COVID-19 had detrimental effects on mental health, with worldwide rates of major depressive disorders (MDD) and anxiety disorders rising to 27.5 and 25.6%, respectively.1 Fear of the virus itself and lockdowns implemented by governments around the globe have caused greater mental distress and lower quality of life in the general population.Reference Aknin, Andretti, Goldszmidt, Helliwell, Petherick and De Neve2–Reference Fancourt, Steptoe and Bu5 In particularly, young people, who are known to experience major social role transitions,Reference Sawyer, Azzopardi, Wickremarathne and Patton6 experienced higher levels of depressive and anxiety symptoms than people in older age groups.1,Reference Glowacz and Schmits4,Reference Fancourt, Steptoe and Bu5 The pandemic has also been reported to worsen symptoms of patients with pre-existing mental illness,Reference Lewis, Lewis, Roberts, Richards, Evison and Pearce7,Reference Warne, Heron, Mars, Kwong, Solmi and Pearson8 although contradictory findings have been reported.Reference Pan, Kok, Eikelenboom, Horsfall, Jorg and Luteijn9–Reference Sadeghi, Fors, Eisner, Taigman, Qi and Gorham12 These contradictions and the limitations of studies to date highlight the need for further research that is both longitudinal and focuses on youth.Reference Wade, Prime and Browne13
The psychosocial stress caused by this pandemic has been detrimental to youth around the world, who have experienced adverse lifestyle changes.Reference Lee14,Reference Amerio, Lugo, Stival, Fanucchi, Gorini and Pacifici15 Confinement measures during lockdowns and the associated personal, educational and economic disruptions created pervasive social isolation, increased stress and decreased peer interactions, which may have triggered psychological distress and mental health difficulties in this age group. Indeed, meta-analyses of studies of children and adolescents indicate an increased prevalence of clinically elevated depression and anxiety symptoms compared with pre-pandemic estimates, especially in adolescent females.Reference Racine, McArthur, Cooke, Eirich, Zhu and Madigan16,Reference Ma, Mazidi, Li, Li, Chen and Kirwan17 However, most studies investigated the effects of the pandemic on mental health changes only at the beginning of the pandemic. Although enormously instructive, these studies do not address the longer-term effects of the pandemic. Other limitations include the considerable heterogeneity of studies, which is largely due to differences in assessments and diagnostic criteria.Reference Diaz Gonzalez-Colmenero, Millan-Alanis, Barrera and Saucedo-Uribe18 The focus of most studies on anxiety and depression has also led to a call for more research to consider the effects of the pandemic on other youth mental health conditions that may have been negatively affected by the COVID-19 pandemic, in particular, eating disorders and addiction.Reference Dey, Mansell and Ranu19 More limited evidence available suggests that pre-pandemic disordered eating is a risk factor for poorer mental health during the pandemic.Reference Warne, Heron, Mars, Kwong, Solmi and Pearson8,Reference Hyam, Richards, Allen and Schmidt20 However, interpretations of these findings are limited as, again, assessment of mental health was restricted to the period of eased restrictions following the first lockdown. As for addiction, a decline in substance use has been reported, especially among adolescents initially at higher risk for substance use disorder.Reference Sheikhan, Hawke, Ma, Courtney, Szatmari and Cleverley21,Reference Deeken, Reichert, Zech, Wenzel, Wedemeyer and Aguilera22 It is clear from these limitations that longitudinal trajectory research with comprehensive mental health assessments, spanning the pre-pandemic period and across multiple lockdown and release phases, is needed to understand the long-term impact of the COVID-19 pandemic on youth mental health.Reference Wade, Prime and Browne13 Research comparing data from the general population and from patient groups is also needed. Crucially, identifying the most vulnerable and resilient groups will be important for the design and delivery of the most appropriate targeted interventions.
Aims
Our study addresses these needs by using data collected before and throughout the COVID-19 pandemic in two pre-existing youth cohorts: IMAGEN, a longitudinal population-based adolescent cohort; and ESTRA/STRATIFY, a clinical cohort with diagnoses of MDD, alcohol use disorders (AUD) and eating disorders. Our repeated assessments, based on the CoRonavIruS Health Impact Survey (CRISIS)Reference Nikolaidis, Paksarian, Alexander, Derosa, Dunn and Nielson23 and standardised mental health questionnaires, aimed to (i) establish trajectories of behaviours and mental health symptoms throughout stages of the pandemic in these cohorts; (ii) compare these trajectories to identify the most vulnerable groups; and (iii) identify pre-pandemic predictors of these mental health trajectories.
Method
Study design
Participants were drawn from three existing cohorts located in the UK, France and Germany: IMAGEN, STRATIFY and ESTRA. IMAGEN was a longitudinal population cohort, whereas STRATIFY and ESTRA were case–control cohorts. To be eligible for inclusion, participants needed to respond to our invitation and provide informed consent through an online form sent via email. Data collection was conducted through online questionnaires, with the initial round taking place during the first national lockdown in the UK and Europe (April–May 2020). Subsequent follow-up surveys were administered when the first lockdown was released (July 2020) and when the second lockdown was imposed (November 2020). The design and reporting of our study were in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Participants
Population cohort
These participants, with no known history of mental illness, were drawn from the IMAGEN study, a longitudinal cohort of over 2000 adolescents recruited at age 14 years from eight study sites in Europe, with follow-up assessments at ages 16, 19 and 23 years. For detailed study protocols, please refer to Schumann et al.Reference Schumann, Loth, Banaschewski, Barbot, Barker and Büchel24 Our survey was sent to those who had completed the follow-up assessment at age 23 (N = 1350). A total of 458 IMAGEN participants recruited from the UK, France and Germany (London, Nottingham, Paris, Mannheim and Berlin) who completed the COVID-19 survey at baseline were included in our analyses.
Clinical cohort
This cohort was derived from two studies, STRATIFY and ESTRA, of participants aged 18–30 years (N = 628). STRATIFY participants included in this study comprised participants recruited in the UK and Germany (London, Southampton and Berlin) who met diagnostic criteria for MDD and AUD, as assessed by self-report via online computerised screening. Participants were included if they had scores ≥15 (moderate to severe) on the Patient Health Questionnaire (PHQ-9)Reference Kroenke, Spitzer and Williams25 and Alcohol Use Disorders Identification Test (AUDIT)Reference Bush, Kivlahan, McDonell, Fihn and Bradley26 for MDD and AUD, respectively. ESTRA consisted of participants recruited in London and meeting the DSM-5Reference Asken, Grossman and Christensen27 diagnostic criteria for anorexia nervosa or bulimia nervosa. All were female. Their eating disorder symptoms were assessed using the Eating Disorder Diagnostic Scale (DSM-5 version) over a screening phone call by study researchers.Reference Stice, Telch and Rizvi28 A total of 211 STRATIFY/ESTRA participants (80 MDD, 51 AUD, 47 anorexia nervosa and 33 bulimia nervosa) who completed the COVID-19 survey at baseline were included in our analyses.
Ethics statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by King's College London Research Ethics Committee (17/LO/0552) for IMAGEN, London Westminster Research Ethics Committee (PNM/10/11-126) for STRATIFY and North West–Greater Manchester South Research Ethics Committee (20/NW/0143) for ESTRA. All adult participants provided written/online signature informed consent to participate in this study.
Survey and assessments
The COVID-19 survey
We adapted the CoRonavIruS Health Impact Survey (CRISIS v0.1 http://www.crisissurvey.org)Reference Nikolaidis, Paksarian, Alexander, Derosa, Dunn and Nielson23 to examine changes to individuals’ mental health and behaviours induced by the pandemic. The survey encompassed data collection at various time points, specifically: pre-pandemic (3 months prior, pre-LD1), during the first lockdown (LD1), after the first lockdown (after-LD1) and during the second lockdown (LD2). This questionnaire assessed a range of data domains including COVID-19-related health status and life changes, daily behaviours and emotions, and worries due to the COVID-19 crisis (see Supplementary Information available at https://doi.org/10.1192/bjo.2023.601 for details).
Mental health assessments
The severity of mental disorder symptoms was assessed with validated questionnaires, including the PHQ-9 for depressive symptoms, the Eating Disorder Examination Questionnaire (EDE-Q)Reference Fairburn and Beglin29 for eating disorder symptoms and the AUDIT ConsumptionReference Bush, Kivlahan, McDonell, Fihn and Bradley26 for alcohol misuse (see Supplementary Methods for details). Questionnaires were administered at three time points: (a) at the previous recruitment wave, ~3 years prior to the pandemic (pre-PD), (b) during the first lockdown (LD1) and (c) during the second lockdown (LD2). The exception was the EDE-Q, which was administered at only two time points (i.e. LD1 and LD2) in the clinical cohort.
Pre-pandemic mental health
Pre-PD symptom severity scores were used to classify participants from the population cohort, based on the following criteria. For depression, PHQ-9 scores of 0–4, 5–9 and 10+ were used to indicate minimal, mild and moderate to severe depression, respectively.Reference Kroenke, Spitzer and Williams25 For alcohol misuse, AUDIT scores of 0–7 and 8+ were used to indicate low and high risk, respectively.Reference Piccinelli, Tessari, Bortolomasi, Piasere, Semenzin and Garzotto30 For eating disorders, EDE-Q global scores <2.8 (for females) or 1.68 (for males) were used to indicate low risk; higher scores were considered to indicate probable eating disorders.Reference Schaefer, Smith, Leonard, Wetterneck, Smith and Farrell31,Reference Mond, Myers, Crosby, Hay, Rodgers and Morgan32 For body mass index (BMI), we used the following categories: underweight or normal weight, BMI < 25; overweight or obese: BMI > 25.
Statistical analyses
Data were analysed in SPSS version 27 using mixed-effects analysis of variance (ANOVA), with within-subject effect (time) adjusted by country and between-subjects effects (cohort and sex) adjusted by country and age. For each analysis, participants were included if they had no missing data for any variable needed. Separate analyses were conducted, as detailed in the Supplementary Methods, on the whole sample or on each cohort separately. Statistical significance was set at P < 0.05.
Trajectories of lifestyle changes, worries and mental health symptoms during the pandemic
Scores from the COVID survey and mental health questionnaires were analysed across time points. In addition to time effects, we investigated cohort and sex effects, along with interaction effects (i.e. time × cohort, time × sex) in the whole sample. Given the strong cohort effects, we also investigated these trajectories in the population and clinical cohorts separately.
Trajectories of mental health symptoms based on pre-pandemic symptom severity
These analyses were performed in the population cohort only. Subgroups based on the severity of pre-PD symptoms (see above) were included in mixed-effects ANOVAs. Three analyses were run to investigate interactions between time and pre-PD severity of mental health symptoms (i.e. depression, alcohol misuse or eating disorder) during the pandemic.
Results
Sample description and participants’ characteristics
A flowchart outlining the recruitment and follow-up of participants for this study is provided in Fig. 1. In total, 669 individuals (31.5% clinical cohort; 69.4% females) completed the COVID survey at pre-LD1, 471 (29.9% clinical cohort) at LD1 and 429 (27.0% clinical cohort) at LD2 (Supplementary Table 1). As expected, immediately prior to the pandemic, symptoms of depression (F(1,615) = 156.26, P < 0.001, ηp2 = 0.203) and alcohol misuse (F(1,630) = 30.11, P < 0.001, ηp2 = 0.046) were higher in the clinical cohort. BMI was higher in the population sample (F(1,543) = 17.76, P < 0.001, ηp2 = 0.032). Females reported higher levels of depressive (F(1,615) = 13.95, P < 0.001, ηp2 = 0.022) and eating disorder symptoms (F(1,411) = 45.92, P < 0.001, ηp2 = 0.100), whereas males reported higher levels of alcohol misuse (F(1,630) = 23.38, P < 0.001, ηp2 = 0.036).

Fig. 1 Recruitment flowchart for study participants. Analysis 1 examined trajectories of behaviours, emotions and COVID-related worries during the pandemic. Analysis 2 investigated mental health trajectories during the pandemic. Analysis 3 explored the impact of pre-pandemic symptom severity on mental health trajectories during the pandemic. For each analysis, participants were excluded if they had missing data for any required variable. ADHD, attention-deficit hyperactivity disorder; LD1, first lockdown; LD2, second lockdown; RecAN, recovered from anorexia nervosa; RecBN, recovered from bulimia nervosa.
Behavioural, emotional and mental health trajectories during the pandemic
We compared behavioural, emotional and mental health trajectories during the pandemic in our two cohorts using mixed-effects ANOVA. Significant main effects of time on behaviours (i.e. positive lifestyle changes, frequency of media use, average daily food consumption and frequency of substance use; all P < 0.001; Fig. 2(a–e) and Supplementary Table 2) were observed when analysing both samples together, but there were no significant time × cohort interactions. Similarly, there were significant main effects of time on emotional health, as assessed by the ‘emotions and worries’ and ‘worries about COVID’ sections of the survey (all P < 0.001; Fig. 3(a–e) and Supplementary Table 2) but no significant time × cohort interactions (all detailed in the Supplementary Material).
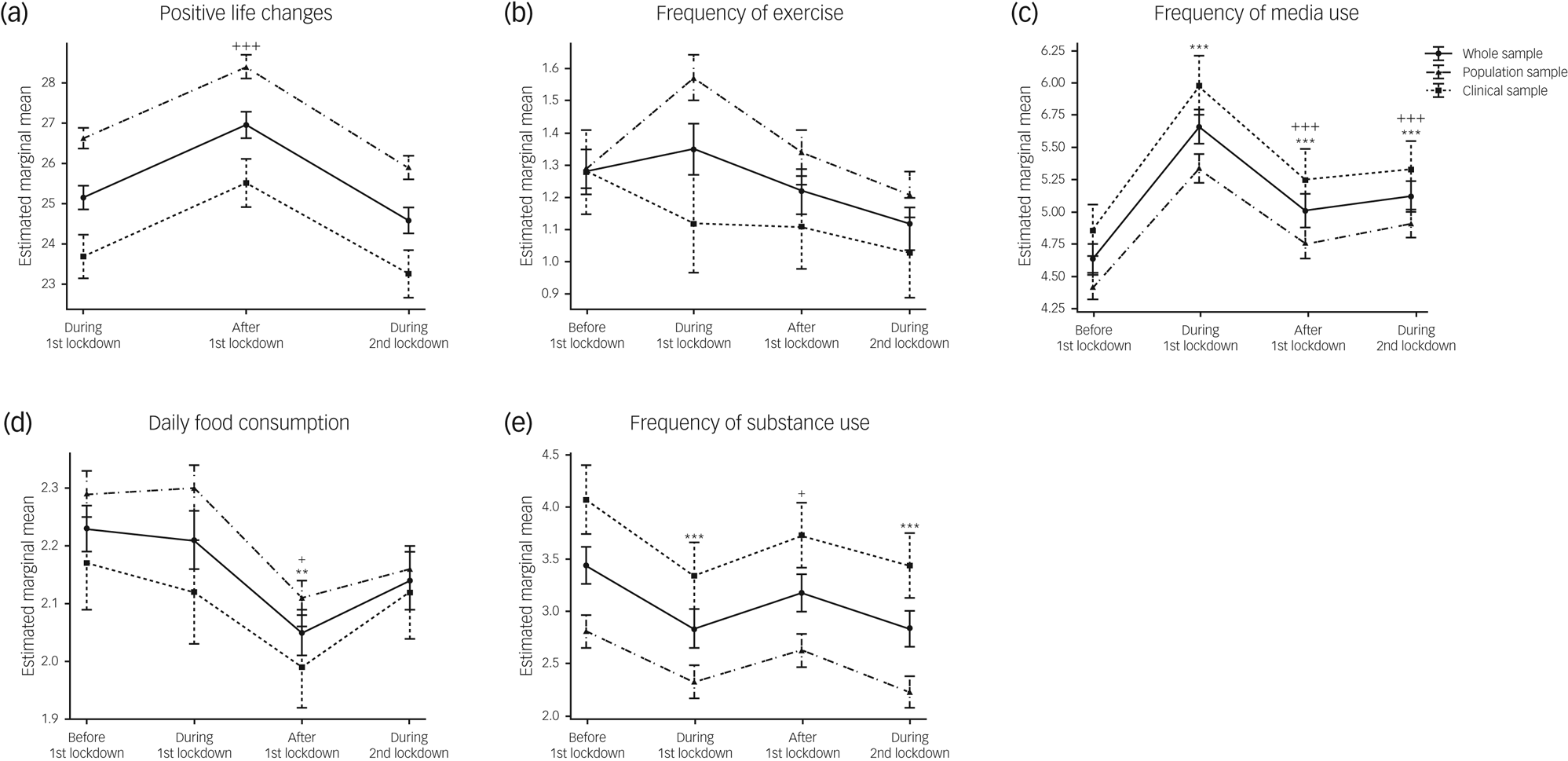
Fig. 2 Behavioural trajectories during the pandemic, including (a) positive life changes; (b) frequency of exercising; (c) frequency of media use; (d) daily food consumption; and (e) frequency of substance use, in the whole sample and stratified by cohort. Data are expressed as mean and standard error. Time effects from mixed-effects ANOVA in the whole sample were estimated by comparing data collected before the first lockdown with data collected at other time points (*P < 0.05, **P < 0.01, ***P < 0.001) and by comparing data collected during the first lockdown with data collected afterwards (+P < 0.05, ++P < 0.01, +++P < 0.001).

Fig. 3 Emotional trajectories during the pandemic, including (a) emotions and worries; (b) worries about oneself being infected; (c) worries about friends or family being infected; (d) worries about own physical health; and (e) worries about own mental health, in the whole sample and stratified by cohort. Data are expressed as mean and standard error. Time effects from mixed-effects ANOVA in the whole sample were estimated by comparing data collected before the first lockdown with data collected at other time points (*P < 0.05, **P < 0.01, ***P < 0.001) and by comparing data collected during the first lockdown with data collected afterwards (+P < 0.05, ++P < 0.01, +++P < 0.001).
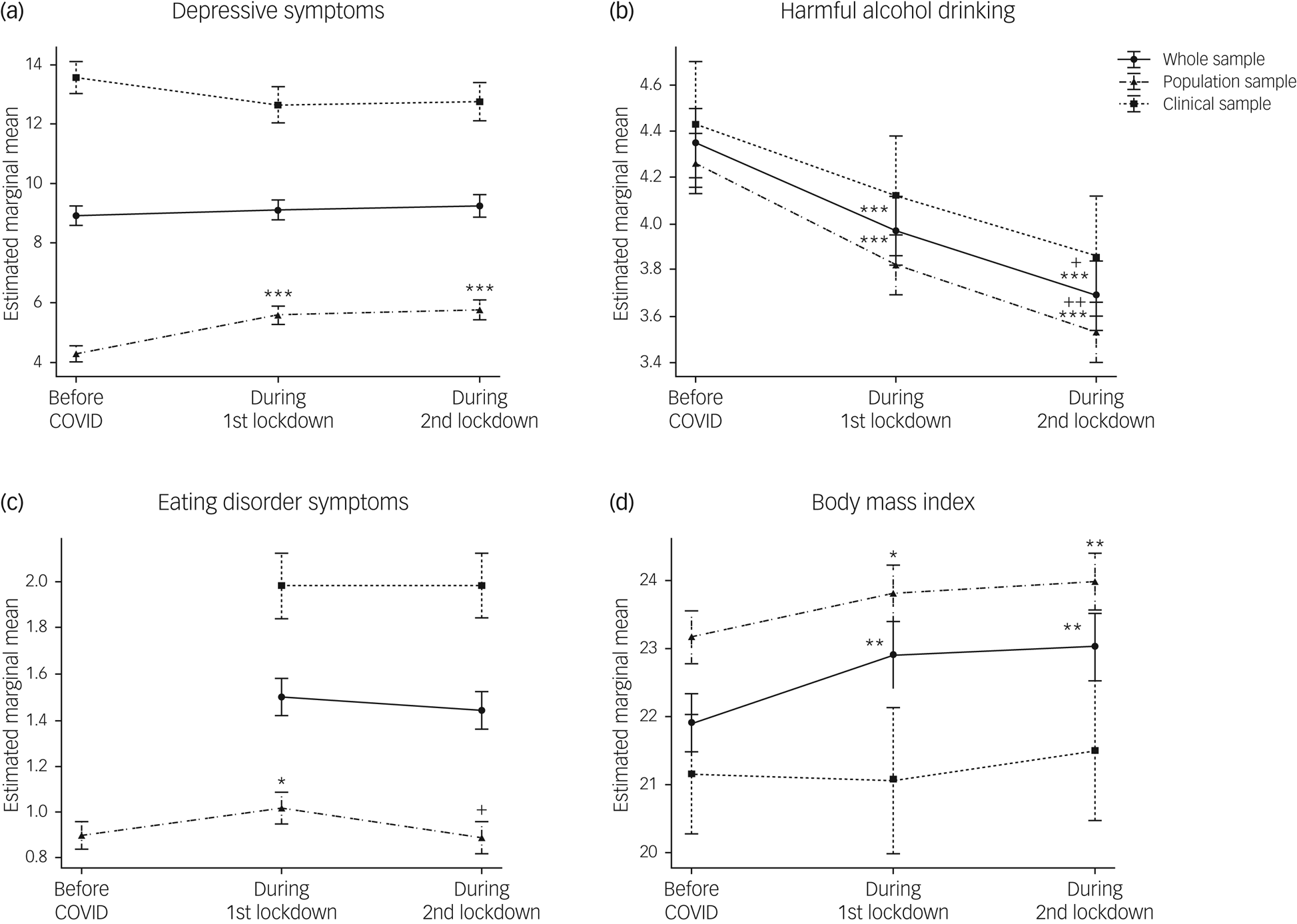
Comparisons of mental health symptoms (i.e. depression, alcohol drinking and eating disorders) and BMI just prior to the pandemic and during both lockdowns revealed the differential impact of the COVID-19 crisis on the cohorts (Fig. 4(a–d) and Supplementary Table 3).

Fig. 4 Mental health trajectories during the pandemic. Trajectories of (a) depressive symptoms; (b) harmful alcohol drinking; (c) eating disorder symptoms (d) and body mass index are indicated for the whole sample and for each cohort separately. Data are expressed as mean and standard error. Time effects from mixed-effects ANOVA in the whole sample were estimated by comparing data collected before the pandemic with data collected at other time points (*P < 0.05, **P < 0.01, ***P < 0.001), and by comparing data collected during the first lockdown with data collected afterwards (+P < 0.05, ++P < 0.01, +++P < 0.001).
There was no significant main effect of time on depressive symptoms (F(2,836) = 0.43, P = 0.65, ηp2 = 0.001) in the whole sample. As expected, there were sex (F(1,417) = 11.84, P < 0.001, ηp2 = 0.028) and large cohort effects (F(1, 1417) = 213.05, P < 0.001, ηp2 = 0.338), depressive symptoms being higher in females and in the clinical cohort. A significant time × cohort interaction was also found (F(2,836) = 7.41, P < 0.001, ηp2 = 0.017), indicating that the trajectories of depressive symptoms significantly differed between the population and the clinical samples (Fig. 4(a)). Analyses of these trajectories in the two cohorts separately revealed a significant main effect of time only in the population cohort (F(2,606) = 16.98, P < 0.001, ηp2 = 0.053). Depressive symptoms increased by 33.9% (95% CI, 31.78–36.57) during the lockdowns, with severity increasing to mild depression compared with minimal depression prior to the pandemic. In the clinical cohort, depressive symptoms remained high and constant across time (F(2,220) = 0.10, P = 0.91, ηp2 = 0.001).
A significant main effect of time in harmful alcohol drinking was found (F(2,838) = 14.06, P < 0.001, ηp2 = 0.032), with symptoms decreasing during the pandemic to reach their lowest levels (i.e. a 15.2% decrease) during the second lockdown (pre-PD > LD1 > LD2; P < 0.05). Males drank more than females (F(1,418) = 41.90, P < 0.001, ηp2 = 0.091). There were no significant cohort or time × cohort interactions (Fig. 4(b)). Nonetheless, a time × diagnosis interaction in the clinical cohort (F(6,220) = 4.25, P < 0.001, ηp2 = 0.104) revealed that the significant the decline in harmful drinking in the clinical cohort (i.e. a 23.04% decrease) was driven by participants with AUD.
For eating disorder symptoms, as the EDE-Q was only administered at two time points in the clinical cohort, we analysed the two cohorts separately. In the population cohort, there was a significant main effect of time on eating disorder behaviours and attitudes, as assessed by the EDE-Q global score (F(2,550) = 4.31, P = 0.01, ηp2 = 0.015). Eating disorder symptoms increased by 15.6% (95% CI, 15.39–15.68) during the first lockdown, returning to pre-pandemic levels during the second lockdown (Fig. 4(b)). As expected, eating disorder symptoms were significantly higher in females than males (F(1, 274) = 33.26, P = <0.001, ηp2 = 0.108), but there were no significant time × sex interactions (F(2, 550) = 1.14, P = 0.32, ηp2 = 0.004). In contrast to our findings in the population cohort, eating disorder symptoms did not significantly differ between the two lockdowns in the clinical cohort (F(1,110) = 0.09, P = 0.77, ηp2 = 0.001). Limiting analyses to the eating disorder subgroups also revealed no significant time effects (F(1,30) = 2.35, P = 0.14, ηp2 = 0.073 and F(1,18) = 0.03, P = 0.87, ηp2 = 0.002), for anorexia nervosa and bulimia nervosa, respectively.
Analyses of BMI trajectories revealed a significant main effect of time in the whole sample (F(2, 638) = 6.85, P < 0.001, ηp2 = 0.032), with higher BMIs during the pandemic (pre-PD < LD1 and LD2, P < 0.01) (Fig. 4(d)). A significant time × sex interaction (F(2,638) = 3.81, P < 0.05, ηp2 = 0.012) indicated that BMI significantly increased in females but not in males. No time × cohort interaction was found, but analyses of the two cohorts separately indicated that these findings were driven by the population cohort (F(2,428) = 9.61, P < 0.01, ηp2 = 0.043). Although no main effect of time was found in the clinical cohort, analyses within each diagnostic group revealed a time × sex interaction (F(2,50) = 5.48, P < 0.01, ηp2 = 0.180) in the AUD group, with significant BMI increases observed only in females (pre-PD < LD2, P < 0.05).
Re-running the analyses described above while controlling for other potential confounders generated largely similar results (Supplementary Table 3).
Effects of pre-pandemic symptom severity on mental health trajectories during the pandemic
The following analyses were performed to identify participants from the population cohort most vulnerable to COVID-induced mental illness. We categorised participants from this cohort based on their pre-pandemic symptom severity with respect to depression, alcohol misuse, eating disorders and BMI and re-ran analyses with these categories as predictors (Fig. 5(a–e) and Supplementary Table 4).

Fig. 5 Effects of pre-pandemic symptom severity on pandemic-induced mental health trajectories. (a) Effects of pre-pandemic severity for depression (minimal, mild and moderate to severe) on trajectories of depressive symptoms; (b) effects of pre-pandemic risk for alcohol misuse (low or high risk) on trajectories of harmful alcohol drinking; (c) effects of pre-pandemic risk for eating disorders (low risk or probable eating disorder) on trajectories of eating disorder symptoms; (d) effects of pre-pandemic risk for eating disorders on body mass index (BMI) trajectories, and (e) effects of pre-pandemic BMI (low or normal and overweight or obese) on BMI trajectories. Data are expressed as mean and standard error. Mixed-effects ANOVA revealed significant time × group (i.e. pre-pandemic risk levels) interactions in all comparisons. Time effects in each group were estimated by comparing data collected before the pandemic with data collected at other time points (*P < 0.05, **P < 0.01, ***P < 0.001) and by comparing data collected during the first lockdown with data collected afterwards (+P < 0.05, ++P < 0.01, +++P < 0.001).
Effects of pre-pandemic depression symptom severity on depressive symptom trajectories
There were significant interactions between time and pre-pandemic symptom severity for pandemic-related depressive symptoms (F(4,602) = 21.35, P < 0.001, ηp2 = 0.124). Post hoc analyses revealed notable group differences in trajectories (Fig. 5(a) and Supplementary Table 4). Participants with minimal pre-pandemic depression symptoms reported significant changes over time (F(2,300) = 41.73, P = <0.001, ηp2 = 0.218), with symptoms increasing during the first lockdown and remaining higher afterwards (pre-PD < LD1 or LD2, P < 0.001). By contrast, participants with moderate to severe depression reported the opposite trend (F(2,300) = 17.54, P = <0.001, ηp2 = 0.105), with symptoms being lower during the first and second lockdowns (pre-PD > LD1 or LD2, P < 0.001). Participants with mild depression did not report significant symptom changes with time (F(2,300) = 1.59, P = 0.21, ηp2 = 0.010).
Effects of pre-pandemic risk for alcohol misuse on trajectories of alcohol misuse
There were significant group differences in trajectories of harmful drinking during the pandemic (F(2,606) = 16.26; P = <0.001, ηp2 = 0.051; Fig. 5(b) and Supplementary Table 4). Participants initially more at risk of harmful drinking (i.e. prior to the pandemic) reported a significant decrease in alcohol misuse at all time points during the pandemic (pre-PD > LD1 > LD2, all, P < 0.001). A decrease was also observed for participants at low risk, and this became significant during the second lockdown (pre-PD > LD2, P < 0.001; LD1 > LD2, P < 0.05).
Effects of pre-pandemic risk for eating disorder on eating disorder symptoms and BMI trajectories
Similarly, significant group differences in trajectories of eating disorder symptoms during the pandemic were observed (F(2, 548) = 18.07; P = <0.001, ηp2 = 0.062; Fig. 5(c) and Supplementary Table 4). Participants initially at low risk for eating disorders reported a increase in eating disorder symptoms specifically during the first lockdown, with symptoms decreasing during the second lockdown (pre-PD < LD1, P = <0.001; LD1 > LD2, P < 0.01). Conversely, for participants initially scoring higher for eating disorder symptoms (i.e. those with probable eating disorder), symptoms significantly decreased during the first lockdown (P < 0.001), remaining lower during the second lockdown. Unsurprisingly, there were significant group differences in BMI (F(1,195) = 16.03; P < 0.001, ηp2 = 0.076), with participants with probable eating disorder having BMIs in the overweight range and those at low risk having BMIs in the normal range (Fig. 5(d)). No significant group differences in BMI trajectories during the pandemic were observed (F(2,392) = 0.43; P = 0.65, ηp2 = 0.002); a nominally significant increase in BMI was observed in the participants at low risk for eating disorder (pre-PD < LD2, P < 0.05) but not in those with higher eating disorder risk (Fig. 5(d)).
Effects of pre-pandemic BMI on BMI and eating disorder symptoms trajectories
Although no significant group differences on BMI trajectories were observed when comparing participants who were initially underweight/normal weight (BMI < 25) and overweight/obese (BMI > 25) (F(2,426) = 2.09; P = 0.13, ηp2 = 0.010), significant increases in BMI were observed in the underweight/normal weight group (pre-PD < LD1, P = 0.005; pre-PD < LD2, P < 0.001) but not in the overweight/obese group, for which BMI remained constant during the pandemic (Fig. 5(e) and Supplementary Table 4). Consistent with the analyses above, the increase in BMI in the underweight/normal weight group was paralleled by a significant increase in eating disorder symptoms, specifically during the first lockdown (BMI < 25; F(2,270) = 5.74, P = 0.004, ηp2 = 0.041; pre-PD < LD1, P = 0.003; LD1 > LD2, P = 0.045).
When re-running analyses controlling for other potential confounders, minor differences emerged in post hoc tests, probably owing to increased degrees of freedom and reduced sample size after adding numerous covariates. However, the overall pattern remained – participants with high levels of pre-pandemic symptoms showed improvement during lockdowns, whereas those with minimal pre-pandemic depression symptoms reported significant increases over time.
Discussion
This comparative study following population and clinical cohorts during the pandemic revealed the differing impact of the pandemic in youth with and without pre-existing mental illness. Whereas symptoms of depression and eating disorders increased during the pandemic in young people from the population, these symptoms remained high and stable in the clinical cohort. Pre-pandemic symptom severity predicted mental health trajectories in the population cohort. Participants initially at higher risk for depression, alcohol misuse or eating disorders reported a lasting decrease in their symptoms over the course of the pandemic. By contrast, being relatively healthy (i.e. having the lowest scores for depression or eating disorder) was a significant risk for deterioration in mental health during the pandemic; this was associated with relative increases in depressive symptoms throughout the pandemic and in eating disorder symptoms during the first lockdown. Being non-overweight or non-obese predicted the observed rise in eating disorder symptoms and was associated with weight gain (i.e. BMI increase).
Our findings corroborate previous research showing an increase in depression symptoms in all age groupsReference Fancourt, Steptoe and Bu5,Reference Amerio, Lugo, Stival, Fanucchi, Gorini and Pacifici15,Reference Racine, McArthur, Cooke, Eirich, Zhu and Madigan16,Reference Devoe, Han, Anderson, Katzman, Patten and Soumbasis33 from the population during the pandemic, but particularly in young and more physically active individuals.Reference Amerio, Lugo, Stival, Fanucchi, Gorini and Pacifici15 This observation may reflect greater changes in lifestyle habits in this group or a reduced tolerance of uncertainty. Our findings also highlight the contrasting effects of the pandemic on other mental health outcomes in young people: a long-term negative impact on depressive symptoms lasting until the second lockdown, in contrast to the transient increase in eating disorder symptoms and continuous decrease in alcohol misuse.
Our findings also shed light on the contradictory debate concerning pre-existing mental illnesses.Reference Lewis, Lewis, Roberts, Richards, Evison and Pearce7–Reference Sadeghi, Fors, Eisner, Taigman, Qi and Gorham12,Reference Ambrosetti, Macheret, Folliet, Wullschleger, Amerio and Aguglia34 Contrary to previous reports of worsening symptoms during the pandemic in patients with a history of mental illnessReference Lewis, Lewis, Roberts, Richards, Evison and Pearce7 or pre-existing disordered eating,Reference Warne, Heron, Mars, Kwong, Solmi and Pearson8 our findings indicated that although symptoms remained higher in the clinical sample, they did not worsen because of the pandemic. These discrepancies may be due to a lack of diagnostic measurement of mental illness and lack of repeated assessments to measure symptom changes during the pandemic in the relevant studies. Our observations of differences in mental health trajectories between young people from the general population and those with a clinical diagnosis suggest that pre-pandemic symptoms may have been a protective factor, and that the general population was more likely to be affected by the lockdowns than patients, which our analyses confirmed. The clinical cohort seemed to be resilient in the face of the pandemic, confirming previous reports for depression from the early stages of the pandemicReference Pinkham, Ackerman, Depp, Harvey and Moore10,Reference Hamm, Brown, Karp, Lenard, Cameron and Dawdani11,Reference Steff, Godinot, Gourlan, Robinson, Vidya and Winterer35 and further indicating that this effect persisted as the pandemic progressed. By contrast, and in agreement with previous assessments of depression in adultsReference Pan, Kok, Eikelenboom, Horsfall, Jorg and Luteijn9 and adolescents,Reference Sadeghi, Fors, Eisner, Taigman, Qi and Gorham12 young people without depressive or eating disorder symptoms showed an increase in these symptoms during the pandemic, whereas those with the highest pre-pandemic risk experienced a decrease. However, it should be noted that symptoms in the higher-risk groups remained much higher than those of individuals without prior symptoms, and that patients are more vulnerable to some stressful situations due to the pandemic.Reference Ambrosetti, Macheret, Folliet, Wullschleger, Amerio and Aguglia34
In contrast to our findings for depression and eating disorders, we observed a decline in alcohol and substance misuse during the pandemic, consistent with previous evidence.Reference Deeken, Reichert, Zech, Wenzel, Wedemeyer and Aguilera22,Reference Layman, Thorisdottir, Halldorsdottir, Sigfusdottir, Allegrante and Kristjansson36 This decline during lockdown periods was similar in participants with and without mental health diagnoses. Among the general population, this decline could be attributed to both those at high risk and those at low risk for alcohol misuse and may reflect closures of shops, bars and pubs during lockdown.
Participants from the general population and patients differed in the intensity of their behavioural or emotional responses to the pandemic but not in their trajectories. That young people are not equally at risk from the psychosocial stress brought about by COVID-19 was to be expected; however, counterintuitively, our findings indicate that healthier individuals tended to be the most vulnerable to the negative effects of the pandemic on mental health, not those with a higher burden. Possible explanations for this are that heightened fears and worries during periods of confinement, as highlighted in this study, and increased social isolation may have contributed to deterioration of mental health in healthier individuals. By contrast, those with depression and eating disorders might have felt relief owing to reduced exposure to psychosocial stressors (e.g. social interactions). They may also have felt less isolated given the global increase in fears and worries. As for alcohol and substance use, as noted above, the general reduction may reflect restriction policies such as closures of shops, bars and pubs, which would have limited access to those substances, as evidenced by a return to pre-pandemic levels after confinement measures were lifted. In addition, the more time young people spent at home with their families, the less likely they were to gain access to these substances.
Strengths and limitations
Strengths of our study include the use of longitudinal data collected over a period of up to 3 years prior to the pandemic and further assessments covering the two lockdowns, which allowed for a more comprehensive understanding of the impact of the pandemic. A clear strength is also the combination of data from the youth population as well as from patients with pre-existing mental illness, with both groups assessed under the same study protocol. This enabled the investigation of vulnerability and resilience and improved our understanding of how distinct groups of people may respond to challenging circumstances. However, some limitations should be acknowledged. First, our study had a relatively low response rate and high attrition during the data collection phase. It did not include underrepresented groups, such as participants from ethnic minorities that may have been disproportionately affected by the pandemic. Moreover, our clinical sample was relatively small, with the majority of participants being females. All of this may limit the generalisability of our findings. In addition, although our study used validated instruments (PHQ-9, AUDIT and EDE-Q) to measure psychiatric symptoms, these are not diagnostic tools but only measure a greater risk of the presence of clinical illness. Finally, psychiatric assessments were only conducted during periods of confinement, which precluded investigation of mental health changes once restrictions were lifted.
Clinical implications
In summary, our study revealed opposite effects of the pandemic on mental health in youth with and without mental illness. Improvements in depression, alcohol misuse or eating disorder symptoms were observed over the course of the pandemic for participants with a higher pre-pandemic risk for these disorders, suggesting that the pandemic and lockdown measures decreased the mental health burden specifically in this population group. By contrast, the increases in depressive and eating disorder symptoms in those with low prior risk suggest the detrimental effects of such measures on healthier youth. If confirmed by future studies in a more representative sample, our findings could support personalised mental health interventions to help young people to cope better with the challenges of psychosocial stress and reduce the associated healthcare burden.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2023.601.
Data availability
The data that support the findings of this study are available from the corresponding author (S.D.) on reasonable request.
Author contributions
S.D. conceived and designed the study. L.R., M.B., C.G., J.W., R.A., K.A., Y.Z., S.K., E.A., T.B., A.L.W.B., M.J.B., R.B., H.F., J.H.F., H.G., A.G., A.H., S.H., M.-L.P.M., S.M., F.N., B.M.N., L.P., J.S., M.N.S., R.W., A.S., H.W., J.-L.M., G.S., U.S. and S.D. collected the data. D.P.O. managed the data. L.Q. analysed the data and drafted the initial output. L.Q., Z.Z. and S.D. contributed to the interpretation of findings. S.D. will serve as a guarantor for the contents of the paper. All authors have read and approved the final version of the manuscript.
Funding
This work received support from the following sources: the Medical Research Council and Medical Research Foundation (‘ESTRA’ – Neurobiological underpinning of eating disorders: integrative biopsychosocial longitudinal analyses in adolescents: grant MR/R00465X/; ‘ESTRA’ – Establishing causal relationships between biopsychosocial predictors and correlates of eating disorders and their mediation by neural pathways: grants MR/S020306/1), the European Union-funded FP6 Integrated Project IMAGEN (LSHM-CT-2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), the Medical Research Council (grant MR/W002418/1: ‘Eating Disorders: Delineating illness and recovery trajectories to inform personalized prevention and early intervention in young people (EDIFY)’ and the National Institute for Health and Care Research Maudsley Biomedical Research Centre. This work was co-funded by UK Research and Innovation under the UK Government's Horizon Europe funding guarantee (10041392 and 10038599) as part of Horizon Europe HORIZON-HLTH-2021-STAYHLTH-01 under European Union grant agreement number 101057429 (environMENTAL). Z.Z. is supported by a fellowship from the Medical Research Foundation (MRF-058-0014-F-ZHAN-C0866). Further support was provided by grants from: the National Institutes of Health (NIH) (consortium grant 5U54EB020403-05-‘ENIGMA’) and National Institute on Aging 1R56AG058854-02-‘ENIGMA World Aging Center’), the Human Brain Project (HBP SGA 2, 785907, and HBP SGA 3, 945539), the Medical Research Council grant ‘c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the NIH (R01DA049238, A decentralized macro and micro gene-by-environment interaction analysis of substance use behavior and its brain biomarkers), the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B; Forschungsnetz IMAC-Mind 01GL1745B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940, TRR 265, NE 1383/14-1), NSFC grant 82150710554, the ANR (ANR-12-SAMA-0004, AAPG2019 – GeBra), the Eranet Neuron (AF12-NEUR0008-01 – WM2NA; and ANR-18-NEUR00002-01 – ADORe), the Fondation de France (00081242), the Fondation pour la Recherche Médicale (DPA20140629802), the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, the Fondation de l'Avenir (grant AP-RM-17-013), the Fédération pour la Recherche sur le Cerveau; the NIH, Science Foundation Ireland (16/ERCD/3797) and USA (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1).
Declaration of interest
T.B. has served in an advisory or consultancy role for Eye Level, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche and Takeda. He has received conference support or speakers’ fees from Janssen, Medice and Takeda, and royalties from Hogrefe, Kohlhammer, CIP Medien and Oxford University Press; the present work is unrelated to these relationships. L.P. has served in advisory or consultancy roles for Roche and Viforpharm and has received speakers’ fees from Shire. She has received royalties from Hogrefe, Kohlhammer and Schattauer. The present work is unrelated to the above grants and relationships.








eLetters
No eLetters have been published for this article.