High blood uric acid (hyperuricaemia) is the strongest determinant risk factor for gout, an inflammatory arthritis caused by uric acid crystals, and its prevalence is higher in males compared with females( Reference Richette and Bardin 1 ). Hyperuricaemia is also common in patients who develop diabetes( Reference Chien, Chen and Hsu 2 ), obesity( Reference Remedios, Shah and Bhasker 3 ), hyperglycaemia( Reference Nakanishi, Okamoto and Yoshida 4 , Reference Lv, Meng and He 5 ), hypertension( Reference Grayson, Kim and LaValley 6 ) and stroke( Reference Li, Hou and Zhang 7 ), although it is often unattended until their first, if any, gout attack. Gout prevalence increased approximately from 0·5 to 3 % between 1960 and 2010 in the USA( Reference Roddy and Choi 8 ) and other areas( Reference Mikuls, Farrar and Bilker 9 ) accompanied by a parallel increase in the number of individuals with hyperuricaemia( Reference Luk and Simkin 10 , Reference Grassi, Ferri and Desideri 11 ). The fact that 25–34 years is the age group with the highest blood uric acid level( Reference Qiu, Cheng and Wu 12 ) may suggest that hyperuricaemia precedes the development of metabolic syndromes( Reference Krishnan, Kwoh and Schumacher 13 ). Interestingly, allopurinol, a uric acid-lowering agent used in gout therapy, has a protective effect on hypertension, which suggests that excess uric acid synthesis is a causal factor for developing hypertension( Reference Feig, Soletsky and Johnson 14 ).
Some dietary factors including purines, alcohol and fructose( Reference Dalbeth, House and Gamble 15 – Reference Rho, Zhu and Choi 18 ) also elevate blood uric acid levels – for example, chronic exposure to fructose can lead to the development of hyperuricaemia( Reference Lim, Mietus-Snyder and Valente 19 ). Fructose phosphorylation by fructokinase causes intracellular phosphate depletion, leading to the activation of deaminase, which converts adenosine monophosphate to inosine monophosphate. The consumption of ATP activates transformation of inosine monophosphate to inosine, the precursor of uric acid metabolism. Chronic hyperuricaemia may also up-regulate fructokinase expression, leading to the amplification of the lipogenic effects of fructose in human hepatocytes( Reference Lanaspa, Sanchez-Lozada and Cicerchi 20 ). Xanthine oxidoreductase (also called xanthine oxidase or xanthine dehydrogenase depending on proteolytic processing) catalyses the final step in uric acid production. Inhibition of this enzyme has been the target of uric acid-lowering drugs such as allopurinol( Reference Becker, Schumacher and Wortmann 21 ). Studies in both healthy humans( Reference Facchini, Chen and Hollenbeck 22 , Reference Vuorinen-Markkola and Yki-Jarvinen 23 ) and animal models( Reference Zhu, Hu and Huang 24 ) substantiate the importance of increased insulin resistance to hyperuricaemia, and vice versa, providing a link to excess fructose intake.
Quercetin is a dietary flavonoid, which is particularly abundant in black tea and apples, and occurs predominantly as quercetin-4′-O-glucoside or quercetin−3,4′-O-diglucoside in onions and as quercetin 3-O-rutinoside in tea( Reference Neveu, Perez-Jimenez and Vos 25 ). The bioavailability of quercetin in humans has been extensively studied, and in plasma multiple conjugates of quercetin appear post-prandially. In healthy subjects, using urine as a biomarker, we have previously demonstrated that 500 mg quercetin aglycone, as provided in supplements used here, is comparable with the quercetin present in approximately 100 g of fresh red onion( Reference Shi and Williamson 26 ). Quercetin and its metabolites inhibit xanthine oxidoreductase in vitro ( Reference Day, Bao and Morgan 27 ) and regulate blood uric acid levels in vivo in animal studies( Reference Haidari, Rashidi and Eshraghian 28 – Reference Auclair, Silberberg and Gueux 30 ), yet whether uric acid metabolism could be similarly affected in humans is still highly debatable( Reference Egert, Wolffram and Bosy-Westphal 31 – Reference Kimira, Arai and Shimoi 36 ).
Therefore, we performed this randomised, double-blinded, placebo-controlled, cross-over trial to test the hypothesis that 4 weeks of quercetin supplementation might result in a reduction in plasma uric acid levels in male subjects with non-optimal blood uric acid levels.
Methods
Subjects
A total of twenty-two healthy males were eligibly assigned and were compliant to successfully complete the study. Selection criteria included the following: being apparently healthy, aged between 19 and 65 years, having a BMI between 18·5 and 29·9 kg/m2 and a non-smoker and not a heavy drinker (<3 units of alcohol regularly/d). Volunteers with diagnosed gout and/or kidney stone, who were experiencing intestinal disorders, or whose plasma uric acid concentration was lower than 300 µmol/l, were excluded. All the data were collected from February 2013 to April 2014 and analysed in the School of Food Science and Nutrition at the University of Leeds, UK. The study was conducted according to the guidelines laid down in the Declaration of Helsinki of 1975, as revised in 1983, and all the procedures involving human subjects were approved by the University of Leeds, MaPS and Engineering joint Faculty Research Ethics Committee (MEEC12-019), UK. Written informed consent was obtained from each of the subjects before commencement of the study (Clinicaltrials.gov identifier: NCT01881919).
Study design
The main goal and primary objective of the present study was to examine the chronic effect of quercetin on plasma uric acid concentration. For this purpose, the study was a randomised, double-blinded, placebo-controlled, cross-over, 4-week intervention trial with two treatment groups, with daily consumption of either quercetin dihydrate in tablet form (500 mg stated on the label, actual measured 544 (sd 45) mg quercetin dihydrate aglycone, purchased from Nature’s Best, and containing small amounts of calcium carbonate, cellulose, methylcellulose, glycerine, stearic acid, silicon dioxide, cross-linked cellulose gum and magnesium stearate)( Reference Shi and Williamson 26 ) or placebo (the placebo formulation was a white oval tablet and contained lactose monohydrate, magnesium stearate and cellulose, purchased from Fagron). There was a 4-week washout period between each treatment. Blood and urine samples were collected before, during and at the end of each study phase. Each participant was independently and randomly assigned to one of the two groups, receiving both treatments in one order or another.
During the protocol, volunteers made six visits to the research unit at day 0, 14 and 28 of each experimental period for measurement and sample collection. In practice, with 24-h urine samples collected at home during the day and night before the visit, overnight-fasted subjects arrived at the research unit between 07.00 and 10.00 hours. A fasting blood sample was collected, followed by administration of questionnaires and measurements of weight, height and blood pressure. Subjects received a light meal and the study tablets before leaving the research unit. Subjects were asked to maintain their lifestyle and normal dietary habits from 4 weeks before the first visit until the end of the entire study. Compliance was assessed at the end of each 4-week period by call-back questionnaires, recording date of missing dose (if any), changes in physical activity and intensity, use of exotic diet or non-routine medications and the occurrence of any side-effects. Subjects were also asked to return the unconsumed tablets at each follow-up visit.
Intervention was randomised independently by a coin toss for each volunteer who received a random three-digit code. A decode list (participant identification and subject code) was maintained by a third person in order to blind the researcher assessing outcomes. The size and shape of the study tablets were the same, but of different colour, and the participants were not aware of the identification of the two types of study tablets. The quercetin-containing tablet was light green in colour and the placebo tablet was off-white. As quercetin is light yellow, it is not immediately obvious as to which tablet is the active one, and subjects were not informed whether the tablets were placebo or active. Analysis of the blood and urine samples was also blinded to the researcher using codes held by a third party.
Sample collection and assay
Blood pressure was measured on the upper left arm in a quiet room at normal room temperature, using a cuff-less upper-arm blood pressure monitor (Panasonic Co.). Before blood pressure recordings were made, participants rested for 15 min in a seated position. At each assessment, three consecutive blood pressure readings were recorded at 5-min intervals. The average of these measurements was used for the analysis.
Venous blood samples were collected following a standard venepuncture protocol into sodium fluoride/potassium oxalate-containing blood collection tubes (Greiner BioOne). Blood samples were immediately centrifuged at 3000 g at 4°C for 10 min, and aliquots were stored at −80°C until analysis; 24-h urine samples were collected by volunteers in 3-litre sterile urine containers (Simport), which contained 3 g of l-ascorbic acid (MP Biomedicals). The urine samples were weighed before centrifugation at 2000 g at 4°C for 10 min before storage at −20°C. Urine samples for uric acid assay were diluted 10-fold before storage at −80°C.
Analytical methods
Assessment of uric acid in plasma and urine samples was by a specific coupled enzyme reaction, followed by colourimetric determination at 520 nm( Reference Fossati, Prencipe and Berti 37 ). The protocol was modified for use in a 96-well plate reader (BMG Labtech) for high-throughput and improved accuracy. Within-run variation was 1·99 (sd 1·20) %, and the between-run variation was 2·17 (sd 0·52) %. Recovery was 92·8 (sd 1·6) % for plasma and 80·4 (sd 3·8) % for 10-fold diluted urine. Calibration curves were prepared every time for each plate, with a slope of 0·550 (sd 0·003)/mmol per litre of uric acid, with R 2≥0·999 up to a maximum concentration of 1·0 mmol/l.
Plasma glucose level was measured using a commercial hexokinase-based assay kit for d-glucose (Sigma-Aldrich). The protocol was modified for use in a 96-well plate reader. Within-run variation was 4·29 (sd 2·21) %, and the between-run variation was 3·33 (sd 2·51) %. Recovery was 104 (sd 8) %. Calibration curves were prepared every time for each plate, with a slope of 0·923 (sd 0·006)/g per litre d-glucose, with R 2≥0·999 up to a maximum concentration of 1·50 g/l.
Urinary quercetin was quantified by HPLC-ESI/MS as previously described( Reference Shi and Williamson 26 ).
Sample size
A minimum sample size of seventeen was estimated to be required to detect a 10 % difference for the primary efficacy variable, plasma concentration of uric acid, and to achieve 80 % power to meet the two-tailed equality criteria between quercetin and placebo. A significance level of 0·05 from paired two-sample t test was set for this two-sequence, two period cross-over design( Reference Machin, Campbell and Fayers 38 ). The CV of the blood uric acid level among the population was approximately 20 % according to previous cohort reports( Reference Egert, Bosy-Westphal and Seiberl 39 – Reference Forman, Choi and Curhan 41 ), and 10 % of CV among study population was estimated as we pre-screened and selected the upper 50 % of the volunteers for plasma uric acid analysis.
Statistics
Normality of data distribution was tested by Shapiro–Wilk tests. The paired two-sample t test was used for comparison of normally distributed data. Data that were not normally distributed were compared using the Wilcoxon signed-rank test. Relationships between variables were evaluated using Pearson’s correlation coefficient. In all cases, a value for P<0·05 (two-tailed) was considered to indicate a significant effect. Unless otherwise indicated, results are expressed as mean values and standard deviations. All the statistical analyses were performed using SPSS statistics software (version 21; International Business Machines Corp.).
Results
A total of fifty-four male volunteers made contact through advertisements (Fig. 1); fifty-two of them provided blood samples at the screening stage, with a mean plasma uric acid concentration of 316 (sd 56) µmol/l (range 194–472 µmol/l, n 52). Among them, twenty-three subjects were selected, and twenty-two of them completed the study with the following characteristics at baseline: healthy adult males, 29·9 (sd 12·9) years, mean BMI of 24·8 (sd 3·0) kg/m2, blood pressure of normal to (pre-) hypertensive range (systolic 122·9 (sd 8·1) mmHg and diastolic 74·3 (sd 9·0) mmHg), fasting blood glucose level of normal to impaired fasting glycaemia range with a mean of 5·04 (sd 0·56) mmol/l and plasma uric acid level of 339 (sd 51) µmol/l. No significant change of lifestyle or medication occurred during the study based on the lifestyle maintenance questionnaire, and no adverse events after receiving quercetin or placebo were reported; 24-h urinary excretion of quercetin was 0·810 (sd 0·704) µmol during quercetin treatment and 0·200 (sd 0·366) µmol during placebo treatment. According to the returned unconsumed tablets, participant self-reports and urinary quercetin, none of the participants was classified as non-compliant.
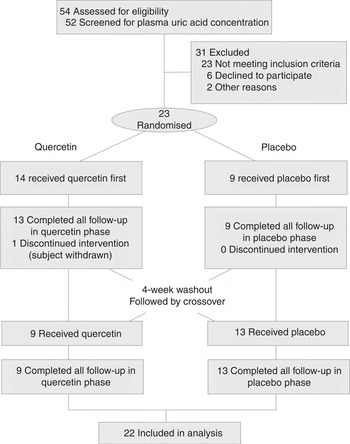
Fig. 1 Participant flow diagram of the progress through this double-blinded, placebo-controlled, randomised, cross-over trial.
Plasma uric acid levels progressively lowered over time among participants during quercetin supplementation. From baseline to 2 weeks, the mean plasma uric acid level showed a downward trend (−15·9 µmol/l; 95 % CI 0·9, −32·8; P=0·06). From baseline to 4 weeks, the mean plasma uric acid level decreased significantly by −26·5 µmol/l (95 % CI −7·6, −45·5; P=0·008). Plasma uric acid levels remained unchanged throughout the placebo period: 95 % CI −8·9, 30·0; P=0·27 at the 2-week interval and 95 % CI −15·1, 25·5; P=0·60 after 4 weeks. No difference was observed between the baselines of each arm (P=0·21) (Table 1, Fig. 2).
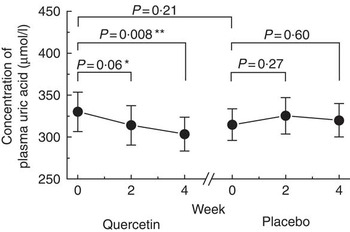
Fig. 2 Effect of consumption of quercetin on plasma uric acid. Comparison of plasma uric acid at baseline, 2 and 4 weeks after consuming quercetin (containing 500 mg of quercetin) or a placebo daily in twenty-two healthy subjects. Error bars indicate 95 % CI. Trend (* P<0·1) and significant difference (** P<0·05) when compared with baseline by paired t test.
Table 1 Effect of quercetin and placebo treatments on plasma biomarkers and blood pressureFootnote † (Mean values and standard deviations; mean difference from baseline and 95 % confidence intervals; n 22)

* P<0·1; ** P<0·05 when compared with baseline.
† Two-tailed paired t test were used if not stated otherwise.
‡ Wilcoxon’s signed-rank test was used as the data were not normally distributed.
There was a trend for mean diastolic blood pressure to decrease by −2·0 mmHg (95 % CI 0·1, −4·1; P=0·07) during the quercetin phase, whereas there was no change during the placebo phase. No change was observed in fasting glucose levels or in systolic blood pressure in either group by either treatment (Table 1). Renal excretion of uric acid was assessed by total 24-h urinary uric acid values and did not significantly vary between the two time points after either treatment: from 2·15 (sd 1·80) to 1·61 (sd 1·56) mmol after quercetin treatment (P=0·11, Wilcoxon’s signed-rank test) and from 1·42 (sd 1·33) to 1·64 (sd 1·42) mmol after placebo treatment (P=0·35, Wilcoxon’s signed-rank test).
Discussion
In this randomised-controlled trial, supplementation with quercetin at 500 mg/d for 4 weeks progressively reduced plasma concentrations of uric acid without inducing changes in BMI, in fasting blood glucose or showing any adverse effects. The reduction in plasma uric acid was equivalent to approximately 8 % with high significance (P value of 0·008 after 4 weeks). The dose of quercetin was carefully considered based on both realistic food composition and a bioavailability test that we have previously reported on healthy volunteers. In this comparison, we showed that quercetin (as glycoside conjugates) in 100 g fresh red onion provides a similar amount of bioavailable quercetin to the tablet used here (500 mg of pure quercetin aglycone), as assessed by urinary excretion( Reference Shi and Williamson 26 ). This dose was sufficient to produce the observed change after 4 weeks and provided a more reproducible, practical and acceptable form of consuming quercetin. Similar approaches have been reported recently( Reference Dower, Geleijnse and Gijsbers 42 , Reference Dower, Geleijnse and Gijsbers 43 ).
There are several possible mechanisms for the observed change in plasma uric acid. The most likely one is the direct inhibition of xanthine oxidoreductase activity, as, in vitro, bovine xanthine oxidoreductase is inhibited strongly by quercetin (K i =1·40 (sd 0·78) µmol/l)( Reference Lin, Chen and Chen 44 ). The drug, allopurinol, is comparable (K i =0·34 (sd 0·22) µmol/l)( Reference Lin, Chen and Chen 44 ), and furthermore some conjugates such as quercetin-4′-O-glucuronide also inhibited xanthine oxidoreductase (K i =0·25 (sd 0·03) µmol/l)( Reference Day, Bao and Morgan 27 ). Additional mechanisms are also possible, including promoted renal excretion of uric acid, which could be as a result of an increased glomerular filtration of uric acid. Some drugs such as losartan directly inhibit URAT1, involved in uric acid re-absorption, and thereby decrease plasma uric acid levels( Reference Hamada, Ichica and Hosoyamada 45 ), whereas some treatments down-regulate mURAT1 and mGLUT9 in mice( Reference Hu, Zhang and Wang 46 ). Up-regulation of transporters such as mOAT1( Reference Hu, Zhang and Wang 46 ), rOAT1( Reference Hu, Wang and Li 47 ) and hOAT1( Reference Hong, Seo and Lim 48 ), which increase kidney urate secretion in the proximal tubules of the renal cortex, is also possible. However, a change in urinary excretion is unlikely as 2 weeks of quercetin administration did not change renal excretion, as assessed using the 24 h urine method. This implies an overall effect of quercetin on uric acid production rather than an increase in excretion. Other additional mechanisms could involve an indirect antioxidant effect that reduces microvascular ischaemia in glomeruli and leads to increased local blood flow, dilation of afferent arterioles and competition for re-absorption with ions such as Na and K that exert osmotic effects( Reference Fabre, Bayach and Berka 49 ). A trend for reduction in diastolic blood pressure after quercetin supplementation lends partial support to this hypothesis. The −2·0 mmHg (95 % CI 0·1, −4·1; P=0·07) trend in reduction is potentially noteworthy, as a decrease of similar magnitude has been calculated to result in a substantial decrease in the prevalence of hypertension in population studies( Reference Cook, Cohen and Hebert 50 , Reference Whelton, He and Appel 51 ). We found no significant effect on systolic blood pressure in this study. Quercetin has been shown to reduce systolic and diastolic blood pressure in hypertensive subjects( Reference Edwards, Lyon and Litwin 52 ), but our subjects were chosen for their high blood uric acid levels and not specifically for exhibiting hypertension.
Quercetin has demonstrated some effects on various biomarkers in intervention studies, but the results are dependent on dose, nature of the cohort and the length of time of treatment( Reference Dower, Geleijnse and Gijsbers 42 , Reference Dower, Geleijnse and Gijsbers 43 , Reference Loke, Hodgson and Proudfoot 53 – Reference Conquer and Holub 55 ). Some effects of quercetin may only be seen for defined genotypes( Reference Pfeuffer, Auinger and Bley 56 ). A very limited number of studies have examined changes in plasma uric acid levels as a result of quercetin supplementation or high flavonol diets, but none as a primary outcome. For example, 150 mg/d for 6 weeks showed no change in plasma uric acid levels( Reference Egert, Bosy-Westphal and Seiberl 39 ), and a diet high in onions and tea for 2 weeks did not change plasma uric acid levels in patients with type 2 diabetes( Reference Lean, Noroozi and Kelly 33 ).
The present study was intentionally designed to be carried out on a homogeneous population with higher-than-average blood uric acid levels to minimise confounding influences of sex, medication, diet or other lifestyle factors. Therefore, our result may be valid only for male individuals who are mildly or pre-hyperuricaemic but otherwise healthy, and we cannot predict whether the findings will extend to populations that have lower plasma uric acid levels, females, hypertensive individuals and older or younger populations. The role of habitual diet should also be considered. The intervention in the present study was designed to provide proof of principle and only one dose was tested, but there were no adverse events. Quercetin is part of the normal diet and consumed in very different amounts by individuals according to their dietary patterns.
It is noteworthy in our study that the hypouricaemic effect of quercetin is more significant in subjects with higher uric acid levels (Fig. 3), which is in accordance with animal models( Reference Hu, Zhang and Wang 46 ). These findings have served implications. Dietary quercetin could help maintain a healthy blood uric acid level and help prevent the formation of uric acid crystals (gouty arthritis)( Reference Schlesinger 57 ). Although hyperuricaemia alone is not sufficient to cause gout, a dose–response relationship between serum uric acid and the risk of developing gout is well documented( Reference Campion, Glynn and DeLabry 58 ). These findings may also help recovering gout patients where the primary treatment is to achieve an end point of serum uric acid levels <360 µmol/l over a period of 3 months( Reference Schlesinger 57 ). This includes the use of the drug allopurinol to inhibit xanthine oxidoreductase and uric acid production, or the use of uricosuric drugs that increase renal excretion of uric acid. However, for patients also presenting kidney disease, liver disease, diabetes, congestive heart failure or hypertension, the dosage of allopurinol has to be adjusted in this stage( Reference Becker, Schumacher and Wortmann 21 ). Once restored, patients are often advised to make comprehensive dietary modifications for prevention against recurrent gout attacks. In the above situations, adoption of one quercetin tablet that has the efficacy to reduce blood uric acid levels in the habitual diet is easier to adhere to compared with making major dietary changes. Therefore, quercetin may be a promising approach to lower uric acid levels in individuals with above-optimal blood uric acid, for those at high risk and have not yet developed any disease or for patients recovering after therapy.

Fig. 3 Changes in plasma uric acid levels from observations in relation to baseline plasma uric acid levels. The magnitude of plasma uric acid reduction was higher in individuals with higher baseline plasma uric acid levels in both treatments. Plasma uric acid in the majority of subjects declined after 4 weeks of treatment with quercetin (17/22) but not placebo (10/22). The correlation coefficient r was calculated by the Pearson test. Quercetin: ![]() , 2 weeks;
, 2 weeks; ![]() , r −0·37;
, r −0·37; ![]() , 4 weeks;
, 4 weeks; ![]() , r −0·56; placebo:
, r −0·56; placebo: ![]() , 2 weeks;
, 2 weeks; ![]() , r −0·32;
, r −0·32; ![]() , 4 weeks;
, 4 weeks; ![]() , r −0·45.
, r −0·45.
Acknowledgements
The authors express their sincere gratitude to all the subjects who participated in this trial.
This work was supported by the China Scholarship Council and the University of Leeds as a PhD studentship to Y. S. The funders had no role in designing and conducting the study or in the collection, management, analysis and interpretation of the data or preparation, review or approval of the manuscript. This work did not receive funding from any commercial organisation, but G. W. has recently, or currently, received other research funding from Nestle and Florida Department of Citrus, and conducted consultancy for Nutrilite, USA.
Y. S.: study concept and design, data interpretation, volunteer recruitment, clinical study management, protocol implementation, sample acquisition, data collection and analysis, statistical analysis, writing and revision of the manuscript; G. W.: supervision of the study, study concept and design, writing and revision of the manuscript. Y. S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. G. W. had primary responsibility for the final content. Both the authors have read and approved the final version of the manuscript.







