Undernutrition leading to poor anthropometric status and Zn deficiency remain significant global risk factors for death and disease. The WHO(1) reports that globally, childhood underweight is the leading cause of disease burden, while Zn deficiency is the 17th leading cause of both death and disability. Children recovering from protein–energy malnutrition (PEM) do not regain muscle mass as efficiently as fat mass(Reference Graham, Cordano and Blizzard2, Reference MacLean and Graham3), and there may be histological differences in the muscle structure, including a significantly smaller muscle fibre size than age- and sex-matched controls(Reference Montgomery4, Reference Hansen-Smith, Picou and Golden5).
During nutritional rehabilitation, high rates of weight gain have been associated with suboptimal serum Zn concentrations and a high cost of growth, indicating adipose tissue synthesis(Reference Golden and Golden6). Addition of Zn to the refeeding regimen of children with PEM has decreased the cost of growth, indicating muscle tissue synthesis(Reference Golden and Golden7) and increased N retention(Reference Golden and Golden8). Similarly, it has been shown that adequate dietary Zn is required for improved weight gain and lean body mass during recovery from undernutrition in rodent models(Reference Morgan, Keen and Calvert9, Reference Glore, Orth and Knehans10). These findings have led to questions about the interaction of Zn with various anabolic hormones and peptides, and the quality of lean mass recovery.
Insulin-like growth factor-1 (IGF-1) is an anabolic peptide produced in many tissues including the liver and skeletal muscle. In skeletal muscle, IGF-1 has been associated with normal growth(Reference Jennische and Olivercrona11), hypertrophy(Reference Zanconato, Moromisato and Moromisato12) and repair(Reference Adams and Haddad13). Serum IGF-1 concentrations are influenced by nutritional state, particularly PEM and Zn status(Reference Cesur, Yordaman and Doğan14–Reference Doherty, Crofton and Sarkar16). Serum IGF-1 concentrations are reduced in models of severe energy restriction (50 % of ad libitum intake) or protein restriction (5 v. 15 % lactalbumin) in young growing rats(Reference Prewitt, D'Ercole and Switzer17). Supplemental Zn improves serum IGF-1 levels in children with clinical Zn deficiency(Reference Cesur, Yordaman and Doğan14, Reference Doherty, Crofton and Sarkar16); however, IGF-1 infusion is not effective for restoring carcass growth in protein-restricted young rats(Reference Thissen, Underwood and Maiter18), indicating that it is the combination of nutrient substrate availability and IGF-1 that is important. Serum IGF-1 concentrations are reduced in weanling rats fed a Zn-deficient diet ( < 1 mg Zn/kg diet), compared with pair-fed control rats(Reference Cossack19, Reference Dorup, Flyvbjerg and Everts20), and in force-fed Z rats that receive the same amount of energy and protein as the control group(Reference Roth and Kirchgessner21). Furthermore, serum IGF-1 has been reported to respond to increasing Zn intake, but not protein intake, when rats have previously been fed a combined Z and low-protein diet(Reference Cossack19). Although Zn status and serum IGF-1 have been studied in relation to growth, liver and bone metabolism, less is known about the interaction of Zn status and serum IGF-1 with skeletal muscle.
The present study was designed to address the effects of different types of malnutrition in combination with Zn deficiency on growth and skeletal muscle development during a deficiency phase followed by nutritional rehabilitation. An important goal was to determine whether recovery from malnutrition occurs equally among the different levels of cellular organisation, i.e. from whole body to muscle histology. Since IGF-1 is an important factor in skeletal muscle physiology(Reference Ketelslegers, Maiter and Maes22) and is sensitive to Zn status(Reference Prewitt, D'Ercole and Switzer17, Reference Cossack19, Reference Dorup, Flyvbjerg and Everts20), it has been hypothesised that previous Zn deficiency may be more detrimental to the recovery of muscle mass and muscle fibre diameter (MFD) than previous macronutrient deficiency of protein or energy. The objectives of the study were to (1) investigate the effects of Zn, protein and energy deficiencies on growth, body composition, gastrocnemius muscle weight and fibre diameter in relation to serum IGF-1 and Zn concentrations (deficiency phase) and (2) examine these parameters after a standard (30 mg Zn/kg diet) recovery diet (repletion phase). Weanling rats were chosen for the present study as they are poised for rapid growth and development, analogous to children. Zn deficiency was studied alone and in combination with protein deficiency, as inadequate protein intake is a major contributor to inadequate Zn intake in the human population(Reference Mares-Perlman, Subar and Block23). MFD was used to assess the quality of muscle repair and hypertrophy(Reference Sarnat24).
Experimental methods
Design
Male weanling Sprague–Dawley rats (3 weeks old, n 88; Charles River Laboratories, St Constant, PQ, Canada) were used to conduct the 6-week study which consisted of a deficiency phase (0–3 weeks) followed by a repletion phase (4–6 weeks). The rats were randomly assigned to a baseline (B) group and killed on day 0 (n 8), or to one of the following five dietary treatment groups (sixteen animals per group) and fed for 3 weeks: Zn- and protein-deficient (ZP, 20 g protein+ < 1 mg Zn/kg diet); Zn-deficient (Z, 170 g protein+ < 1 mg Zn/kg diet); protein-deficient (P, 20 g protein+30 mg Zn/kg diet); energy-deficient (E, 170 g protein+30 mg Zn/kg diet); control (C, 170 g protein+30 mg Zn/kg diet). The diet composition (Table 1) was based on the AIN-93G formulation(Reference Reeves, Nielsen and Fahey25). The ZP, Z, P and C groups were given free access to the diet, whereas the E rats were individually pair-fed the amount of diet consumed by the Z rats on the previous day, and this was equivalent to 50 % of the cumulative feed intake by the C group during the deficiency phase. Thus, the E group was a pair-fed control group to control for the anorexia of Zn deficiency, and it was a model for energy deficiency via diet restriction. At the end of the 3-week deficiency phase, tissues were collected from eight rats per group. The remaining forty rats entered the 3-week recovery phase and were given free access to the control diet containing 30 mg Zn/kg diet. During the study, the rats were housed in stainless-steel wire-bottomed cages with free access to double-distilled water from a polyethylene bottle with a stainless-steel sipper tube. Feed intake was determined daily and the rats were weighed weekly. Animal care was provided in accordance with a protocol approved by the University of Manitoba Animal Care Committee in accordance with Canadian Guidelines for the Care and Use of Laboratory Animals(26).
Table 1 Diet formulation*
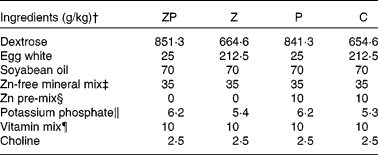
ZP, zinc- and protein-deficient; Z, zinc-deficient; P, protein-deficient; C, control diet.
* The energy-deficient group was fed restricted amounts of the C diet as described in the Methods section. Formulations are based on the American Institute of Nutrition (AIN)-93G diet for growing rats; the Zn content of each diet was verified by atomic absorption spectroscopy as described previously(Reference Lepage, Giesbrecht and Taylor29).
† Diet ingredients were purchased from Harlan Teklad (Madison, WI, USA) with the exception of soyabean oil (Vita Health, Winnipeg, MB, Canada) and dextrose (Moonshiners, Winnipeg, MB, Canada).
‡ AIN-93M Zn-free mineral mix (Harlan Teklad).
§ Zn pre-mix contained 5·77 g of zinc carbonate/kg dextrose.
∥ Adjustment for equivalency to the AIN-93G mineral mix and differing amounts of egg white.
¶ AIN-93 vitamin mix (Harlan Teklad).
Tissue collection
Rats were asphyxiated with CO2, weighed and decapitated for the collection of trunk blood. Then, one gastrocnemius muscle was excised and frozen in isopentane ( − 150°C) cooled in liquid N2(Reference Dubowitz and Brooke27, Reference Estes and Goss28). The other gastrocnemius muscle and inguinal fat pads were excised, weighed and frozen in liquid N2. Tissues and serum were stored at − 80°C.
Biochemical assays
Serum IGF-1 was determined using a rat serum IGF-1 double antibody RIA kit (DSL-2900; Diagnostic Systems Laboratories, Inc., Webster, TX, USA) and serum albumin using a kit (no. 631; Sigma Diagnostics, St Louis, MO, USA). Liver lipid, and Zn concentrations in serum, femur and diets were determined as described previously(Reference Lepage, Giesbrecht and Taylor29).
Muscle histology
Muscle fibre types were identified by ATPase activity at pH 9·4(Reference Sarnat24, Reference Dubowitz and Brooke27, Reference Estes and Goss28). Briefly, cross-sectional muscle sections (10 μm) were incubated in a CaCl2 and sodium bicarbonate enhancing solution (15 min at room temperature) and an alkaline solution of Ca and ATP (20 min at 37°C). The ATPase enzyme splits the terminal phosphate from ATP, and the free phosphate is immediately bound by Ca, creating calcium phosphate that is insoluble in the alkaline solution and which is deposited at the site of ATPase activity. The muscle sections were rinsed in three changes of 1 % CaCl2, incubated with 2 % cobalt chloride for 3 min (Co replaces Ca forming cobalt phosphate), and rinsed with six changes of distilled water. The muscle sections were exposed to 1 % ammonium sulphate for 1 min, resulting in insoluble black cobaltous sulphide at the original site of ATPase activity. Thus, type 1 (slow twitch) and type 2 (fast twitch) muscle fibres appear light (low ATPase activity) or dark (high ATPase activity) in colour, respectively. Finally, the slides were washed six times with distilled water and lightly counterstained with eosin. Digital images of the muscle sections were used to determine MFD (>75 fibres/rat) with IBAS software (version 2.0; Kontron Electronics, Kaufbeuren, Germany) calibrated with a micrometre slide.
In addition, the muscle samples were subjected to haematoxylin and eosin staining for identification of histological structures, and modified Gomori trichrome for assessment of mitochondria, oil red O for the presence of TAG and neutral fat in muscle, and periodic acid Schiff with and without diastase staining for detection of glycogen(Reference Estes and Goss28).
Statistical analysis
Data were analysed by ANOVA (SAS software 6.04; SAS Institute, Cary, NC, USA). Duncan's multiple-range test was used for means testing. Differences were deemed significant at P < 0·05.
Results
In the present study, the main effects of diet, time and diet × time interaction were significant (P < 0·05) for all parameters except the gastrocnemius muscle:body weight ratio (no significant main effects) and type 2 MFD (T2-MFD; diet and diet × time interaction were significant but not time) as described below. The significant diet × time interaction for all parameters (except the gastrocnemius muscle:body weight ratio) means that the response of a particular diet regimen (ZP, P, Z, E, C) was different depending on time (deficiency and repletion phases), as described in the following sections.
The feed intake of the C group was greater (P < 0·05) than that of the other groups during the deficiency and repletion phases (Table 2). During repletion, the feed intake of the Z group was greater (P < 0·05) than that of the ZP and P groups while the E group was intermediate. Feed efficiency during the deficiency phase was highest (P < 0·05) in the E group, followed by the P and C groups, and then the Z group. The ZP group had a negative feed efficiency during the deficiency phase; however, during repletion, this group had a greater (P < 0·05) feed efficiency than the P, E and C groups.
Table 2 Effects of dietary zinc, protein and energy deficiencies and repletion on cumulative feed intake and feed efficiency of growing rats
(Mean values with their standard errors)
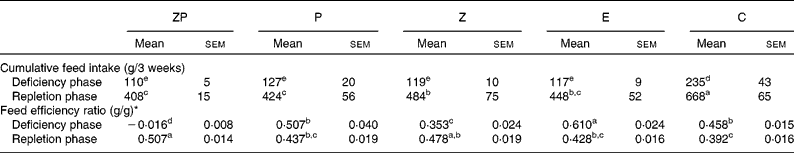
ZP, Zn- and protein deficient; P, protein deficient; Z, Zn deficient; E, energy deficient; C, control diet.
a,b,c,d,e Mean values with unlike superscript letters were significantly different (P < 0·05).
* Feed efficiency ratio = body weight gain (g)/feed intake (g). Main effects (diet, time and diet × time) were significant for each parameter (P < 0·05).
At the end of the deficiency phase, the ZP and P groups had body weights that were 34 % of the C group (P < 0·05), while the Z and E groups had body weights that were 50–56 % of the C group (P < 0·05; Fig. 1(A)). At the end of the repletion phase, the ZP and Z groups had body weights that were 66–69 % of the C group (P < 0·05) and the P and E groups had body weights that were 78–80 % of the C group (P < 0·05).

Fig. 1 Effects of dietary zinc, protein and energy deficiencies and repletion on (A) body weight (bwt), (B) inguinal fat pad:bwt ratio and (C) liver lipid concentration in growing rats. Values are means, with their standard errors represented by vertical bars (eight animals per group). Main effects (diet, time and diet × time) were significant for each parameter (P < 0·05). a,b,c,d,e,f,g Mean values with unlike letters were significantly different from each other (P < 0·05). B, baseline; ZP, zinc- and protein-deficient; P, protein-deficient; Z, zinc-deficient; E, energy-deficient; C, control.
Adipose stores were monitored via the inguinal fat pad weight, and no fat pads could be excised from the E group at the end of the deficiency phase, confirming energy-type malnutrition. The inguinal fat pad:body weight ratio of the Z group was similar to the B group and lower (P < 0·05) than the ZP, P and C groups (Fig. 1(B)). After the repletion phase, the Z group had approximately two-thirds (P < 0·05) the inguinal fat pad:body weight ratio of the P group.
After the deficiency phase, the ZP and P groups had elevated (P < 0·05) liver lipid concentrations (Fig. 1(C)) and lower (P < 0·05) serum albumin concentrations (Fig. 2(A)), compared with the other groups including B, confirming protein-type malnutrition.

Fig. 2 Effects of dietary zinc, protein and energy deficiencies and repletion on serum (A) albumin and (B) insulin-like growth factor-1 (IGF-1) concentrations in growing rats. Values are means, with their standard errors represented by vertical bars (eight animals per group). Main effects (diet, time and diet × time) were significant for each parameter (P < 0·05). a,b,c,d,e Mean values with unlike letters were significantly different from each other (P < 0·05). B, baseline; ZP, zinc- and protein-deficient; P, protein-deficient; Z, zinc-deficient; E, energy-deficient; C, control.
At the end of the deficiency phase, the Z and E groups had lower (P < 0·05) serum IGF-1 concentrations than all the other groups including B (Fig. 2(B)). The serum IGF-1 concentrations of the Z and E groups were 11 and 22 %, respectively, of the C group. The serum IGF-1 concentration of the P group was higher (P < 0·05) than the B and ZP groups, but it was lower (P < 0·05) than the C group. Following the repletion phase, all groups had similar serum IGF-1 concentrations.
Following the deficiency phase, the ZP, P and Z groups had serum Zn concentrations that were similar and 51–81 % lower (P < 0·05) than the B, E and C groups (Fig. 3(A)). After the repletion phase, all groups had similar serum Zn concentrations. The changes in serum Zn concentration were paralleled by the changes in femur Zn concentration (Fig. 3(B)). Despite similar dietary Zn intake, the ZP group had a higher (P < 0·05) femur Zn concentration than the Z group.

Fig. 3 Effects of dietary zinc, protein and energy deficiencies and repletion on (A) serum and (B) femur zinc concentrations in growing rats. Values are means, with their standard errors represented by vertical bars (eight animals per group). Main effects (diet, time and diet × time) were significant for each parameter (P < 0·05). a,b,c,d,e,f Mean values with unlike letters were significantly different from each other (P < 0·05). B, baseline; ZP, zinc- and protein-deficient; P, protein-deficient; Z, zinc-deficient; E, energy-deficient; C, control.
Gastrocnemius muscle weights after the deficiency and recovery phases (Fig. 4(A)) paralleled body weight as there were no differences when gastrocnemius muscle mass was expressed relative to body weight (Fig. 4(B); no significant main effects).

Fig. 4 Effects of dietary zinc, protein and energy deficiencies and repletion on (A) gastrocnemius muscle weight, (B) gastrocnemius muscle:body weight (bwt) ratio, (C) type 1 muscle fibre diameters (T1-MFD) and (D) type 2 muscle fibre diameters (T2-MFD) in growing rats. Values are means, with their standard errors represented by vertical bars (eight animals per group). Main effects (diet, time and diet × time) were significant for each parameter (P < 0·05), but not time for T2-MFD and there were no significant main effects for the muscle:bwt ratio. a,b,c,d,e,f,g Mean values with unlike letters were significantly different from each other (P < 0·05). B, baseline; ZP, zinc- and protein-deficient; P, protein-deficient; Z, zinc-deficient; E, energy-deficient; C, control; ND, not determined.
ATPase histochemical staining at pH 9·4 of the gastrocnemius muscle was used to differentiate type 1 (low ATPase activity, light in colour) and type 2 (high ATPase activity, dark in colour) muscle fibres after the deficiency (Fig. 5) and repletion (Fig. 6) phases. After the deficiency phase, the type 1 MFD (T1-MFD) of the C group increased 1·3-fold (P < 0·05) from the 3-week-old B group (Fig. 4(C)). The ZP, P and Z groups had T1-MFD values that were not different from the B group but were 80–82 % of the C group (P < 0·05). The E and C groups had similar T1-MFD values that were greater (P < 0·05) than the other groups. By the end of the repletion phase, the T1-MFD of the C group had increased 1·2-fold (P < 0·05) from the 6-week-old C group value. The T1-MFD values of the P and E groups were 16 and 9 %, respectively, less (P < 0·05) than the C group. The T1-MFD values of the ZP and Z groups were not different from that of the C group. At the end of the deficiency phase, the T2-MFD of the C group had increased by 1·5-fold (P < 0·05) from the B group, and it was higher (P < 0·05) than the other groups (Fig. 4(D)). The 6-week-old ZP, P and Z groups had T2-MFD values that were not different from the 3-week-old B group but were 69–78 % of the C group (P < 0·05). The T2-MFD of the E group was similar to the Z group and 14 % less (P < 0·05) than the C group. After the repletion phase, the T2-MFD of the ZP, Z and E groups were not different from that of the C group. Only the P group had a smaller (P < 0·05) T2-MFD that was 15 % less than the C group. There were no differences among groups for the other histological assessments of mitochondria, neutral fat or glycogen in skeletal muscle (data not shown).

Fig. 5 Representative images of dietary zinc, protein and energy deficiencies on ATPase histochemical staining at pH 9·4 of the gastrocnemius muscle. (A) Baseline group, (B) control group, (C) zinc- and protein-deficient group, (D) protein-deficient group, (E) zinc-deficient group and (F) energy-deficient group. Type 1 muscle fibres (low ATPase activity) are light in colour and type 2 muscle fibres (high ATPase activity) are dark in colour. Scale bar = 100 μm. The baseline group was 3 weeks old and all other groups were 6 weeks old at the end of the deficiency phase.

Fig. 6 Representative images of the effects of nutritional repletion on ATPase histochemical staining at pH 9·4 of the gastrocnemius muscle. (A) Control group, (B) zinc- and protein-deficient group, (C) protein-deficient group, (D) zinc-deficient group and (E) energy-deficient group. Type 1 muscle fibres (low ATPase activity) are light in colour and type 2 muscle fibres (high ATPase activity) are dark in colour. Scale bar = 100 μm. All groups were 9 weeks old.
Discussion
In the present study, Zn, energy and protein deficiencies had divergent negative effects on muscle mass (Fig. 4(A)), MFD (Fig. 4(C) and (D)) and serum IGF-1 (Fig. 2(B)). The reductions in gastrocnemius muscle mass in the deficient groups were proportional to body weight (Fig. 4(B)) but they did not reflect the differences in MFD. The Zn, protein and combined Zn+protein deficiencies arrested T1- and T2-MFD growth at baseline measurements, and energy deficiency compromised T2-MFD, but not T1-MFD. Serum IGF-1 concentrations did not parallel these differences in MFD, and in the context of this experiment, there was a greater reduction of serum IGF-1 concentrations in energy-type malnutrition (Z and E groups) than protein-type malnutrition (ZP and P groups). The IGF-1-lowering effect of Zn deficiency in the present study (Z group) was partially blunted by concomitant protein deficiency (ZP group; Fig. 2(B)). Typically, IGF-1 levels rely on both protein and Zn intake(Reference Cesur, Yordaman and Doğan14–Reference Prewitt, D'Ercole and Switzer17, Reference Cossack19, Reference Dorup, Flyvbjerg and Everts20); thus, the higher serum IGF-1 concentrations in the Zn+protein-deficient animals compared with the Zn-deficient animals in the present study are surprising. Likewise, the higher femur Zn concentrations of the ZP v. Z group during the deficiency phase were not anticipated as both groups consumed the same amount of diet with < 1 mg Zn/kg as confirmed by atomic absorption spectroscopy. It is possible that the concomitant deficiency of two nutrients, Zn and protein, results in some type of metabolic efficiency not observed with a single nutrient deficiency. Future studies are needed to investigate IGF-1 mRNA and protein levels in muscle tissue as well as the IGF-1 signalling pathway.
During the deficiency phase, the ZP, P and Z groups had the smallest T1- and T2-MFD but serum IGF-1 concentrations varied six-fold among these groups. The Z and E groups had similar serum IGF-1 concentrations (11–22 % of the C group), but T1-MFD was smaller in the Z group compared with the E group (pair-fed control). It is generally believed that type 2 muscle fibres reflect changes in nutrition and physical activity more readily than type 1 muscle fibres(Reference Sarnat24). Histochemical staining of muscle sections to quantify T1- and T2-MFD provides further characterisation of the consequences of nutritional deficiencies on skeletal muscle and the quality of lean mass recovery with nutritional rehabilitation.
Others have reported reductions in serum IGF-1 concentrations in rodent models of Zn, protein or energy deficiencies(Reference Prewitt, D'Ercole and Switzer17, Reference Cossack19, Reference Dorup, Flyvbjerg and Everts20), but relationships between IGF-1 and muscle wasting or recovery were not the focus of those studies. When effects of dietary energy and protein were compared, dietary protein was the more influential factor on serum IGF-1 concentrations(Reference Prewitt, D'Ercole and Switzer17). IGF-1 measurements in serum are relevant to clinical applications; however, serum IGF-1 concentrations do not always reflect tissue IGF-1 status due to the localised production and actions of IGF-1, and the role of binding proteins(Reference Ketelslegers, Maiter and Maes22, Reference Backeljauw, Bang and Dunger30). Undernutrition reduces skeletal MFD and cross-sectional area, but fibre number is maintained(Reference Glore and Layman31). Failure of type 1 and 2 muscle fibres to grow has previously been reported in the extensor digitorum longus muscle of 25-week-old rats fed a 1·5 % protein diet for the previous 19 weeks(Reference Oldfors, Mair and Sourander32).
The recovery data indicated that previous nutritional status and not serum IGF-1 was the primary factor determining the degree of muscle mass and muscle fibre recovery. Although there were differences in gastrocnemius muscle mass recovery (ZP and P < E < Z < C; Fig. 4(A)), the ratios for gastrocnemius muscle mass:body weight were equivalent among the groups (Fig. 4(B)). Recovery of MFD was not dependent on recovery of body weight or gastrocnemius muscle mass. The P and E groups had better recovery of body weight than the ZP and Z groups (Fig. 1(A)); however, the Z and ZP groups experienced full recovery of T1-MFD (Fig. 4(C)) and T2-MFD (Fig. 4(D)), while the P group did not for either fibre type and the E group recovered only T2-MFD compared with the C group. Although further research is required to understand the effects of nutritional status on IGF-1 signalling in skeletal muscle, there are in vitro data that Zn treatment activates IGF-1-receptor phosphorylation in H9c2 cardiac cells(Reference Lee, Chanoit and McIntosh33).
The higher inguinal fat pad:body weight ratio in the P group after recovery (Fig. 1(B)) suggested that a greater proportion of the weight gain in this group may be due to adipose tissue. The higher fat accretion by the P and E groups compared with the Z group during the repletion phase may also explain their better catch-up growth compared with the Z group (Fig. 1(A) and (B)). Other than achieving expected weight-for-height, children with PEM experience the same incomplete and disorganised recovery of adipose and muscle tissues and muscle histology(Reference MacLean and Graham3, Reference Hansen-Smith, Picou and Golden5, Reference Golden and Golden8) as observed in the P and E rats. The muscle fibre cross-sectional areas of children apparently recovered from PEM were still significantly smaller than age- and sex-matched controls(Reference Montgomery4, Reference Hansen-Smith, Picou and Golden5), and much of the weight gained by children with PEM during compensatory growth was fat(Reference MacLean and Graham3).
The animals in the present study did appear to have muscle recovery relative to their body weight (Fig. 4(B)) despite differences in final body weight and fat accretion (Fig. 1(A) and (B)) among the groups. Glore & Layman(Reference Glore and Layman31) reported that rehabilitation with a 20 % protein diet for 28 weeks was insufficient for the recovery of body weight, skeletal muscle weight, soleus MFD and cross-sectional area (76, 84, 92 and 84 % of control, respectively) in rats that had been feed-restricted for the first 120 d of life(Reference Oldfors, Mair and Sourander32). The control diet with 30 mg Zn/kg diet, which is designed for normal growing rats, may have been unable to meet the nutritional requirements for full gastrocnemius recovery during compensatory growth. Adequate Zn during recovery in children (5–10 mg Zn/kg body weight) and mice (40 v. 5 and 10 mg Zn/kg diet) has been reported to improve rates of weight gain and recovery of lean tissue mass(Reference Golden and Golden8, Reference Morgan, Keen and Calvert9). The nutritional programming theory has been used to explain the negative impacts of protein and energy deficiencies during fetal and neonatal development on various organ systems later in life; however, there is an absence of studies focusing on skeletal muscle. Both starvation and obesity have been shown to decrease satellite cell proliferation in skeletal muscle(Reference Halevy, Geyra and Barak34, Reference Peterson, Bryner and Always35). The present study demonstrating differential effects of previous protein or energy deficiency on recovery of MFD suggests that malnutrition type may have differential effects on impairments in various step(s) for proliferation of satellite cells and the myogenesis process.
In summary, Zn, protein and energy deficiencies resulted in retarded growth and development of the biochemical, cellular and whole-body levels. Previous protein or energy deficiency, but not Zn or combined Zn+protein deficiency, limited the recovery of T1-MFD (P and E groups) or T2-MFD (P group) during nutritional repletion. The quality of skeletal muscle recovery in the malnourished groups could not be predicted from body weight, muscle mass, serum Zn or serum IGF-1 concentrations. Further research is required to delineate the effects of sub-optimal postnatal nutrition and nutritional metabolism on skeletal muscle structure and function.
Acknowledgements
The present study was supported by the Natural Sciences and Engineering Research Council of Canada (grant to C. G. T.) and the University of Manitoba Graduate Fellowship (to A. L. V. P.). A. L. V. P. and C. G. T. contributed to the study design, completion and analysis and manuscript preparation. W. C. H. contributed to the analysis and interpretation of muscle histology. There were no conflicts of interest. Thanks also to the staff of the Science Animal Care facility and the Pathology Laboratory, Health Sciences Centre, Winnipeg, MB, Canada; and Sergio Mejia, Department of Geological Sciences, University of Manitoba for assistance with the image analysis.










