Introduction
The function and functional morphology of shells have been a topic of debate for many decades. Shells are thought to have evolved early in the history of biomineralizing metazoans during the Ediacaran and Cambrian to protect soft tissue (“the Verdun Syndrome” [Dzik Reference Dzik2005, Reference Dzik2007]). Among the phyla that developed a shell during that period were molluscs (e.g., Pojeta Reference Pojeta1987; Dzik Reference Dzik2005), including Cambrian bivalves (e.g., Runnegar and Pojeta Reference Runnegar and Pojeta1992; Elicki and Gürsu Reference Elicki and Gürsu2009; Zong-Jie and Sánchez Reference Zong-Jie and Sánchez2012). Whereas the shell as a whole may be primarily for protection of the soft tissue inside the bivalve shell, ornamentation on the shell may serve a variety of purposes. Bivalve ornamentation has been postulated to be important for a variety of functions including maintaining a stable life position in the sediment, burrowing, shell strengthening, directing inhalant and exhalant currents, and protecting against predators (e.g., Trueman et al. Reference Trueman, Brand and Davis1966; Carter Reference Carter1967, Reference Carter1968; Kauffman Reference Kauffman1969; Stanley Reference Stanley1970, Reference Stanley1981, Reference Stanley1988; Aller Reference Aller1974; Thomas Reference Thomas1975; Arua and Hoque Reference Arua and Hoque1987; Harper and Skelton Reference Harper and Skelton1993; Kelley and Hansen Reference Kelley and Hansen1996; Stone Reference Stone1998; Harper and Kelley Reference Harper and Kelley2012). An anti-predatory function of ornamentation has also been suggested for Paleozoic and Mesozoic brachiopods (Leighton Reference Leighton2001, Reference Leighton2003; Vörös Reference Vörös2010; Johnsen et al. Reference Johnsen, Ahmed and Leighton2013), Cretaceous and Paleogene ostracods (Reyment Reference Reyment1967; Reyment et al. Reference Reyment, Reyment and Honigstein1987), Jurassic nautiloids and ammonoids (Bardhan and Halder Reference Bardhan and Halder2000; Kröger Reference Kröger2002), modern barnacles (Palmer Reference Palmer1982), and modern and fossil marine and continental gastropods (e.g., Bertness and Cunningham Reference Bertness and Cunningham1981; Arua and Hoque Reference Arua and Hoque1987; Donovan et al. Reference Donovan, Danko and Carefoot1999; Paul et al. Reference Paul, Das, Bardhan and Mondal2013; Liew and Schilthuizen Reference Liew and Schilthuizen2014), although Yochelson et al. (Reference Yochelson, Dockery and Wolf1983) suggested that predation intensity on the sub-Holocene scaphopod Dentalium laqueatum increased with more ribs.
Various types of bivalve ornamentation have been proposed to serve an anti-predatory function, although Leonard-Pingel and Jackson (Reference Leonard-Pingel and Jackson2013) suggested ornamentation was not effective against drilling predation in a study that lumped all types of ornamentation and used a variety of bivalves. Spines have been argued to prevent predation (Carter Reference Carter1967; Stone Reference Stone1998); spines may promote epibiont settling, thus camouflaging epifaunal bivalves from predators (Feifarek Reference Feifarek1987; Carlson Jones Reference Carlson Jones2003; Mackensen et al. Reference Mackensen, Brey, Bock and Luna2012; but see Willman Reference Willman2007). An increase in the strength of radial ribs in Cretaceous exogyrine bivalves was linked to durophagous predation (Dietl et al. Reference Dietl, Alexander and Bien2000). The latter was supported by Alexander and Dietl (Reference Alexander and Dietl2003), who argued that radial ribs are more common in modern tropical bivalves experiencing a higher intensity of predation than in bivalves in more temperate waters. Concentric ornamentation has received relatively little study with regard to drilling predation, with some indications that concentric ribs may affect drilling behavior in that drill holes tend to be located between smooth-topped concentric ribs (Arua and Hoque Reference Arua and Hoque1989; Klompmaker Reference Klompmaker2011) or concentric lamellae (Ansell and Morton Reference Ansell and Morton1985; Anderson et al. Reference Anderson, Geary, Nehm and Allmon1991). Drill holes between this type of ornament have also been figured in the literature for ribs (e.g., Hayasaka Reference Hayasaka1933: Pl. 1; Simões et al. Reference Simões, Rodrigues and Kowalewski2007: Fig. 5B,C; Ottens et al. Reference Ottens, Dietl, Kelley and Stanford2012: Fig. 2C) and lamellae (e.g., Robba and Ostinelli Reference Robba and Ostinelli1975: Pl. 43.1; Hingston Reference Hingston1985: Fig. 6A; Roopnarine and Beussink Reference Roopnarine and Beussink1999: Fig. 1). These observations may suggest that ribs and lamellae are ineffective against this type of predation. However, the strength of concentric ornament varies greatly among bivalves and the effectiveness of drill holes on ribs is largely unknown. Thus, it is premature to suggest that concentric ornament fails to serve as a protection against drilling predators such as muricid and naticid gastropods, which are important predators of bivalves in modern and ancient oceans (e.g., Kabat Reference Kabat1990; Kowalewski Reference Kowalewski1993; Kelley and Hansen Reference Kelley and Hansen2003; Dietl et al. Reference Dietl, Herbert and Vermeij2004; Klompmaker Reference Klompmaker2009; Sawyer and Zuschin Reference Sawyer and Zuschin2010; Chattopadhyay et al. Reference Chattopadhyay, Zuschin and Tomašových2013).

Figure 1 Specimens of the four studied Cenozoic bivalve species showing complete and incomplete drill holes. A, B. Astarte radiata from the Miocene of Miste, Breda Formation, The Netherlands (RGM.794165K–794165L). C–F, Astarte goldfussi from the Miocene of Miste, Breda Formation, The Netherlands (RGM.607538K–607538N). G–J, Lirophora glyptocyma from the Miocene of Oak Grove, Oak Grove Sand, Florida (UF 244747–244750). K–L, Lirophora latilirata from the Pliocene of the Acline Borrow Pits 01, Tamiami Formation, Florida (UF 244751–244752). Scale bars, 2.0 mm.
We here address the degree to which ornamentation is effective against drilling predation by studying several bivalve species with varying strengths of smooth-topped concentric ribs. We investigate (1) the percentage of incomplete drill holes per species, (2) the stereotypy of drill hole site location per species, (3) the drilling frequency of two congeneric species with different ornamentation from the same locality, (4) the proportion of incomplete drill holes on ribs versus between ribs, and (5) drill hole size on ribs versus between ribs. Our results show that concentric ribs do influence the drilling behavior and success of gastropods. We also assess whether this type of shell ornamentation serves as an adaptation or exaptation to drilling predation (see discussion in Harper Reference Harper2006 and Harper and Kelley Reference Harper and Kelley2012). The definitions of adaptation and exaptation used herein follow Gould and Vrba (Reference Gould and Vrba1982): adaptations are features built by natural selection for their current role, whereas exaptation refers to characters that evolved for other usages or for no particular function and were co-opted later for their current role.
Materials and Methods
To test the effectiveness against drilling predation of smooth-topped concentric ribs that are symmetrical in cross-section, we examined gastropod drill hole size, position, and completeness for four Cenozoic bivalve species (Fig. 1). All display ribs that are approximately equal in width to the spaces (valleys) between them, but they differ in coarseness of ribs (Astarte radiata, A. goldfussi, Lirophora glyptocyma, and L. latilirata, ordered by increasing rib strength). All four species are relatively sedentary because of their concentric ribs (Stanley Reference Stanley1970), so morphology rather than mobility should be of prime importance in defense. Specimens of Astarte were collected at the Miste locality near Winterswijk, Gelderland province, the eastern Netherlands (Janssen Reference Janssen1984), from middle Miocene (“Hemmoorian”) sediments assigned to the Miste Bed, Aalten Member, Breda Formation (Reference Van den Bosch, Cadee and JanssenVan den Bosch et al. 1975). More specifically, specimens of A. goldfussi and A. radiata were collected from the Hiatella arctica acme zone or the base of the A. radiata acme zone within the Miste Bed. Specimens of L. glyptocyma were collected in the lower Miocene Alum Bluff Group (Oak Grove Sand) at the Oak Grove locality, Okaloosa County, Florida, U.S.A.; specimens of L. latilirata were collected from the Pliocene Tamiami Formation (Pinecrest beds) at the Acline Borrow Pits 01 locality, Charlotte County, Florida, U.S.A.
Antero-posterior shell length was measured using calipers accurate to ≤0.03 mm. Because drilling parameters may vary with prey size (Ottens et al. Reference Ottens, Dietl, Kelley and Stanford2012), interspecific comparisons of drilling parameters were conducted at a standardized shell length. A length of 5.1–10.0 mm was selected for data standardization because of the abundance of specimens of this range in these faunas.
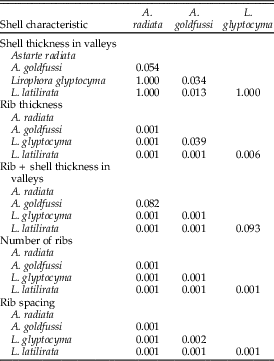
Rib characteristics were compared for ten undrilled specimens in the length range of 9.1–10.0 mm for each species. The number of concentric ribs was tallied and total shell thickness (including the rib) was measured at the second rib from the commissure. Shell thickness between ribs was measured midway between the anterior and posterior margin in the valley dorsal or ventral to the second rib from the commissure, depending on which was more accessible. Rib thickness of each specimen was calculated by subtracting the shell thickness between the ribs from the total shell thickness. Rib spacing was determined by measuring the distance from the top of the rib to the next rib around rib 2 or 3 from the commissure. Shell thickness between ribs, rib thickness, the number of ribs per species, and rib spacing were compared using the nonparametric Kruskal-Wallis test instead of ANOVA, because not all data followed a normal distribution. The null hypothesis is that the samples are taken from populations with equal medians for the three parameters tested. The subsequent Bonferroni corrected Mann-Whitney pairwise comparisons tests were used in PAST. For all tests, alpha is 0.05.
Drill holes were categorized as complete (penetrating to the interior of the valve) or incomplete; incomplete drill holes generally indicate failed predation attempts (Kelley and Hansen Reference Kelley and Hansen2003; Visaggi et al. Reference Visaggi, Dietl and Kelley2013). The percentage of drill holes that were incomplete (termed prey effectiveness, PE, by Vermeij [Reference Vermeij1987]) was determined for each species. Drilling frequencies (DF) at the standardized size were determined only for the two species of Astarte, both from the same locality and stratigraphic age, by dividing the number of completely drilled valves by one-half the total number of valves, to account for the fact that each Astarte individual consists of two valves. We compared drilling frequencies for these two species by using a Pearson's chi squared test. DFs were not calculated for the two other species because samples differed in age and/or locality, implying that comparing those values is not meaningful because differences in DF would not be attributable solely to rib strength.
The location of the center of each complete or incomplete drill hole was categorized as “between ribs,” “on rib,” or “other” for drilled specimens of each species. The latter category refers to those holes that were centered on the boundary between a valley and rib, those that were too large relative to the ribs to determine the location of the center (see Fig. 1A), or those situated on the lunule or escutcheon. Six pairwise Fisher’s exact tests compared the number of occurrences in the three drill hole categories for each of the four species, based on the null hypothesis that proportions of drill holes are equal for the three drill hole locations. A Bonferroni corrected alpha of 0.0083 (instead of 0.05) was used.
For the subset of drill holes small enough to fit completely between adjacent ribs, the category “between ribs,” “on rib,” or “middle” was assigned for each drill hole; those that were placed on the lunule or escutcheon were excluded from this analysis. “Middle” refers to drill holes that are located partly in the valley and partly on the rib. This analysis was not possible for Astarte radiata because of the close spacing of ribs. For A. goldfussi we used additional specimens from the same collection, horizon, and locality to increase sample size of drill holes that fit between ribs; no additional specimens were available for L. glyptocyma. A Fisher’s exact test was performed to compare A. goldfussi and L. latilirata given their sufficient sample size (~30 specimens each).
We tested the effect of ribs on the completeness of drill holes for several coarse-ribbed species as described below. Samples with at least 30 drilled specimens with holes of any size relative to ribs that were centered either on or between the ribs were studied for this purpose. Positions of incomplete and complete drill holes were compared for A. goldfussi and L. glyptocyma (length 5.1–10.0 mm). In order to increase the number of samples, we also conducted comparisons for several additional samples of Lirophora spp., using all drilled specimens regardless of length: L. athleta from the Jackson Bluff locality, Jackson Bluff Formation (Cancellaria zone), upper Pliocene, Florida, U.S.A.; L. athleta from the Richardson Road Shell Pit locality, Tamiami Formation (Pinecrest beds), Pliocene, Florida, U.S.A.; L. glyptocyma (locality and age as above); L. latilirata from the Register Quarry near Old Dock (lower Waccamaw Formation), lower Pleistocene, North Carolina, U.S.A. Pairwise Fisher’s exact tests were conducted on the number (and percentage) of incomplete and complete drill holes for drill holes on ribs and between ribs using an alpha of 0.05. The null hypothesis is that the percentage of incomplete drill holes on ribs is equal to that between ribs.
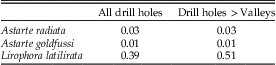
To investigate whether complete and incomplete drill holes differed in size, we measured with calipers the outer drill hole diameters of drilled specimens of A. goldfussi and A. radiata (The Netherlands) and L. glyptocyma and L. latilirata from Florida (all in the length range of 5.1–10.0 mm) and of all sizes of L. latilirata from the Register Quarry in North Carolina. Where permitted by sample size (restricted to at least 30 drilled specimens, at least ten of which exhibited an incomplete drill hole), results were analyzed separately for holes of any size relative to the ribs, holes larger than the valley between ribs, and holes that fit between the ribs. We used Mann-Whitney U-tests with alpha of 0.05 to test the null hypothesis that the sizes of incomplete and complete drill holes do not differ within a sample.
To test whether the rib strength of Astarte spp. changed during the time interval in which drilling became more abundant (Late Cretaceous–Paleocene [see Kowalewski et al. Reference Kowalewski, Dulai and Fürsich1998; Huntley and Kowalewski Reference Huntley and Kowalewski2007]), figured occurrences of Jurassic–Paleogene Astarte from North America were classified from 1 (weak ribs) to 5 (strong ribs) at the period-level and the mean was calculated for each period. We used a Kruskal-Wallis test to investigate the hypothesis that the medians are equal using an alpha of 0.05. For data on occurrences and references to articles containing the figures we consulted the Paleobiology Database (2014): Jurassic (Cragin and Stanton Reference Cragin and Stanton1905; Jaworski Reference Jaworski1929; Fürsich Reference Fürsich1982; Poulton Reference Poulton1991; Vega and Lawton Reference Vega and Lawton2011); Cretaceous (Imlay Reference Imlay1940; Stoyanow Reference Stoyanow1949; Cserna Reference Cserna1956; Richards et al. Reference Richards, Whyte Cooke, Garner, Howell, Jeletzky, Miller, Miller, Ramsdell, Reeside, Roberts and Wells1958; Perrilliat et al. Reference Perrilliat, Vega and Corona2000); Paleogene (Dockery Reference Dockery1982; Rossbach and Carter Reference Rossbach and Carter1991). North American occurrences of Astarte for this time interval were common and figured frequently, relative to those from northwestern Europe. The same method could not be used for Lirophora because its earliest occurrence is Cenozoic in age (Paleobiology Database 2014).
Specimens exhibiting more than one drill hole, which was rare, were excluded from all analyses in all methods used in this research. All statistical analyses were performed in PAST 2.17 (Hammer et al. Reference Hammer, Harper and Ryan2001).
Institutional abbreviations: UF – University of Florida, Florida Museum of Natural History (Invertebrate Paleontology), Gainesville, Florida.; RGM – Naturalis Biodiversity Center, Leiden, The Netherlands.
Results
Shell Characteristics
Kruskal-Wallis tests comparing shell thickness between the ribs, rib thickness, total shell thickness, and the number of ribs all returned p-values <0.05, suggesting that the medians differ among species. Results of the subsequent Mann-Whitney tests are as follows: Astarte radiata, Lirophora glyptocyma, and L. latilirata have a statistically indistinguishable shell thickness between the ribs for the length range of 9.1–10.0 mm, whereas this thickness is less for A. goldfussi than for Lirophora spp. (Table 1, Fig. 2A). Rib thickness, and consequently total shell thickness, significantly increases from A. radiata to L. latilirata; nearly all pairwise comparisons between species are statistically significant. Conversely, the number of ribs decreases from A. radiata to L. latilirata (Table 1, Fig. 2B). Rib spacing increases significantly from A. radiata to L. latilirata (Table 1, Fig. 2C). As the thickness of ribs increases so does rib width and spacing, leaving space for fewer ribs, which results in an inverse relationship of rib thickness and number of ribs.

Figure 2 Rib thickness, number, and spacing based on ten specimens for each of the four species within the length range 9.1–10.0 mm. A, Total mean thickness of the shell consisting of the thickness of the rib and the shell thickness between the ribs. B, Mean number of ribs. C, Mean rib spacing. Error bars are±1 SD.
Table 1 p-values using the Mann-Whitney pairwise comparison test for four shell characteristics.
Incomplete Drill Holes, Drilling Frequency, and Placement of Drill Holes
The percentage of drill holes that are incomplete (i.e., prey effectiveness) is <10% for all species when data are size standardized. Strongly ribbed species of Lirophora have PE values <4% (Fig. 3). Although the prey effectiveness in the stronger-ribbed Astarte goldfussi appears higher than that of A. radiata (10.3 vs. 8.7%), the difference is not statistically significant (χ2=0.23, p=0.8911). Drilling frequency for A. radiata within the length range 5.1–10.0 mm is 0.343 (181 drilled valves of a total of 1055). In contrast, the stronger-ribbed shells of A. goldfussi have a significantly lower DF of 0.271 (183 / 1353) (χ2=6.09, p=0.013).

Figure 3 Percentage of drill holes that are incomplete for the four species, based on drilled specimens in the length range 5.1–10.0 mm.
The position of drill holes relative to ribs could not be determined for A. radiata because of the small size of the ribs relative to the drill holes. Our results for all other species indicate that the percentage of drill holes for which the center is located between the ribs increases with rib strength for drill holes of any size, from 10% (A. goldfussi) to 84% (L. latilirata). In contrast, the percentage of drill holes with the center on top of ribs decreases with increasing rib strength from A. goldfussi (40%) to L. latilirata (3%) (Table 2, Fig. 4A). Six pairwise Fisher’s exact tests on the number of occurrences in the three drill hole categories of the four species all returned p-values <0.0083 (= Bonferroni corrected alpha using 0.05), indicating that the drill holes are placed differently for every pair of species. The percentage of drill holes classified as “other” is lower in more strongly ribbed species. When only drill holes that could fit between the adjacent ribs are taken into account, A. goldfussi has a higher percentage of holes on ribs and a lower percentage of holes between ribs than L. latilirata (Table 2, Fig. 4B); the difference in drill hole placement is statistically significant (Fisher’s exact test: p<0.05).

Figure 4 Location of drill holes relative to the ribs of bivalve species with increasing rib strength (left to right) for specimens with length of 5.1–10.0 mm. A, The proportion of drill holes with the center located between the ribs increases, whereas the proportion of drill holes on ribs decreases, as rib strength increases. Specimen count: Astarte radiata=150; A. goldfussi=202; Lirophora glyptocyma=86; L. latilirata=38. B, The proportion of holes fitting entirely between the ribs is markedly higher for L. latilirata than for A. goldfussi. A bar could not be shown for A. radiata because none of the drill holes fit between the ribs; sample size for Lirophora glyptocyma was only 3. Specimen count: A. goldfussi=32; L. latilirata=28. “Other” refers to those holes that were centered on the boundary between a valley and rib, those that were too large relative to the ribs to determine the location of the center (see Fig. 1A), or those situated on the lunule or escutcheon. “Middle” refers to drill holes that are located partly in the valley and partly on the rib.
Table 2 Location of drill holes in specimens of four species.

As shown by the results of the Fisher’s exact tests, significantly more incomplete holes of any size occur on ribs than between ribs for half of the tests. Results appear influenced by the relatively low number of specimens, as two of the nonsignificant results are significant when Fisher’s exacts tests were performed on percentages instead of numbers (Table 3). In the latter case, all samples except Lirophora glyptocyma with a length range of 5.1–10.0 mm returned significant differences (Fig. 5).

Figure 5 Proportions of incomplete and complete drill holes with the center on the rib and between ribs for eight samples with at least 30 drilled specimens. A–D, Specimens of all sizes included. A, Lirophora athleta, Jackson Bluff Formation, upper Pliocene, Florida (upper: 15 specimens; lower: 19 specimens). B, L. athleta, Tamiami Formation, Pliocene, Florida (22 and 42 specimens). C, L. glyptocyma, Alum Bluff Group (Oak Grove Sand), lower Miocene, Florida (20 and 92 specimens). D, L. latilirata, Waccamaw Formation, lower Pleistocene, North Carolina (121 and 138 specimens). E–F, Specimens with lengths from 5.1–10.0 mm. E, Astarte goldfussi, Breda Formation, middle Miocene, The Netherlands (82 and 21 specimens). F, L. glyptocyma (as above but with restricted length range of 5.1–10.0 mm) (9 and 30 specimens).
Table 3 Results of Fisher’s exact tests comparing the occurrence of complete vs. incomplete drill holes on ribs and between ribs for numbers and percentages.

Drill Hole Size and Ribs
Statistical comparisons of the drill hole diameter of complete and incomplete holes could be carried out only for the North Carolina samples of Lirophora latilirata and the two species of Astarte. We could not use Florida samples of L. latilirata or L. glyptocyma because the minimum number of incomplete drill holes was not reached, as was the case for drill holes that could fit between the ribs for both species of Astarte and L. latilirata (NC). For categories with sufficient numbers of drilled specimens, our results indicate that all mean drill hole sizes for incomplete drill holes are smaller than those for complete drill holes. The results are significant for both species of Astarte but not for L. latilirata (Table 4, Fig. 6), including when the size range is restricted to 25.1–30.0 mm. Data on sizes of drill holes present in each species can be found in the Supplementary Table.

Figure 6 Mean outer drill hole diameter for complete and incomplete drill holes. A, Astarte radiata, 5.1–10.0 mm in length. B, Astarte goldfussi, 5.1–10.0 mm. C, Lirophora latilirata (North Carolina), all lengths. Error bars are±1 SD.
Table 4 p-values using the Mann-Whitney U-test comparing drill hole size of complete and incomplete drill holes. “Drill holes >Valleys” means that the outer diameter of the drill holes is greater than the width of the valley.
Rib Strength in Astarte
Despite a limited sample size, rib strength of North American Astarte remained relatively stable through the studied time interval: Jurassic (2.4, 7 occurrences), Cretaceous (2.5, 6 occurrences), and Paleogene (2.5, 4 occurrences). The Kruskal-Wallis test returned a p-value of 1, suggesting that the medians are not statistically different. Because rib strength and rib spacing are positively correlated (r 2=0.997; p=0.001) based on data underlying Figures 2A and 2C, we can conclude that rib spacing also did not change.
Discussion
Identity of the Predator
The (subcircular) outline of the drill holes, three-dimensional shape, size, and orientation perpendicular to the shell surface all point to drill holes produced by gastropods. The beveled shape of most drill holes (e.g., Fig. 1) suggests predation by naticid gastropods, though muricid drillers likely produced some of the holes studied here. Specimens of both Muricidae and Naticidae are common in all stratigraphic levels studied (Janssen Reference Janssen1984; Florida Museum of Natural History Invertebrate Paleontology Online Database 2013).
Comparing Species from Multiple Localities and Stratigraphic Levels
This study uses material from multiple locations and stratigraphic levels because we are unaware of an assemblage of related species from the same locality and age displaying such a broad range in rib morphology as studied herein. This approach raises the question of whether results can be compared among samples. Because the number and identity of prey and predators can differ among sedimentary environments, we do not compare drilling frequencies of taxa from different localities and ages. However, we argue that other measures such as the location of complete and incomplete drill holes and the percentage of incomplete drill holes can be compared in this case because (1) the taxa have a generally similar shape (Fig. 1), suggesting similarity of life mode; (2) a similar size range (5.1–10.0 mm length) was used for many analyses; (3) the ribs are comparable in shape across taxa; (4) the ratio of rib width to valley width is about equal regardless of rib size; (5) some analyses are within a taxon/sample; and (6) an overlapping range of drill hole sizes is present (Supplementary Table), suggesting that predator size may have been largely comparable. We assume that drilling gastropods had a similar drilling behavior for the localities studied. The common gastropod drilling predators, naticids and muricids, were both present in all deposits (see above).
Incomplete Drill Holes and Placement of Drill Holes
The percentage of incomplete drill holes tends to be lower in strongly ribbed species (Fig. 3). In contrast, Hingston (Reference Hingston1985: Table 4) noted that the heavily ornamented bivalve Placamen subroboratum had a high percentage of incomplete drill holes relative to moderately ornamented to smooth bivalves from a Pliocene assemblage from SE Australia, but results were not standardized with respect to bivalve shape and size and the shell thickness without the ornamentation is not provided. Our results can be explained by the placement of drill holes: in stronger-ribbed species more drill holes tend to be placed between ribs, where the shell thickness to penetrate is less (Figs. 2, 4). Thus as rib strength and spacing increased, gastropod predators selected the drill hole site with increasing stereotypy. This result suggests that natural selection favors gastropods that select drill hole sites between the ribs, thus saving time and energy, as rib strength increases.
Drill Hole Frequency in Astarte spp
The stronger-ribbed shells of Astarte goldfussi have a significantly lower DF than the congeneric A. radiata from the same formation, horizon, and locality for the studied length range, implying that gastropods favored the smoother A. radiata. The similar shell shape and close phylogenetic relationship suggests that both species had a similar life mode and should have been equally susceptible to drilling. Both species are very common in the museum collections examined (>500 specimens), suggesting that relative abundance did not control drilling frequency. Therefore the lower DF in A. goldfussi likely can be attributed to the increased rib height (Fig. 1), given that the gastropods did not preferentially select a drill hole site between the ribs in either species (Fig. 4). This conclusion may be supported by the higher percentage of incomplete drill holes in A. goldfussi, although the difference is not statistically significant. Thus, the moderate-sized ribs in A. goldfussi appear effective against drilling predation because of the lack of stereotypy in drill hole site selection.
Drilling Success and Ribs
Drilling is generally more successful between the ribs than on the ribs in most cases (Table 3, Fig. 5), as might be expected given the greater overall shell thickness on ribs. Greatly thickened bivalve shells are known to inhibit drilling (e.g., Vermeij Reference Vermeij1978), and Kelley (Reference Kelley1989) reported that increases in shell thickness in several Miocene bivalves correlated with decreases in successful drilling and increases in prey effectiveness. Addition of ribs provides a cost-effective means of increasing apparent shell thickness when drill holes are placed on ribs (see e.g. Ansell and Morton Reference Ansell and Morton1985). Fisher’s exact tests on the percentages yield more significantly different results than tests based on the number of specimens, suggesting that more of these tests using the number of specimens would be significant if sample size were increased. Our result is based on Miocene–Pleistocene samples of Lirophora spp. and Astarte goldfussi from a variety of localities, suggesting that this pattern is consistent across time and space.
Drill Hole Size and Ribs
Juvenile gastropods have been reported to drill prey from different phyla than those attacked by adult individuals (e.g., Maddocks Reference Maddocks1988; Klompmaker Reference Klompmaker2012). Young gastropods also preyed upon different taxa within the same phylum and class compared to adults (Broom Reference Broom1983, for bivalves). Drill hole position may also change during ontogeny of the driller (e.g., Calvet i Catà Reference Calvet i Catà1992; Złotnik Reference Złotnik2001). Specifically, Złotnik (Reference Złotnik2001) noted that when drilling Corbula gibba, large naticids displayed greater site selectivity than smaller individuals. The latter observation is in line with our results, as the outer drill hole diameter is usually significantly smaller for incomplete drill holes, suggesting that younger individuals produced these holes, which were often placed on ribs. Alternatively, adults of taxa with a small maximum size drilled preferentially on ribs, an explanation that seems less likely.
Drilling Behavior and Ornamentation
Drill hole site selection by naticid gastropods has been studied widely and was reviewed by Kabat (Reference Kabat1990), who concluded that no factor (shape of the prey, shell thickness, ornamentation, location of tissue, prey handling by predator, and prey size) solely determines the drill hole site for bivalves as a group. However, studies on individual bivalve prey have made strong cases for ornamentation playing an important role in determining the siting location and DF. For example, Ansell and Morton (Reference Ansell and Morton1985) found that Bassina calophylla exhibiting pronounced, raised, sharp, concentric lamellae was not drilled by Glossaulax didyma. However, when the lamellae were removed this naticid gastropod did drill B. calophylla. They hypothesized that these lamellae may have confused predators by offering false valve margins, interfered with prey manipulation, and increased the perceived shell thickness. From their examination of empty shells from Mirs Bay (Hong Kong), Ansell and Morton (Reference Ansell and Morton1985) further showed that drill holes were mainly located between the lamellae (their Fig. 5). This observation is in line with that of Anderson et al. (Reference Anderson, Geary, Nehm and Allmon1991), who qualitatively noted that naticid drill holes were located between the concentric lamellae of Chione cancellata. Comparable results are also seen in most bivalves with smooth-topped ribs. Arua and Hoque (Reference Arua and Hoque1989) showed that 58.1% of the 308 drill holes in Glans costaenodulensis were located between the ribs, 82.2% (n=17) of the drill holes in G. triparticostata were situated between the ribs, and 14.3% (n=14) of the drill holes in G. costaeirregularis were located between the ribs. All species of Glans exhibit strong, seemingly smooth-topped radial ribs. One of the five (20%) drill holes in Protonoetia nigeriensis was found between the ribs (Arua and Hoque Reference Arua and Hoque1989), and P. nigeriensis showed less pronounced ribs (Arua Reference Arua1986: Pl. 9, 10). Furthermore, based on a small sample of Miocene Astarte anus, Klompmaker (Reference Klompmaker2011) noted that five of seven drill holes were located between the strong, smooth-topped concentric ribs.
These and other studies have not addressed the influence of space between ribs in detail. This factor becomes especially important here, because rib strength and rib spacing increase together (Fig. 2), raising the question as to which feature is most important in determining the siting of drill holes. Several aspects suggest that ornamentation strength is the most important trait. (1) We minimized the importance of rib spacing first by analyzing only those drill holes that fit between ribs and then by excluding drill holes that fit between ribs. In each case, the pattern of drill hole siting is similar to that when all sizes of drill holes are used (compare Fig. 4A with 4B; Table 2). (2) A higher DF is observed in the weaker ribbed of two Astarte species. Because no drill hole site is favored in these two species (Fig. 4A), rib spacing is not a complicating factor. However, for Lirophora, both rib strength and spacing may be important. (3) More incomplete drill holes occur on ribs than between ribs (Fig. 5). (4) Although Ansell and Morton (Reference Ansell and Morton1985) studied a different type of concentric ornamentation, they found that the naticid Glossaulax didyma did not drill the bivalve Bassina calophylla when concentric lamellae were present, but did when lamellae were removed. Thus our results support the hypothesis that ornamentation influences the siting of drill holes, as siting between ribs is much more pronounced in bivalves with stronger concentric ribs. Spacing between ribs appears less important.
Function of Concentric Ornamentation
Stanley (Reference Stanley1970) suggested that concentric ornamentation is resistant to burrowing by astartid and venerid bivalves, species of which are analyzed in this study. In a more detailed study of venerids, however, Stanley (Reference Stanley1981) reported that concentric ribs that are asymmetrical in cross-section help in burrowing, whereas symmetrical concentric ribs do not. The latter arrangement decreases scouring, so specimens remain buried more effectively. All species studied here exhibit symmetrical concentric ribs, suggesting that they may have been buried shallowly with the ribs serving to reduce scour (however, rib size varies significantly among species, and ribs are generally more robust than those tested by Stanley Reference Stanley1981). Whether smooth-topped concentric ribs in bivalves function against durophagous predation has not been investigated in detail, although this interpretation was suggested cursorily by Alexander and Dietl (Reference Alexander and Dietl2003) and appears plausible given that other types of ornament are effective against predation (e.g., Stanley Reference Stanley1988). More specifically, radial ribs and ornamentation in general appear to deter durophagous predation for bivalves (Dietl et al. Reference Dietl, Alexander and Bien2000; Alexander and Dietl Reference Alexander and Dietl2003).
To determine whether a morphological characteristic that functions to deter predation is an adaptation or an exaptation, previous work has examined the first occurrence of the antipredatory trait. For example, conchiolin layers in corbulid and lucinid bivalves have been considered to be an antipredatory exaptation by Kardon (Reference Kardon1998) and Ishikawa and Kase (Reference Ishikawa and Kase2007), but Harper (Reference Harper1994) argued these layers were an adaptation to drilling predation. Both Harper (Reference Harper1994) and Kardon (Reference Kardon1998) used the earliest occurrence of conchiolin in the fossil record to come to their conclusions. Concentric ribs in bivalves evolved in the Paleozoic and became more common in the Mesozoic (Stanley Reference Stanley1981; Vermeij Reference Vermeij1995). Concentric rib-bearing astartid and venerid bivalves, species of which are studied herein, became diverse during the Jurassic and Cretaceous, respectively (Cox et al. Reference Cox, Newell, Boyd, Branson, Raymond, Chavan, Coogan, Dechaseaux, Fleming, Haas, Hertlein, Kauffman, Keen, LaRocque, McAlester, Moore, Nuttall, Perkins, Puri, Smith, Soot-Ryen, Stenzel, Trueman, Turner and Weir1969; Paleobiology Database 2013). Although Lirophora spp. are known from the Cenozoic only (e.g., Cox et al. Reference Cox, Newell, Boyd, Branson, Raymond, Chavan, Coogan, Dechaseaux, Fleming, Haas, Hertlein, Kauffman, Keen, LaRocque, McAlester, Moore, Nuttall, Perkins, Puri, Smith, Soot-Ryen, Stenzel, Trueman, Turner and Weir1969), Cretaceous venerids show weak to moderate-sized concentric ornamentation (e.g., Wade Reference Wade1926; Imlay Reference Imlay1961; Sealey and Lucas Reference Sealey and Lucas2003). Astarte is known from the Paleozoic and became speciose in the Jurassic (Paleobiology Database 2013); moderate-sized concentric ribs, comparable to those in the Miocene Astarte spp. studied here, were present since at least the Triassic for this lineage (McRoberts and Blodgett Reference McRoberts and Blodgett2000; Yin and McRoberts Reference Yin and McRoberts2006). Conversely, drilling frequencies were generally low in the Neoproterozoic, Paleozoic, and Mesozoic, but were higher in the Late Cretaceous–Cenozoic (Vermeij Reference Vermeij1987; Kelley and Hansen Reference Kelley and Hansen1993, Reference Kelley and Hansen2003; Kowalewski et al. Reference Kowalewski, Dulai and Fürsich1998; Huntley and Kowalewski Reference Huntley and Kowalewski2007).
Ishikawa and Kase (Reference Ishikawa and Kase2007) used absence of a temporal trend, among other reasons, as an argument that conchiolin layers were an exaptation to drilling predation. Similarly, our analysis shows that rib strength of North American Astarte remained stable during the Jurassic–Paleogene as drilling gastropods diversified. All of the above evidence suggests that smooth-topped, symmetrical ribs did not evolve as an anti-predatory trait against drilling predation. Our results show that, when ribs are moderately strong and when drill holes are placed on top of ribs, concentric ribs can be effective against drilling predators. Furthermore, Stanley (Reference Stanley1981) suggested that concentric ribs in bivalves are adaptations to burrowing and/or stabilizing the sediment. It is also possible that these ribs evolved as an adaptation to durophagous predation. In sum, the concentric ribs herein can be viewed as a likely example of exaptation with regard to drilling predation. Smooth-topped, symmetrical concentric ribs can thus fulfill a variety of functions.
Our conclusion that smooth-topped concentric ribs are likely an antipredatory exaptation with regard to drilling predation would be strengthened if phylogenies were available for these species (see Blackburn Reference Blackburn2002). Nevertheless, claims concerning adaptations versus exaptations by Kardon (Reference Kardon1998), Ishikawa and Kase (Reference Ishikawa and Kase2007), and Harper (Reference Harper1994) were made without a phylogenetic context, most likely because of the lack of phylogenies at low taxonomic ranks. Other examples without a phylogenetic framework hypothesizing that ornamentation evolved through adaptation to drilling predation include work on Paleozoic brachiopods (Leighton Reference Leighton2001, Reference Leighton2003). Adaptation against durophagous predators through ornamentation on bivalves was suggested by Dietl et al. (Reference Dietl, Alexander and Bien2000) and Han et al. (Reference Han, Todd, Chou, Bing and Sivaloganathan2008), but only the former made use of a phylogenetic framework (i.e., “lineages”).
To our knowledge, this study is one of the first examples investigating the effectiveness of drilling predation across prey with a range of the same type of ornamentation. This type of study could be extended to asymmetrical concentric ribs, radial ribs, spines, and other types of ornamentation in a variety of mollusks. Data on the first appearance and abundance of these characters may help determine whether these ornaments are exaptive or adaptive traits or ineffective against drilling and/or durophagous predation in bivalves and other marine invertebrates. Whether ornamentation such as concentric ribs played a role in drill hole site selection by early representatives of naticid and muricid gastropods during the late Mesozoic could also be addressed by other studies. If a different stereotypic drilling pattern or no stereotypy at all were found, then the effectiveness of ribs in deterring drilling predation might have been different during that time.
Conclusions
1. The percentage of drill holes located between the ribs on bivalve species with concentric ribs increases with increasing rib strength, whereas the percentage of drill holes on top of ribs decreases. This result supports the hypothesis that ornamentation influences the siting of drill holes, as siting between ribs is much more pronounced in bivalves with stronger concentric ribs. Thus, natural selection favors gastropods that select drill hole sites between the ribs.
2. The lack of stereotypy in drill hole site selection suggests that the moderately sized ribs in Astarte goldfussi were effective against drilling predation.
3. The percentage of drill holes that are incomplete is generally lower in strongly ribbed species.
4. The proportion of drill holes that are located on top of ribs is greater for incomplete than complete holes, implying that ribs can be effective against predators in these cases.
5. The outer drill hole diameter is usually significantly smaller for incomplete drill holes, suggesting that younger individuals produced these holes.
6. Moderately strong ribbing considered herein serves as a likely exaptation against drilling predation, especially when drill holes are placed on top of ribs.
7. The functions of smooth-topped concentric ribs are manifold, as they can not only deter durophagous and drilling predation, but also serve to stabilize the bivalve in the sediment.
Acknowledgments
R. Pouwer and F. P. Wesselingh (both Naturalis Biodiversity Center, Leiden, The Netherlands) are thanked for access to and shipping of specimens of Astarte spp. We thank K. Hattori, N. Moore, S. Simpson, and A. Zappulla for assistance in collecting and measuring specimens from Register Quarry, North Carolina; C. Visaggi also helped with collecting in North Carolina. R. W. Portell and C. M. Robins (both Florida Museum of Natural History, Gainesville) are thanked for assistance with the collections of the Florida Museum of Natural History. Input from two anonymous reviewers, G. Dietl (Paleontological Research Institution and Cornell University, Ithaca, New York), and E. M. Harper (University of Cambridge, Cambridge, U.K.) improved this paper substantially. This work was supported by the Jon L. and Beverly A. Thompson Endowment Fund to the first author. This is University of Florida Contribution to Paleobiology 664, and PBDB publication 206.
Supplementary Material
Supplemental materials deposited at Dryad: doi:10.5061/dryad.f79jb







