Introduction
West Nile virus (WNV) infection is endemic in North America. More than 41 000 cases of WNV-related illnesses and 2000 deaths have been reported in the USA between the introduction of the virus in 1999 and 2015 [1]. During the same period, 5310 cases have been reported in Canada [2]. In Québec, the first documented cases happened in 2002. After a quiet period (2004–2010), the province experienced an outbreak between 2011 and 2013 [Reference Ouhoummane3].
WNV causes an asymptomatic infection in 80% of cases and flu-like symptoms (West Nile fever, WNF) in 20%. In one out of 150 infections, a severe illness occurs with neurological involvement like aseptic meningitis, encephalitis or acute flaccid paralysis (AFP) [Reference Carson4, Reference Mostashari5]. The risk of severe illness increases in older persons, in those with compromised immune systems and those with underlying medical conditions, like hypertension and diabetes [Reference Lindsey6–Reference Murray8]. Surveillance data from the province of Québec indicate that the incidence of neurological disease increases approximately 1.6-fold for each decade of life [Reference Ferrouillet9]. WNV infection and particularly neurological disease have been associated with mild-to-severe clinical manifestations [Reference Sejvar10]. WNF and West Nile meningitis (WNM) are generally associated with a favourable outcome. This is different for West Nile encephalitis (WNE) where altered consciousness is present in up to 75% of patients and 10–30% of them may die [Reference Ferrouillet9–Reference Murray13]. WNE patients may also require rehabilitation services or support at home after acute care discharge [Reference Pepperell11–Reference Murray13].
Unlike acute morbidity and mortality, long-term physical, cognitive as well as functional sequelae associated with WNV disease are less well known. A recent systematic review, which included 29 studies on long-term sequelae of WNV-related illness, showed that the most common persistent physical sequelae reported were weakness, fatigue, myalgia and headache [Reference Patel, Sander and Nelder14]. The most common persistent cognitive sequelae were memory loss, depression and difficulty concentrating and the most common persistent functional sequelae was difficulty doing activities of daily living [Reference Patel, Sander and Nelder14]. In studies comparing sequelae between neuroinvasive and non-neuroinvasive patients, a poorer physical and functional prognosis was noted for neuroinvasive patients, while cognitive status was similar in both groups. However, these studies varied by study design, sample size, methods used to measure sequelae (subjective vs. objective measurements), duration of follow-up and outcomes measured. In addition, few of these studies compared WNE and WNM. The authors of the review highlighted the need for more primary studies with larger sample size, longer follow-up periods and use of matched controls [Reference Patel, Sander and Nelder14].
The objectives of this study are (1) to describe the clinical characteristics of patients according to illness severity, and (2) to prospectively document physical, mental and functional status up to 24 months after symptom onset using standardised questionnaires. The results of this study will help in the understanding of WNV infection burden in Québec to support planning and prevention efforts.
Methods
Study population
WNV infection is a reportable disease in Québec. Physicians and laboratories must report all WNV-positive cases to the regional public health boards, who conduct an epidemiological investigation in order to document the infection, determine the likely place of acquisition and collect socio-demographic and clinical information, such as date of illness onset and clinical syndrome (i.e. uncomplicated fever, meningitis, encephalitis and AFP). In this investigation, the treating physician provides clinical information to the public health nurse while the patient or his family clarifies where the exposure occurred. The data are entered into the integrated system for public health monitoring of WNV (ISPHM-WNV), a provincial electronic surveillance system for WNV disease that includes information on humans, mosquitoes and animals [Reference Gosselin15]. During the period 2012–2013, 155 symptomatic WNV cases were reported in Québec. We asked regional public health boards to approach them to see whether they were willing to be contacted about the study. Written consent to participate in a phone interview and to get access to their medical chart was then obtained from eligible patients by a research nurse. For those younger than 18 years old, the consent was obtained from the parents or legal guardians, and for deceased persons, the consent to get access to their medical chart was obtained from the families. These latter were included only for morbidity and mortality evaluation but excluded from phone interview. The study protocol was submitted to the Comité d’éthique de santé publique from the Institut national de santé publique du Québec, which delivered a favourable opinion towards it [16].
Data collection
Chart review
The medical records of patients hospitalised or seen in an emergency department were reviewed by a research nurse to collect information on medical history, clinical and neurological symptoms and diagnostic test results. For patients who consulted in private practice only, medical history and clinical symptoms were collected from the epidemiological investigation form filled by the regional public health board.
Phone questionnaires
For eligible participants, two standardised phone questionnaires were administered by a research nurse 12 months (2013 cases only) and 24 months after symptom onset to document their physical, mental and functional status.
Physical and mental health statuses were assessed using the Short Form (SF)-36 [Reference Saris-Baglama17]. Canadian population norms are available for this instrument, which permits external comparisons [Reference Hopman18]. The SF-36 scale contains 36 items grouped into eight health concepts or domains: physical function (PH), physical role limitations (RP), bodily pain (BP), general health (GH), social functioning (SF), mental health (MH), emotional role limitations (RE) and vitality (VT). Each item was scored from 0 (worst health state) to 100 (best health state), and the scores of each of the eight domains could be tabulated as the physical and mental component summary scores. The physical component summary includes PH, RP, BP and GH domains and the mental component summary includes SF, MH, RE and VT domains.
Functional status was assessed using the Instrumental Activities of Daily Living (IADL) [Reference Lawton and Brody19]. The IADL scale contains eight items that assess a person's ability to perform tasks before and after illness: using a telephone, go shopping, food preparation, doing laundry, housekeeping, using mode of transportation, owning medications and handling finances. Each item was scored as 0 ‘unable or partially able’ to 1 ‘able’. Item responses are summed to derive a scale score with higher scores indicating greater independence.
These two questionnaires are available and have been validated both in English and in French.
Case definitions
In the ISPHM-WNV, all laboratory diagnoses of WNV infection by IgM capture enzyme immunoassays either from serum or cerebrospinal fluid (CSF) samples are included. In Québec, after a first IgM-positive case has been confirmed with plaque reduction neutralization test, all other IgM-positive cases occurring in the same season are considered to be laboratory confirmed. Cases are further classified according to their clinical presentation as WNF (an acute systemic febrile illness), WNM (with stiff neck and CSF pleocytosis), WNE (with altered mental status), West Nile meningo-encephalitis (WNME) and AFP (polio-like myelitis or Guillain–Barre syndrome) [20].
Data analysis
For the analysis, one case with missing information about clinical syndrome was excluded and one case of muscular weakness was classified as AFP after reviewing his medical record. WNME (n = 18) and AFP (n = 3) cases were combined with the WNE (n = 27) cases given the similar clinical presentation. All analyses were performed according to three clinical categories (WNF, WNM and WNE). Proportions were compared using χ 2 test or Fisher's exact test when appropriate; distributions of age and hospital stay were compared with exact Wilcoxon rank-sum (Kruskal–Wallis) non-parametric test.
Mean scores of each of the eight domains of the SF-36 were compared to age- and sex-matched Canadian norms [Reference Hopman18] using t tests [Reference Hays21]. Physical and mental component summary T-scores were standardised to a mean of 50 (s.d.= 10), with a score >50 representing better than average and <50 poorer than average function [Reference Maruish22]. For 2013 participants (n = 20), SF-36 and IADL were administered 12 and 24 months after symptoms onset. Results were similar for both time points and only results at 24 months are thus presented.
Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and a two-sided P < 0.05 was considered statistically significant.
Results
From the 155 symptomatic WNV 2012–2013 patients, 93 (60%) agreed to participate in the study (Fig. 1). The non-participants were the WNV patients who could not be reached by their regional public health board or those who refused to be contacted for the study. Participants and non-participants were comparable with regard to demographic (except for sex where more women agreed to participate (54% vs. 36%, P = 0.026)) and illness severity (hospitalization, 76% vs. 71%, P = 0.437; clinical syndrome, 73% vs. 68% had neurological symptoms, P = 0.502). More families of deceased patients agreed to participate in the study (15% vs. 5%), but the difference was not statistically significant (P = 0.07). Medical records were available for 81 (87%) participants (71 hospitalizations and 10 emergency department visits), and of the 79 known to be alive at the time of survey, 77 (97%) patients completed the phone interview at 24 months.
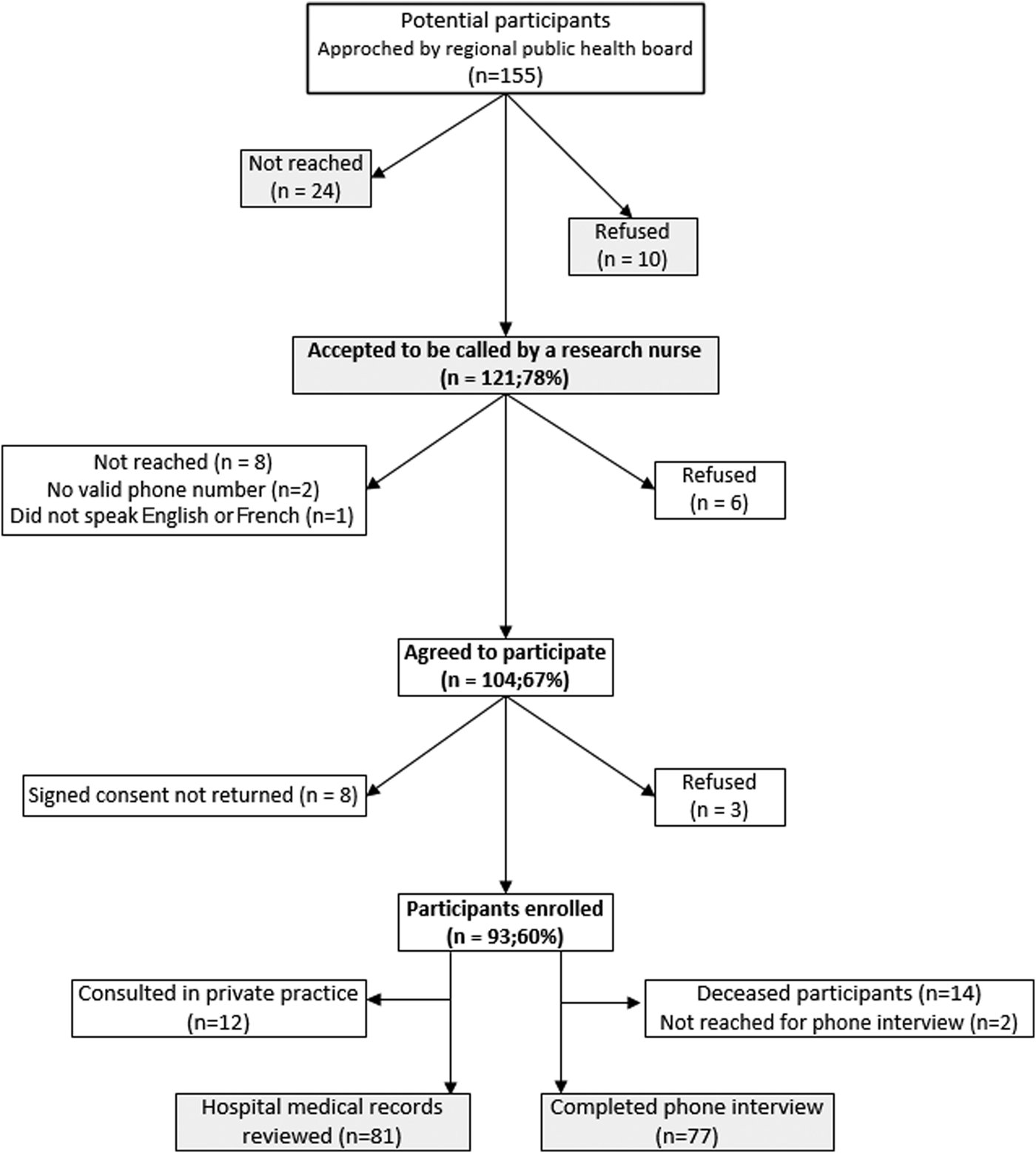
Fig. 1. Study flow diagram.
Clinical characteristics
Demographic and clinical characteristics of participants are presented in Table 1. Among 92 patients, WNF, WNM and WNE accounted for 27%, 20% and 53%, respectively. WNE participants were significantly older that WNM (P = 0.0002) or WNF participants (P = 0.0006). More pre-existing medical conditions (hypertension, heart disease and diabetes) were observed among WNE participants. The majority of WNE (48/49; 98%) and WNM (17/18; 94%) participants were hospitalised, compared with 20% (5/25) of WNF (P < 0.0001). The median hospital stay was however longer among WNE participants compared with WNM (P < 0.0001) or WNF (P = 0.010). In addition, 22/48 (46%) WNE hospitalised patients were admitted to the intensive care (vs. nil WNM or WNF patients). Among them, 12 required intubation.
Table 1. Demographic and clinical characteristics of 92 WNV patients in Québec, 2012–2013
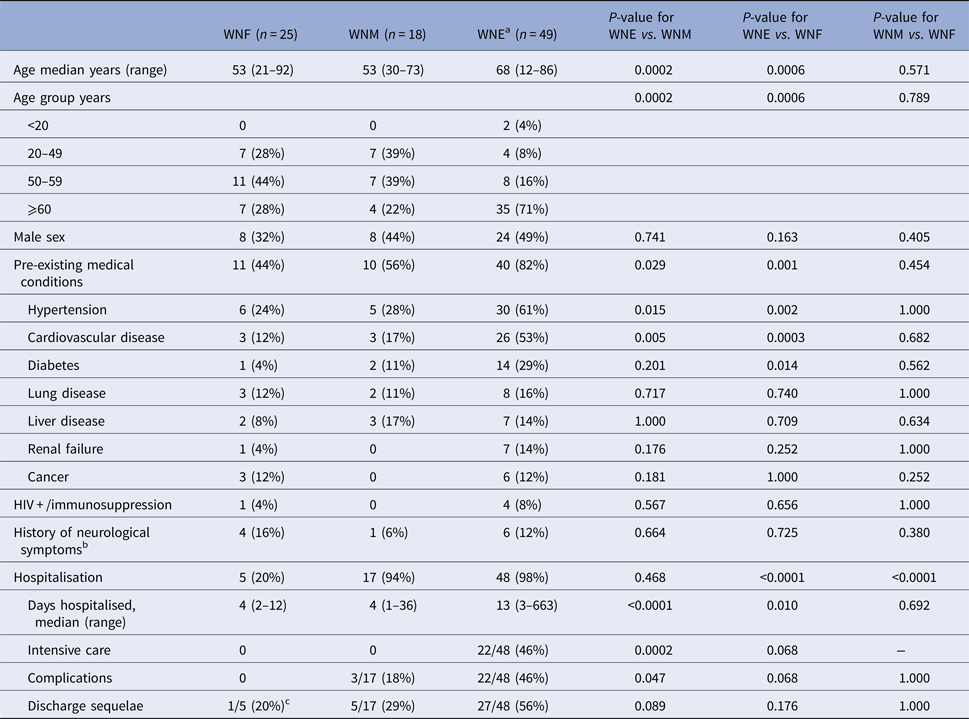
a Include West Nile encephalitis (n = 27), meningo-encephalitis (n = 18) and acute flaccid paralysis (n = 3).
b Headaches, dementia or brain trauma.
c Continue to experience persistent headaches.
Fever, fatigue and headache were frequent and noted in more than 70% of participants (Table 2). Chills, weakness and anorexia were significantly more frequent among WNE and WNM participants, gastrointestinal symptoms among WNM and rash among WNF and WNM participants (Table 2). The association with gastrointestinal symptoms persisted even after controlling for risk factors such as pre-existing medical conditions and age (data not shown). Rash was more frequently observed among younger participants (median age 55 years) compared with those without rash (median age 64 years, P = 0.0034). In addition, when analyses were restricted to participants aged <55 years, no difference in proportion of rash were observed among the three clinical syndromes (data not shown).
Table 2. Clinical manifestation at the presentation of 92 WNV patients in Québec, 2012–2013
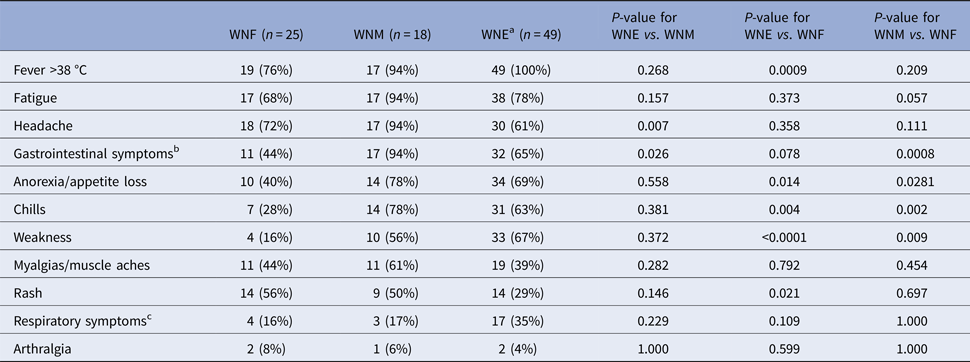
a Including West Nile encephalitis (n = 27), meningo-encephalitis (n = 18) and acute flaccid paralysis (n = 3).
b Nausea, vomiting, diarrhoea or abdominal pain.
c Cough, wheezing or pharyngitis.
Information on neurological symptoms was available only for participants with medical record (n = 81). Overall, neurological symptoms were more common among WNE participants (Table 3). Of the 49 WNE, 44 (90%) had altered consciousness including five patients with coma. Other common neurological symptoms included neuromuscular weakness (76%), cranial neuropathy (67%), cognitive impairment (53%) and neck stiffness (49%).
Table 3. Neurological manifestations of 80 WNV patients with medical charts, Québec, 2012–2013

a Including West Nile encephalitis (n = 27), meningo-encephalitis (n = 18) and acute flaccid paralysis (n = 3).
b Confusion, drowsiness, stupor, delirium or coma (n = 5, WNE).
c Hypo- or hyper-reflexia, myoclonus, weakness or paralysis (n = 3, WNE).
d Tremor or vertigo.
e Ocular pain, decreased visual acuity or photophobia.
Among hospitalised participants, only 20 (42%) WNE were discharged home without support compared with 15 (88%) WNM and four (80%) WNF participants, P = 0.001. Ten (21%) WNE, one (6%) WNM and one (20%) WNF participants were discharged home with support. Six (13%) and three (6%) WNE participants were transferred to rehabilitation institution and long-term care hospital, respectively. Ten (21%) WNE and one (6%) WNM participants died during hospitalization, in addition to three WNE participants who died during the follow-up study (data not shown).
Physical and mental health status 24 months after symptom onset
For analyses of physical and mental status, WNE and WNM participants were grouped because they presented similar results (Table 4). Overall, 20 (38%) and 29 (55%) neuroinvasive participants and nine (39%) and 12 (52%) non-neuroinvasive participants had low physical component scale (PCS) and mental component scale (MCS), respectively. Compared with age- and sex-matched Canadian norms, mental health and social functioning were the only affected domains in cases with neuroinvasive disease (P = 0.0025 and 0.0297, respectively). The differences were not statistically significant for other domains of the MCS and all domains of the PCS.
Table 4. Physical and cognitive health status 24 months after WNV symptom onset, Québec, 2012–2013
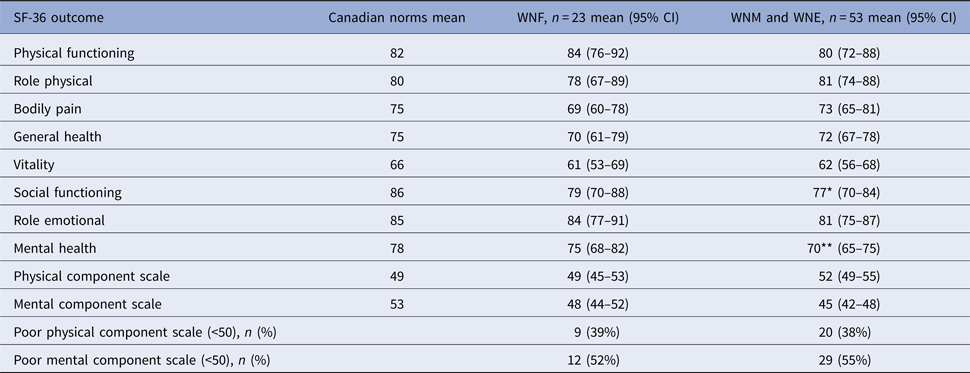
*P = 0.020 for comparison with age- and sex-matched Canadian norms.
**P = 0.003 for comparison with age- and sex-matched Canadian norms.
Analyses of physical and mental status were also made according to the pre-existing medical conditions status (Table 5). Compared with age- and sex-matched Canadian norms, participants with one or more pre-existing medical conditions had significantly lower score in two domains of the PCS (bodily pain; P = 0.039 and general heath; P = 0.030) and two domains of the MCS (mental health; P = 0.009 and social functioning; P = 0.003). While participants without pre-existing medical conditions had significantly higher score in three domains of the PCS (physical functioning, role physical and bodily pain) and similar score in all domains of the MCS than general population.
Table 5. Physical and cognitive health status 24 months after WNV symptom onset according to pre-existing medical conditions, Québec, 2012–2013
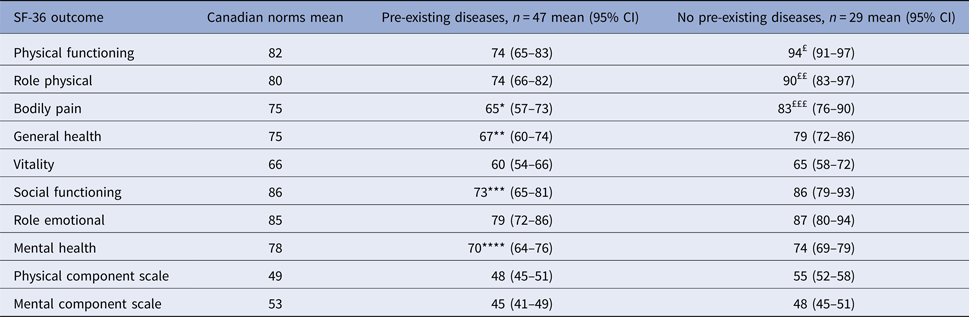
*P = 0.039 for comparison with age- and sex-matched Canadian norms.
**P = 0.03 for comparison with age- and sex-matched Canadian norms.
***P = 0.003 for comparison with age- and sex-matched Canadian norms.
****P = 0.009 for comparison with age- and sex-matched Canadian norms.
£P = 0.0008 for comparison with age- and sex-matched Canadian norms.
££P = 0.038 for comparison with age- and sex-matched Canadian norms.
£££P = 0.048 for comparison with age- and sex-matched Canadian norms.
Functional status 24 months after symptom onset
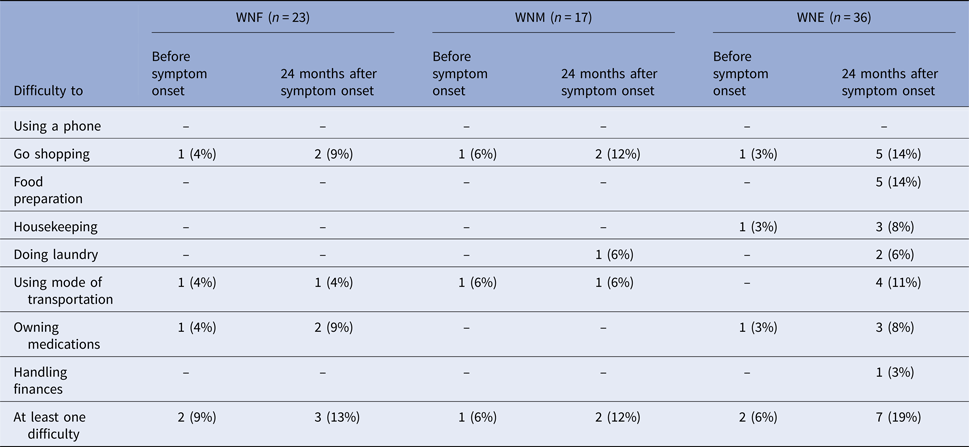
Participants were asked to report their ability to perform tasks before and 24 months after symptom onset (Table 6). Excluding five participants with baseline functional impairment (two WNF, one WNM and two WNE), one (5%) WNF, one (6%) WNM and five (15%) WNE participants reported functional sequelae 24 months post-infection. Most reported difficulties were food preparation, shopping and transportation. These participants were older (median age of 63 years, range of 55–83 years) and all had pre-existing medical conditions.
Table 6. Impaired functional status at baseline and 24 months after WNV symptom onset, Québec, 2012–2013
Discussion
In our study, WNE participants were older, had more underlying medical conditions, more neurological symptoms and worse hospital course (longer hospital stay, intensive care, in-hospital complications and death) than WNM or WNF participants. Nearly half of surviving hospitalised encephalitis participants required extra support upon discharge. These results are consistent with what was found in previous studies [Reference Murray13, Reference Racsa23–Reference Weatherhead26].
Like in other viral infection of the central nervous system, nausea and vomiting occur in WNV infection due to the inflammatory response around the brain. In our study, WNM participants have significantly more gastrointestinal symptoms than WNF or WNE and the association persisted even after controlling for risk factors. It has been reported that WNM patients frequently require hospitalization for pain control for severe headache or rehydration because of prolonged nausea and vomiting [Reference Sejvar10]. In addition, the increased frequency of rash seen in WNF and WNM patients was associated with their younger age, which is consistent with the previous findings [Reference Murray27].
Despite a favourable outcome, 5/25 (20%) WNF participants were hospitalised with a median hospitalization stay of 4 days, one of them needed extra support upon discharge and one reported deterioration of functional status up to 24 months. In another study, 31% of 98 WNF patients required hospitalization and 84% reported limitations in their household activities in the following weeks [Reference Watson28]. In our study, hospitalised WNF had similar demographic and clinical profiles (i.e. mean age, pre-existing medical conditions and clinical symptom at presentation) as WNE participants, which could contribute to their hospitalization and poor outcome. It is also possible that misclassification occurred and that some WNF cases had undetected mild neuroinvasive disease because lumbar puncture and cerebral scans were not performed on most of these patients [Reference Murray13, Reference Gottfried, Quinn and Jones24, Reference Watson28]. In our study, 3/5 hospitalised WNF did not have a lumbar puncture.
One strength of our study is the use of standardised and validated questionnaires to document long-term sequelae of WNV infection, which provided quantifiable measures and allowed comparison with other studies using the same instruments. At 24-month follow-up, only neuroinvasive patients had a lower score in two domains of the mental component, mental health and social functioning, compared with the general population. This result could be attributable to the high proportion of pre-existing medical conditions among them. Indeed, participants with pre-existing medical conditions, such as hypertension, cardiovascular disease and diabetes, had a lower score in both mental (mental health and social functioning) and physical (general health and bodily pain) components, whereas participants without these conditions had better or similar score compared with the general population. These results are consistent with the previous findings indicating that pre-existing medical conditions are a prognostic factors for long-term WNV-related sequelae [Reference Loeb25]. In the study of Loeb et al., 156 Canadian patients were followed up to 36 months to assess long-term physical and mental status [Reference Loeb25]. Most cases recovered after 1 year, but recovery was delayed in patients with neuroinvasive disease (only for physical function) and in those with underlying medical conditions (for both physical and mental function), whereas an absence of underlying medical conditions was associated with a faster rate of recovery. One important difference between this study and our study was the exclusion of patients with AFP, which have poor long-term outcomes [Reference Sejvar29]. Another difference was the younger age and less common underlying medical conditions in this study compared with our study, which could explain the overall favourable outcomes in the Canadian study. In a series of seven AFP cases, mental component score normalised at 2 years, while the physical component score stayed below normal [Reference Johnstone30], but these results are based on patients with this specific neurological manifestation. Sejvar et al. noted significant lower SF-36 scores in all eight physical and mental domains in 22 WNM and 16 WNE patients compared with pre-illness scores at 18 months post-infection [Reference Sejvar31].
We also used IADL to measure functional sequelae. Among our participants, 5/36 (15%) WNE, 1/17 (6%) WNM and 1/23 (5%) WNF had new functional limitations 24 months after symptom onset. Most reported difficulties were food preparation, shopping and transportation. The difference was not statistically significant probably due to the low sample size. Using the same questionnaire, Klee et al. showed that 10/35 (29%) of hospitalised WNV patients still had some functional sequelae 1 year after acute illness [Reference Klee12]. Other studies using self-reported outcomes showed that difficulties with the activities of daily living were the most common functional sequelae in WNV infection [Reference Patel, Sander and Nelder14].
There seems to be further discrepancies among studies between follow-up assessments using standardised questionnaires and self-reported outcomes [Reference Klee12, Reference Murray13, Reference Sejvar32, Reference Carson33]. For example, a prospective study of 157 WNV-infected patients indicated that 40% (18/45) of participants were still reporting persistent physical and cognitive symptoms related to their infection up to 8 years later [Reference Murray13]. Self-reported sequelae studies could be limited by recall bias and the tendency of patients to overestimate their health problems.
Our study has a number of limitations. Although participation rate was high, subgroup analyses were based on a small number of cases and the power to detect statistically significant differences was reduced. Fourteen patients died (including 13 among WNE patients) and were excluded from the evaluation of long-term sequelae. Therefore, the impact of the disease could be underestimated particularly among WNE patients. In our study, we did not ask for persisting symptoms at 24 months. A combination of assessment methods would be most appropriate in order to obtain a full picture of the long-term impact of WNV infection. Finally, functional sequelae 2 years post-WNV infection were more frequent among WNE participants, but it could be a result of advancing age of these patients.
This study provided the first portrait of WNV morbidity and mortality and long-term sequelae in the province of Québec. Our study showed that WNE patients were associated with more severe clinical manifestations and worst short-term outcomes. At 24 months, sequelae reported by these patients seem to be long lasting, but this could be due to the fact that persons with chronic diseases are more susceptible to develop severe WNV manifestations leading to a deterioration in their quality of life. This reinforces the idea that preventive measures against WNV infection need to be well communicated to the population and meticulously followed by persons with chronic diseases.
Acknowledgements
The authors would like to acknowledge the regional public health boards for approaching patients, research nurses for data collection, Melissa Trudeau for data entry and all patients who agreed to participate in this study.
Financial support
This study was funded by a research grant from Ministère de la santé et des services sociaux du Québec.
Declaration of Interest
None.









