment occurring before action) are essential steps in any cognitive process and can even be considered cognitive functions, on which many other cognitive functions rely.Reference Hélie, Shamloo, Novak and Foti7
In this view and considering findings on patients’ deficits in processing information to guide behavior, many cognitive impairments observed in depression (among other neuropsychiatric disorders) are associated with or may be accounted for by deficits in reward processing. The terms reinforcement learning, reward responsiveness and motivation to obtain a reward will be the ones used throughout the manuscript for reasons of simplicity.
Characterization of reward-related impairments in depression
Research on reward processing in depression over the past decade first focused on behavioral responses to reward and punishment. Common findings, mainly obtained from reward reinforcement learning paradigms, indicated that depressed patients respond hyposensitively to a rewardReference Admon and Pizzagalli11, Reference Huys, Pizzagalli, Bogdan and Dayan22 and maladaptively to a punishment (worse performance demonstrating increased sensitivity to punishment and blunted responses to reinforcement). This pattern is related to a dysfunction in the frontostriatal systems modulated by the monoamine systems.Reference Eshel and Roiser23 Reinforcement learning is impaired in both medicated and unmedicated patients with depression (particularly those with high anhedonic symptoms) who fail to modulate behavior as a function of prior reinforcement or feedback in a probabilistic task.Reference Chen, Takahashi, Nakagawa, Inoue and Kusumi6, Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava15, Reference Vrieze, Pizzagalli and Demyttenaere24 They are also observed in healthy individuals with high levels of anhedonia.Reference Admon and Pizzagalli11 Interestingly, reduced reward learning in depressed patients before treatment onset was predictive of a persistent diagnosis of depression 8 weeks later, even when controlling for baseline depression severity and comorbid anxiety, and, thus, may contribute to the persistence of MDD or treatment resistance.Reference Vrieze, Pizzagalli and Demyttenaere24 Further, there is evidence for a persistent reward learning impairment in euthymic individuals with a history of depression in full remission, as compared with healthy controls,Reference Pechtel, Dutra, Goetz and Pizzagalli25, Reference Whitton, Kakani and Foti26 possibly attributable to abnormalities in the neural processes that support reward feedback monitoring (eg, reduced activity in the ACC).Reference Whitton, Kakani and Foti26
However, the underlying mechanisms remain unclear, as an impaired learning rate (ability to update an outcome value and using prediction errors information), in both reward and punishment, has inconsistently been detected in MDD patients, depending on several factors (ie, specific paradigms and patient characteristics).Reference Chen, Takahashi, Nakagawa, Inoue and Kusumi6, Reference Rothkirch, Tonn, Köhler and Sterzer27 Moreover, in a meta-analysis of studies that implemented reinforcement learning tasks, Huys et al (2013) concluded that depression, as well as subclinical anhedonia in healthy controls, primarily affected reward responsiveness rather than learning rate.Reference Huys, Pizzagalli, Bogdan and Dayan22
Reward responsiveness, reflecting hedonic capacity, might indeed be dysfunctional in depression and considered a potential contributory mechanism for depressive symptom.Reference Alloy, Olino, Freed and Nusslock28, Reference Culbreth, Moran and Barch29 For example, patients with unmedicated MDD fail to express a response bias toward a more frequently rewarded stimulus (an indicator of reward responsiveness), this impairment being correlated with self-reported anhedonia.Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava15 Using the same paradigm, lower reward response biases were also found in patients with MDD in full remission, as compared with healthy controls, indicating that a blunted reward responsiveness endures through the different stages of the disease and might be a trait-related abnormality in MDD.Reference Pechtel, Dutra, Goetz and Pizzagalli25 These findings are consistent with reduced striatal reactivity to reward outcomes, possibly associated with disrupted dopamine and opioid/endocannabinoid transmission.Reference Chen, Takahashi, Nakagawa, Inoue and Kusumi6, Reference Vrieze, Pizzagalli and Demyttenaere24
According to Pizzagalli et al, it may be assumed that a blunted reward responsiveness leads to decreased engagement in pleasurable activities and decreased motivational drive to obtain future reward.Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava15 Thus, anhedonia, rather than a reduction in pleasure per se, would consist of an impaired ability to modify behavior as a function of reward or a dissociation between pleasure and the amount of effort expended to achieve it.Reference Admon and Pizzagalli11, Reference Pizzagalli30 In this view, a reduced reward responsiveness and its associated neural correlates could represent an interesting pathway driving effort deficits in depression.Reference Culbreth, Moran and Barch29
Recent findings have also provided compelling evidence of aberrant reward/effort-based decision-making in depression, contributing to motivational impairment. A first study using a handgrip device to assess incentive motivation showed that contrary to healthy controls, patients with MDD failed to increase effort expenditure when more money was at stake.Reference Cléry-Melin, Schmidt, Lafargue, Baup, Fossati and Pessiglione31 In a follow-up study, patients after remission from MDE retrieved a normal sensitivity to incentives (ie, a normal pattern of effort production in response to monetary rewards), associated with an improvement in apathy scores.Reference Mauras, Masson, Fossati and Pessiglione32 Similarly, studies using different ECDM paradigms (binary choice, willingness to accept, and handgrip squeezing tasks) have found consistent evidence for reduced willingness to expend effort to incentives in patients with MDD, as compared with controls.Reference Treadway, Bossaller, Shelton and Zald5, Reference Yang, Huang and Zhu18, Reference Yang, Huang and Lan19, Reference Culbreth, Moran and Barch29, Reference Hershenberg, Satterthwaite and Daldal33, Reference Sherdell, Waugh and Gotlib34 Decreased effort expenditure in depressed patients was correlated with increased self-reported anhedonic symptoms or lower reward expectancy,Reference Yang, Huang and Zhu18, Reference Sherdell, Waugh and Gotlib34 a longer duration of the current episode,Reference Treadway, Bossaller, Shelton and Zald5 and higher apathy scores.Reference Mauras, Masson, Fossati and Pessiglione32 Interestingly, a correlation with depression severity scores (Beck Depression Inventory [BDI] scores), assessed in depressed, remitted, or control subjects, was contradictory among studies,Reference Yang, Huang and Zhu18, Reference Hershenberg, Satterthwaite and Daldal33, Reference Treadway, Buckholtz, Schwartzman, Lambert and Zald35 suggesting that decreased effort expenditure might represent a specific dimension of depression rather than a consequence of depression severityReference Pessiglione, Vinckier, Bouret, Daunizeau and Le Bouc17 and possibly have trait-like characteristics, as discussed below.
Reward-Related Impairments as Vulnerability Markers of the Disease
Some more specific cognitive dysfunctions, particularly for executive functioning, verbal learning, and memory assessed by neurocognitive tests, persist and somehow progress among clinically remitted and depressed patients, potentially representing trait markers of the disorder, even though the mechanisms involved are not yet fully understood.Reference McIntyre, Cha and Soczynska36, Reference Weiland-Fiedler, Erickson and Waldeck37 Considered to be cognitive impairments, a reward-related impairment may also constitute part of an underlying neurobiological vulnerability to MDD and, thus, have useful clinical implications.
Characteristics of reward-related impairments as vulnerability markers
Some studies, mostly studying the ECDM motivational component, support the hypothesis that reward-related impairments are state dependent and, more or less, correlated with symptom severity.Reference Yang, Huang and Zhu18, Reference Mauras, Masson, Fossati and Pessiglione32, Reference Pulcu, Trotter and Thomas38 However, more research on this aspect is required, as consistent findings indicate that they have trait-like, and even endophenotype, characteristics (Table 1). As described above, comprehensive studies showed that reward-related learning impairment at the acute stage of an MDE predicted treatment outcome (above and beyond baseline depression severity and anxiety comorbidity), possibly contributing to the persistence of the disease or treatment resistance.Reference Vrieze, Pizzagalli and Demyttenaere24
Table 1 Reward-related processes: Vulnerability markers and endophenotype characteristics of MDD

ACC, anterior cingulate cortex; AUC, area under the curve; BDHI, Buss-Durkee hostility inventory; BDI-II, Beck; BGLHA, Brown-Goodwin Assessment of Lifetime History of Aggression Depression Inventory II; BIS, Barratt’s Impulsivity Scale; CGI, Clinical Global Impression scale; CI, confidence interval; DASS-21, Depression Anxiety Stress Scale; dlPFC, dorsolateral prefrontal cortex; EEG, electroencephalography; ERP, event-related potentials; FCPS, Fawcett Clarke Pleasure Scale; fMRI, functional magnetic resonance imaging; HAM-D, Hamilton Depression Rating Scale (17 items); IDAS, Inventory of Depression and Anxiety Symptoms; IGT, Iowa Gambling Task; K-SADS-PL, Kid Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version; LORETA, low-resolution brain electromagnetic tomography analyses; MADRS, Montgomery-Asberg Depression Rating Scale; MAThyS, Multidimensional Assessment of Thymic States; OFC, orbitofrontal cortex; OR, odd ratio; PHQ-9, Patient Health Questionnaire; PRT, Probabilistic Reward Task; PSS, Perceived Stress Scale; QIDS, Quick Inventory of Depression Symptomatology; SDS, Sheehan Disability Scale; SHAPS: Snaith-Hamilton Pleasure Scale; TEPS, Temporal Experience of Pleasure Scale.
a Correlation with depression score improvement.
b Significant t-tests (p < 0.05) appear in bold.
Both reward reinforcement learning impairment and blunted reward responsiveness, often concurrently measured in signal detection tasks (eg, probabilistic reward task), are observed in acute MDD and endure when patients are in full remission, even years after the last MDE,Reference Pechtel, Dutra, Goetz and Pizzagalli25, Reference Whitton, Kakani and Foti26 possibly leading to disease recurrence.
Interestingly, consistent neuroimaging findings found abnormalities in reward-related brain activation in not only unmedicated recovered patients with depression but also young people at risk of depression, particularly in striatal or cortical regions playing a role in linking actions to positive and negative feedback (ACC, orbitofrontal cortex [OFC]).Reference Gotlib, Hamilton, Cooney, Singh, Henry and Joormann39, Reference McCabe, Woffindale, Harmer and Cowen40 Moreover, in a prospective study conducted in never-depressed at-risk adolescents, an impaired neural measure of reward responsiveness (using an event-related potential [ERP] component) at baseline predicted the subsequent onset of MDEs and the severity of future depressive symptoms.Reference Bress, Foti, Kotov, Klein and Hajcak41 Finally, blunted reward responsiveness, associated with a high degree of anhedonia, was found in first-degree relatives of patients with MDD experiencing subclinical depressive symptoms, as compared with healthy controls.Reference Liu, Roiser and Wang42
Reward-related processing impairments might be a trait marker related to a patient’s vulnerability to depression, occurring before onset and throughout the different phases of the illness. Its independent expression beyond an acute MDE and its familial association led to consider these impairments as likely endophenotypes for MDD using the criteria of Gottesman and Gould.Reference Gottesman and Gould10 As of 2004, Hasler et al considered an impaired reward function associated with anhedonia as a specific and plausible endophenotype of MDD and being related to a dysfunction in the brain reward system according to molecular, genetic, and epidemiological research.Reference Hasler, Drevets, Manji and Charney43 Anhedonia, as part of the reward-related impairments (reduced responsiveness to rewarding stimuli specifically) and risk factors for depression, has more recently been considered a promising endophenotype of depression. According to Pizzagalli (2014), the anhedonic behavior could be induced by the pervasive effects of stress (chronic stressors and early childhood adversities) on brain reward systems (specifically mesocorticolimbic dopaminergic pathways) implicated in reinforcement learning and incentive motivation.Reference Pizzagalli30 Interestingly, preliminary findings from a twin study indicated that reward responsiveness is heritable, with additive genetic factors contributing to 46% of the variance.Reference Bogdan and Pizzagalli44 Emerging findings also imply that blunted reward reinforcement learning is a promising endophenotype.Reference Whitton, Kakani and Foti26, Reference Webb, Dillon and Pechtel45 According to Webb et al (2016), this reward-related component has emerged as the most promising behavioral endophenotypes of depression, together with neuroticism and cognitive control.Reference Webb, Dillon and Pechtel45 Blunted reward learning was significantly associated with reduced gamma activity in the left dorsolateral prefrontal cortex (dlPFC) and OFC (those regions also involved in reduced reward responsiveness) on resting-state intracranial EEG measurements, even while controlling for the current symptoms and task difficulty. In this study, the 3 putative endophenotypes had partially dissociable resting electroencephalography (EEG) correlates, reflecting underlying neural dysfunctions that will be further reviewed in the next section.
Neurobiological correlates of reward-related processes
Many neuroimaging studies using task-related functional magnetic resonance imaging (fMRI) have focused on activity changes in the regions and circuits involved in the reward processes in MDD (Figure 1) while performing reward-related tasks and found significant differences in patients with MDD, as compared with healthy controls.
First, these studies reported hyporesponsivity and decreased activation in the striatal regions, and their role in the different reward processing sub-components has already been shown,Reference Chase, Kumar, Eickhoff and Dombrovski14, Reference Haber and Knutson46 in both the ventral striatum (including the nucleus accumbens [NAc]) and dorsal striatum (caudate nucleus and putamen) in patients with depression.Reference Admon and Pizzagalli11, Reference Pizzagalli, Holmes and Dillon47
The NAc, as a main component of the dopaminergic mesolimbic pathway through its connections with the ventral tegmental area (VTA) and substantia nigra (SN), plays a central role in reward processes (anticipation and drive to obtain reward) and may even represent a promising target for deep brain stimulation (DBS) as an effective treatment for resistant MDD.Reference Nauczyciel, Robic and Dondaine48 In addition to the massive dopaminergic input from the midbrain (VTA/SN), the ventral striatum receives its main cortical input from the OFC and ACC.Reference Haber and Knutson46 The OFC, together with the ventral striatum and amygdala, is involved in maintaining representations and predicting reward values, necessary to guide action selection for reward and adjust behavior.Reference O’Doherty16, Reference Deng, Rolls and Ji49, Reference O’Doherty50 Neural processing of reward information (eg, discrepancies between expected and actual reward, namely prediction errors) is specifically attenuated in the medial OFC (mOFC) in unmedicated patients with MDD, as compared with healthy participants, even if their behavioral performances (reinforcement learning) does not significantly differ, possibly reflecting the specific role of this region in the ability to experience pleasure from positive outcomes.Reference Rothkirch, Tonn, Köhler and Sterzer27 The ACC receives information from the OFC on the reward value and is involved in the evaluation of effort (cost)/reward (benefit) options to determine the effort required for possible actions. The ACC sends projections to the ventromedial prefrontal cortex (vmPFC) and dlPFC that are involved in decision-making based on reward value, effort, and reinforcement history regarding future actions.Reference Whitton, Kakani and Foti26, Reference Admon, Nickerson and Dillon51–Reference Knutson, Bhanji, Cooney, Atlas and Gotlib53
Several findings on the dorsal striatum (caudate and putamen) indicate that it may be involved in stimulus-response learning (while the ventral striatum is more involved in stimulus-reward learning), responding to the reinforcement of an action and linking action selection to predicted rewardReference O’Doherty16, Reference Whitton, Kakani and Foti26, Reference Pizzagalli30, Reference Admon, Nickerson and Dillon51–Reference Delgado54 The lateral habenula (LHb), a small structure located at the posterior end of the thalamus, within the neural reward circuitry, is coming under scrutiny in a growing number of studies. It is thought to be involved in the avoidance system of the brain, mainly through encoding negative reward prediction errors. Habenular hyperactivity may contribute to anhedonia and symptoms of depression related to reinforcement learning, such as abnormal response to punishment.Reference Admon and Pizzagalli11, Reference Liu, Valton, Wang, Zhu and Roiser55 The habenula, as the ventral striatum, represents a promising target for DBS. Even if further investigation is required, this treatment appears to provide significant clinical improvement in severely affected and refractory patients.Reference Morishita, Fayad, Higuchi, Nestor and Foote56
Functional MRI studies have further suggested that reward-related impairments in depression are related to abnormal cortical-striatal activity and connectivity that may even predict antidepressant treatment outcomes in depressionReference Admon and Pizzagalli11, Reference Morishita, Fayad, Higuchi, Nestor and Foote56, Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor57 For example, in patients with depression, but not in healthy individuals, an acute pharmacological challenge inducing enhancement of dopaminergic transmission (using a single dose of the D2/D3 receptor antagonist, amisulpride) increased striatal activity and cortical-striatal functional activity in response to rewards. Besides, a stronger corticostriatal functional connectivity between the NAc and midcingulate cortex was associated with better reward learning performance.Reference Admon, Kaiser and Dillon58
More recently, specific resting-state fMRI studies, investigating correlations in activity across different regions to delineate large-scale functional networks, have shown abnormal functional connectivity within and between networks (eg, Central Executive Network, Salience Network, Default-Mode Network), involved in reward-related cognitive deficits and depressive mood in patients with MDD.Reference Admon, Kaiser and Dillon58–Reference Quevedo, Ng and Scott62 For example, compared with control subjects, patients with MDD had disrupted functional connectivity of the bilateral NAc networks primarily located in the prefrontal-striatal (OFC, ACC, and caudate) regions that was significantly associated with nonspecific cognitive deficits (Mini-Mental State Examination [MMSE])Reference Folstein, Folstein and McHugh63 and depression severity.Reference Gong, Yin and He59 Interestingly, in a resting-state fMRI study exploring the whole connectome in relation to reward responsiveness across participants with different psychiatric diagnosis (including MDD), Sharma et al (2017) found that reward responsiveness deficits were associated with connectivity abnormalities between the NAc and distinct major cortical functional networks, regardless of clinical diagnosis category.Reference Sharma, Wolf and Ciric64 Moreover, impairment in motivation to obtain a reward, assessed in an effort-based reinforcement task, was associated with deficient mOFC-striatal functional connectivity, as compared with not only healthy controls but also patients with schizophrenia, suggesting that a common symptom such as motivational impairment may involve different prefrontal-striatal pathways in different psychiatric disorders.Reference Park, Lee, Kim, Kim and Koo65
Electrophysiology studies have had interesting findings, using ERP to assess reward-related impairment in MDD. ERP studies have focused on feedback negativity (FN), an index of early evaluation of rewards, as compared with non-rewards, which is usually maximal at the frontocentral electrodes, 300 ms following reward feedback. There is converging evidence that an FN amplitude represents a neural measure of reward sensitivity, one of the main reward-related processes. FN amplitude is blunted in MDD, is related to anhedonia severity and depressive symptoms,Reference Liu, Wang and Shang66 and correlates with blunted ventral striatum activation especially in patients with melancholic features such as impaired mood reactivity.Reference Foti, Carlson, Sauder and Proudfit67 Moreover, a blunted FN amplitude at baseline in adolescent girls who have never had depression independently predicted the subsequent onset of the first episode and severity of symptoms during the follow-up period.Reference Bress, Foti, Kotov, Klein and Hajcak41
With regards to neurotransmitters, dopamine plays a critical role in reinforcement learning and incentive motivation.Reference Pizzagalli30 Dysfunction within the mesolimbic dopaminergic pathway projecting from the VTA to the ventral striatum (NAc) may have a key role in the pathophysiology of depression and particularly in reinforcement learning, as ventral striatal dopamine regulates the prediction and anticipation of rewards, the 2 mechanisms responsible for reinforcement learning.Reference Der-Avakian and Markou52, Reference Admon, Kaiser and Dillon58 Recent evidence in animal and human studies implicated other neurotransmitters in reward processing, including glutamate, gamma-aminobutyric acid (GABA), and serotoninReference Admon and Pizzagalli11, Reference Der-Avakian and Markou52, Reference Gorwood68 The NAc receives glutamatergic input from the amygdala, increasing motivation to achieve a goal-directed action planned in the prefrontal areas. Ketamine (N-methyl-D-aspartate [NMDA], or glutamate receptor antagonist) has an antidepressant effect that may be mediated by glutamatergic signaling, blocking glutamate uptake in the prefrontal cortex, resulting in reward impairment.Reference Skolnick, Popik and Trullas69 Glutamatergic activity and also probably opioid activity in the amygdala are necessary for motivated behavior.Reference Stuber, Sparta and Stamatakis70 Moreover, activation of mu opioid, endocannabinoid,Reference Berridge and Kringelbach71 and GABA–A receptors in the NAc may mediate the hedonic perception of rewards.Reference Faure, Richard and Berridge72 A reduced GABA concentration in the prefrontal cortex has been specifically associated with depression and high anhedonia scores.Reference Gabbay, Mao and Klein73 Serotonin (5-HT) originating from the raphe nuclei and modulating dopamine and opioid releaseReference Yan74 may also regulate reward-related processes. For example, chronic serotonin-noradrenaline reuptake inhibitor antidepressant treatment is reported to increase ventral striatal activity in patients with depression,Reference Ossewaarde, Verkes and Hermans75 and behavioral results in healthy subjects showed that a selective serotonin reuptake inhibitor (SSRI) treatment improved global performance to obtain a reward, through specific diminution of the effort cost, highlighting the role of serotonin in behavioral regulation.Reference Meyniel, Goodwin and Deakin76 The abovementioned description of neurotransmitters is not exhaustive but provides some insight on the possible effects of disruptions in the underlying circuits on reward-related processes.
Clinical implications: assessment and management of reward-related impairments
As we previously mentioned, reward-related impairments have recently become a growing research interest, as they may persist after remission and lead to a higher risk of poorer treatment response and functional outcomes, such as relapse or recurrence in patients with depression. They may be considered as likely endophenotypes for MDD and have consistent neurobiological substrates; thus, they must be considered a legitimate therapeutic target.
However, there is a lack of consensus on the terminology used to describe these impairments in clinical (symptoms such as amotivation, anhedonia, apathy, avolition, and anergia) and neuropsychological (components such as hedonic capacity or reward responsiveness, reward learning, and motivation or incentive salience or ECDM) domains, possibly explaining the lack of consistent epidemiological data and gold-standard assessment tools in this field.Reference Calabrese, Fava and Garibaldi77
Nevertheless, assessment and early management of reward-related impairments would be of most benefit to patients with depression and could ultimately lead to personalized treatment. Recent data suggest that the presence of both reward-related symptoms and impairments (eg, reduced reward learning) in individuals with depression predicts a poor outcome with antidepressant treatment, over and above depression severity and other previously reported predictors of outcome or comorbid anxiety.Reference Uher, Perlis and Henigsberg9, Reference Vrieze, Pizzagalli and Demyttenaere24 This finding could possibly be related to abnormal cortical-striatal activity and connectivity.Reference Admon and Pizzagalli11, Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor57 Interestingly, in a large prospective study, motivation was the most impaired dimension reported at baseline by patients with depression but had the strongest capacity to detect early improvement; the best predictive value for antidepressant treatment response; and the largest global margin of progress,Reference Gorwood, Vaiva, Corruble, Llorca, Baylé and Courtet78 raising the interest for a special emphasis on reward-related processing within the therapeutic process to improve clinical outcomes. However, treatments may confound the interpretation of findings from reward-related processing studies in MDD, as symptoms of reduced emotion and motivation have been reported as a side-effect of antidepressants (especially SSRI)Reference Price, Cole and Goodwin79 and may be worsened by concomitant medication initiation or discontinuation such as benzodiazepines or antipsychotics.
Findings on both biological and nonbiological therapies (eg, behavioral and cognitive remediation or psychotherapy) specifically focusing on reward-related impairment in depression are still lacking. However, cognitive behavioral therapy (CBT) appears to be most beneficial in reducing depressive symptoms for patients with depression who demonstrated decreased reward sensitivity (assessed using ERP) prior to treatment.Reference Burkhouse, Kujawa and Kennedy80 Some DBS targets core reward network regions such as the ventral striatum and lateral habenula for treatment of resistant MDD.Reference Nauczyciel, Robic and Dondaine48, Reference Morishita, Fayad, Higuchi, Nestor and Foote56 Lastly, transcranial direct current stimulation (tDCS) targeting the frontopolar cortex enhanced motivation to exert effort for rewards, therefore, representing interesting therapeutic prospects.Reference Soutschek, Kang and Ruff81
In a comprehensive article summarizing the search for a consensus by a group of experts in the optimal approach to study motivation impairments in mood disorders, Calabrese et al (2014) pointed out that there is currently neither a widely accepted gold-standard assessment scale for measuring reward-related impairment (qualified as “amotivation” by the authors) either in isolation or within the context of neuropsychiatric disorders, nor a rating scale defining the clinically relevant effect of treatment on motivation symptoms.Reference Calabrese, Fava and Garibaldi77 Even if symptoms overlap in different usual depression rating scales (such as Hamilton Depression Scale [HAM-D 17],Reference Hamilton82 Montgomery and Asberg Depression Rating Scale [MADRS],Reference Montgomery and Asberg83 and BDIReference Beck, Ward, Mendelson, Mock and Erbaugh84), they recommended distinguishing amotivation from other constructs such as fatigue (eg, Fatigue Severity Scale [FSS],Reference Demyttenaere, De Fruyt and Stahl85 fatigue visual analog scale [VAS], and Massachusetts General Hospital Cognitive & Physical Functioning Questionnaire [MGH CPFQ] that measures both amotivation and fatigueReference Fava, Iosifescu, Pedrelli and Baer86), anhedonia (eg, Snaith-Hamilton Anhedonia Pleasure Scale [SHAPS]Reference Snaith, Hamilton, Morley, Humayan, Hargreaves and Trigwell87), and cognitive and executive dysfunction (eg, MGH CPFQ). They also recommended assessing the overall impact of reward-related impairment on functional outcomes (eg, Sheehan Disability Scale [SDS]Reference Sheehan, Harnett-Sheehan, Spann, Thompson and Prakash88), quality of life (eg, Quality of Life Enjoyment and Satisfaction Questionnaire [QLESQ]Reference Hope, Page and Hooke89), and distress experienced by caregivers (eg, Apathy Evaluation Scale [AES]Reference Marin, Biedrzycki and Firinciogullari90 and Neuropsychiatric Inventory-Apathy subscale [NPI-a]Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein91).
Some of these scales were used in the studies described in this review, such as the usual depression rating scales; the anhedonia scale, SHAPS; and the apathy scale, AES; some others were used to examine specific aspects of depression/anxiety (eg, Mood and Anxiety Symptom Questionnaire [MASQ]Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava15), anhedonia (eg, Temporal Experience of Pleasure Scale [TEPS]Reference Yang, Huang and Zhu18, Reference Yang, Huang and Lan19, Reference Liu, Roiser and Wang42), specific depressive dimensions such as motivation (eg, Multidimensional Assessment of Thymic States scale [MAThyS]Reference Gorwood, Vaiva, Corruble, Llorca, Baylé and Courtet78), perceived global stress (eg, Perceived Stress Scale [PST]Reference Pechtel, Dutra, Goetz and Pizzagalli25), cognitive symptoms (eg, MMSEReference Gong, Yin and He59, Reference Quevedo, Ng and Scott62), and global functioning (eg, Global Assessment of Functioning Scale [GAF]Reference Sherdell, Waugh and Gotlib34). Relevant measurement tools of reward-related impairments in patients with depression are needed to provide more personalized preventive and therapeutic strategies.
Reward-Related Processes and Suicidal Behavior
While the risk of suicidal acts is increased in depression in comparison with the general population, less than 10% of patients with depression will die from suicide, and less than 50% will attempt suicide.Reference Isometsä92 Literature now suggests that individuals with a history of depression and suicidal behavior represent a subgroup of patients with particular clinical, cognitive, and biological characteristics.Reference Turecki and Brent93 Among these characteristics, an increasing number of studies have underlined the relevance of reward-related processes in suicidal behavior in the context of depression.Reference Jollant, Lawrence, Olié, Guillaume and Courtet94
Indirect arguments were first given in the 1970s when low levels of 5-HIAA, the main metabolite of serotonin, were found in patients with depression and a history of violent suicidal acts.Reference Asberg, Träskman and Thorén95 Subsequently, it was found that low 5-HIAA levels were predictive of future death from suicide.Reference Mann, Currier, Stanley, Oquendo, Amsel and Ellis96 In postmortem studies, individuals who committed suicide had alterations in serotonergic transporter binding in different regions of the prefrontal cortex independently from depression.Reference Mann, Huang and Underwood97 The “short” allele of the gene coding for the serotonin transporter (5-HTTLPR) was also associated with an increased risk of suicide attempts, unrelated to alcohol dependence or comorbid depression.Reference Gorwood, Batel, Adès, Hamon and Boni98 Among many homeostatic functions, serotonin is implicated in the modulation of risky choices through negative outcome-value associative learning and processing of positive reward signals.Reference Rogers99
More direct evidence for a role of reward-related processes in suicidal behavior was given with the investigation of decision-making. In comparison with patients with a history of depression but no personal history of suicidal behavior and with healthy controls, non-currently depressed suicide attempters showed riskier decision-making during the IGT: in uncertain conditions, attempters preferred options yielding high rewards but even higher losses to options yielding low rewards but lower losses.Reference Jollant, Bellivier and Leboyer100 Alterations were particularly marked in those who used a violent suicidal means. A meta-analysis of 9 studies in mood disorders confirmed impaired performance in patients with and without a history of suicidal behavior.Reference Richard-Devantoy, Berlim and Jollant101 These deficits were found in all age groups including the elderlyReference Clark, Dombrovski and Siegle102 and adolescentsReference Bridge, McBee-Strayer and Cannon103 and were found in attempters, but not in “pure” ideators,Reference Sheftall, Davidson and McBee-Strayer104 suggesting a particular role in the transition from ideas to acts. Interestingly, impaired decision-making was also found in relatives of suicide completers with no personal history of suicidal acts, leading to the idea that it may represent an endophenotype.Reference Hoehne, Richard-Devantoy, Ding, Turecki and Jollant105 Moreover, genetic variants in serotonergic genes were found to modulate decision-making performance in suicide attempters, suggesting a significant role of the serotonergic system, as suspected.Reference Jollant, Buresi and Guillaume106 Using functional neuroimaging and the IGT, our group found in both remitted suicide attempters and healthy relatives of suicide victims changes in brain activations according to risk level in dorsal and ventral prefrontal cortex.Reference Ding, Pereira and Hoehne107–Reference Olié, Ding and Le Bars109 Using a reversal-learning task in depressed elderly patients, Dombrovski et al showed higher discounting in suicide attempters (but not in ideators) and suggested that attempters tend to overly focus on current outcomes and ignore past experience to guide their choices.Reference Dombrovski, Clark and Siegle110 In the same population, neuroimaging was able to differentiate changes associated with depression from those associated with suicidal behavior: a history of a suicide attempt was linked to reduced expected reward signals in the ventromedial prefrontal cortex (in turn predicting insensitivity to contingency changes during decision-making), while depression was linked to perturbed encoding of unpredicted rewards in various corticostriatothalamic regions (in turn predicting an oversensitivity to punishment).Reference Dombrovski, Szanto, Clark, Reynolds and Siegle111
Alterations in reward-related processes in suicidal behavior likely extend beyond abstract stimuli (like money) to social situations. For instance, suicide attempters had increased responses to angry, but not happy or sad, faces in the orbitofrontal cortexReference Ding, Pereira and Hoehne107, Reference Olié, Ding and Le Bars109–Reference Jollant, Lawrence and Giampietro112 and impaired responses to social exclusion in the posterior insula and parietal cortex.Reference Olié, Jollant and Deverdun113 Both results have been interpreted as an excessive valuation of signals of social reject and disapproval (social punishments). Moreover, elderly suicide attempters tend to reject unfair offers at the Ultimatum Game, whatever the level of unfairness and sacrifice it represents for themselves.Reference Szanto, Clark, Hallquist, Vanyukov, Crockett and Dombrovski114 These patients may, therefore, value unfairness excessively, a behavior also observed in individuals submitted to acute tryptophan depletion.Reference Young115
In summary, these findings suggest impaired reward-related and valuation processes in individuals at risk of suicidal behavior. It is hypothesized that depression contributes to acute cognitive disturbances that add up to trait deficits (constituting elements of vulnerability) during the suicidal crisis. More research will be necessary to explore the new dimensions of reward-related processes (eg, effort costs), dissect the neurocognitive basis of various suicidal phenotypes (eg, violent vs nonviolent suicidal acts and ideas vs acts), make sense of these alterations within the suicidal processes, understand the link with other cognitive deficits observed in suicidal patients (affecting, for example, memory and verbal fluency), and identify therapeutic and preventive tools targeting risk and reward processing.
Conclusion
Wanting, liking, and learning from/for rewards represent core aspects of MDDs, interestingly, at the border of clinical and neuropsychological approaches. Indeed, anhedonia and amotivation could constitute the clinical face of cognitive functions, which help drive behavior adaptively among the different options of reward-related learning. Three core aspects have been distinguished: (1) the ability to represent the rewarding value of a stimulus and establish predictions of future reward to guide and modify behavior to maximize reward (namely reinforcement learning); (2) capacity to assess or react toward pleasantness (namely reward responsiveness, sometimes quoted as liking); and (3) incentive motivation for a reward (namely motivation, sometimes quoted as wanting). Many paradigms are being used (Table 1) that have the advantage to focus on specific aspects of the reward process; however, as the tasks used frequently have overlapping aspects, it is somewhat difficult to see which aspects could be more basic “risk factors” (for example, because shared in at-risk families or having endophenotype properties), which could represent “state-marker” (and be more related to clinical symptoms) and which could constitute “stigmates” of past depressive episodes. However, there is little doubt that assessing these core functions, with comparable and valid instruments, in everyday practice, would be of considerable help to disentangle what could explain unresolved symptoms (incomplete remission), characteristics of patient subgroups (such as highly recurrent and treatment-resistant patients), and risk factors in vulnerable populations (for example, those having familial history of depression). Such practice would have a large impact, as the neurotransmitters involved and brain area in charge are more precisely described, offering new opportunities for personalized treatments.
Disclosure
Marie-Laure Cléry-Melin and Fabrice Jollant have nothing to disclose. Philip Gorwood reports grants from Ethypharm, grants and personal fees from Servier, personal fees from Janssen, personal fees from Lilly, personal fees from Lundbeck, and personal fees from Otsuka, outside the submitted work.




