Rubella is a mild, exanthematous viral infection of children and older adults but poses a significant threat in pregnancy, causing congenital rubella syndrome (CRS) in the developing fetus. CRS can be significantly reduced and even eliminated by appropriate vaccination strategies [Reference Robinson1, Reference Plotkin2]. In India, rubella remains endemic as MMR vaccine is not part of the universal immunization programme; rubella is not a notifiable disease, there is no reliable surveillance system for CRS and rubella, and the vaccine is available only in the private sector [Reference Yadav, Wadhwa and Chakarvarti3, Reference Ramamurty4].
Epidemiological data on prevalence of CRS and rubella in the country is inadequate. In 2008, Gandhoke et al. [Reference Gandhoke5] reported a rise in in-utero rubella infections in Delhi during 2005–2006, even though women of childbearing age maintained a high level (95%) of immunity to rubella. Tamil Nadu reported 86·5% seropositivity in adolescent girls [Reference Ramamurty4]. Studies from Tamil Nadu have implicated rubella as a serious cause of congenital cataract [Reference Eckstein6, Reference Malathi, Therese and Madhavan7]. Deafness as a sole manifestation of CRS may be an unrecognized cause for retardation of intellectual development.
Elimination of rubella is possible as humans are the only reservoirs and vaccine efficacy is reportedly high [Reference Plotkin8]. In most countries, infant immunization has been the strategy for prevention of CRS. This approach is beneficial in areas with high vaccination coverage; there is a postulated increase in CRS if vaccination coverage falls below 80% as there is a shift of infection to an older age group. Improper vaccination policies coupled with limited availability of rubella vaccine in developing countries (only available in the private sector) increases incidence of CRS as reported from Sri Lanka and Greece [9–Reference Panagiotopoulos, Antoniadou and Valassi-Adam11].
WHO policy on rubella and CRS elimination is through routine immunization of infants and young children, routine surveillance, and ensuring rubella immunity in women of childbearing age [9]. In the USA, universal immunization of 1-year-old children and mass immunization of large numbers of children of pre-school age was adopted, while in the UK, girls aged 12–14 years were immunized. Both these policies were partially successful. In the UK, rubella vaccine later became part of the childhood immunization schedule and in USA, screening and immunization of seronegative women of childbearing age was prioritized. These revised strategies led to a reduction of CRS cases [Reference Robinson1, Reference Plotkin2].
In this paper we used information generated from routine clinical practice at Christian Medical College (CMC), Vellore, India, where serological testing is offered for diagnosis of CRS, postnatal rubella infection, and assessment of susceptibility to rubella. CMC is a non-profit-making, tertiary-care centre located in Vellore, 140 km from Chennai. It caters to a local population of 350 000 with concessional care being given to deserving cases. It extends its primary- and secondary-level care to 300 villages in Vellore through its peripheral hospitals and outreach programmes. Patients with acute illness are predominantly from the local population while those with chronic conditions (e.g. infants with disabilities) come from throughout India.
We attempted to answer three important questions:
(1) Is there any empirical evidence of increase in CRS cases in our institution?
(2) Do we have adult rubella activity in our region in South India?
(3) Does a significant proportion of women reach childbearing age without exposure to rubella?
Data from the diagnostic services of Department of Clinical Virology from January 2000 to December 2008 was analysed and included:
(i) Serum samples (n=646) from infants (0–1 year) presenting with suspected CRS at the Departments of Neonatology and Child Health with any one or more of the following clinical signs and symptoms:
• fever, pneumonia, bone lesions, lethargy;
• cataract, congenital heart disease, hearing deficiency;
• hepatosplenomegaly, jaundice, developmental delay.
A laboratory-confirmed case of CRS is an infant with clinically suspected CRS that is positive for anti-rubella IgM in a sample obtained within the first year of life, preferably before age 6 months.
A laboratory-confirmed postnatal rubella case is a clinically suspected rubella case that is positive for anti-rubella IgM in a sample obtained within 28 days after onset of rash.
(ii) Serum samples (n=329) from patients aged >1 year presenting with symptoms of suspected postnatal rubella (fever and rash) at the Departments of Child Health and Dermatology.
(iii) Serum samples (n=770) from women attending the Departments of Obstetrics and Gynecology and Reproductive Medicine Unit to assess susceptibility to rubella.
All CRS and postnatal rubella cases were tested for the presence of anti-rubella IgM and immunity status for anti-rubella IgG. Rubella-specific IgM and IgG antibodies were detected using commercially available Euroimmun (Lübeck, Germany) kits. Results of the semi-quantitative anti-rubella IgM assay were evaluated by calculating a ratio of the extinction value of the patient's sample over the extinction value of the calibrator. Any ratio ⩾1 was considered positive. Rubella IgG titres of samples are reported and interpretation of titres (protective, not protective or borderline) was according to the manufacturer's instructions. Clinicians at our institution recommend vaccination based on laboratory results. In all the assays performed, the extinction values of the calibrators, positive and negative controls, were within the limits prescribed by the manufacturer.
Of the 646 infants with suspected CRS, 61 (9·4%) had laboratory evidence of congenital rubella infection (Table 1). The ratio of congenitally infected males to females was 3:2. The proportion of suspected CRS cases that were laboratory confirmed increased from 4% in 2000 to 11% in 2008. The clinical charts of 50 of these CRS cases were available for study. Development delay, auditory insufficiency, hepatitis, cataract, hepatosplenomegaly and respiratory distress were the most common clinical features seen. In seven cases, antenatal rash with fever had been documented.
Table 1. Distribution of congenital rubella syndrome (CRS), rubella cases and immunity level in women of childbearing age, 2000–2008
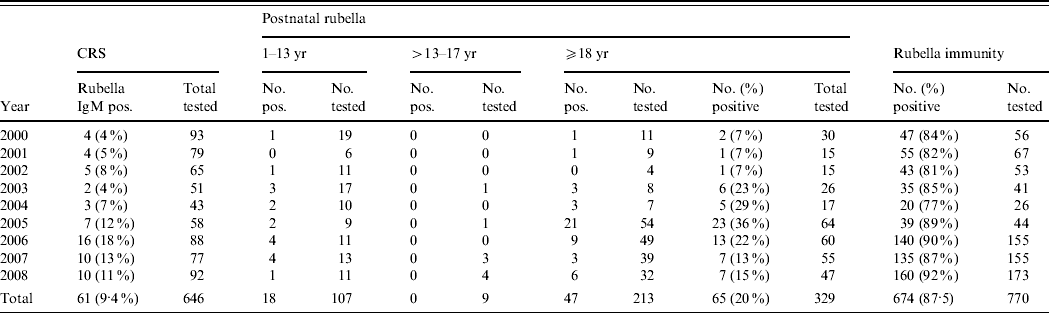
During the same period, samples from 329 clinically suspected postnatal rubella cases were tested of which 65 (20%) were laboratory confirmed. The ratio of clinically suspected rubella cases in males to females was 1:2·3 and that of confirmed rubella cases was 1:2, respectively. The ratio of paediatric postnatal cases to that of adults was 1:3. This could be due to the fact that pediatric cases of fever with rash rarely opt for laboratory confirmation. The average age of infected children was 6 years and 23 years in adults. Almost 91% of these cases occurred during the cooler months of the year (January–March and August–December).
There were no rubella cases in the 13–17 years age groups. There is clinical and laboratory evidence of rubella outbreaks in 2005 and 2006 in our hospital. In 2005, a large number of suspected rubella cases presented at the Staff Students Health Services of the institution. There were 13 laboratory-confirmed cases in healthcare workers and students. In early 2006, there was a small cluster of cases diagnosed at one of the peripheral rural hospitals attached to CMC. Most of these patients were children aged ⩽5 years.
The level of susceptibility to rubella (12·5%) in women of childbearing age (n=770) in this study is similar to previous reports from India. The shift in the mean age of first marriage in India is reflected by the large number of women in the 24–34 years age group (71%) in the study. Women in the 19–23 and ⩾35 years age groups showed better levels of immunity to rubella (91%) than those in the 24–34 years age group (85·5%).
CRS continues to be a cause of concern in developing countries as caring for children with permanent disabilities has high economic cost and social implications. Laboratory-confirmed cases, in paediatric and adult groups, continue to be seen at our institution signifying rubella virus circulation in most age groups. When viewed in the context of a large number of serologically confirmed postnatal cases in 2005, it is evident that a proportion of our population reaches adulthood without being exposed to natural infection or being vaccinated. Similar outbreaks of rubella have been reported from other parts of India [Reference Gandhoke5]. The reasons for few antenatal cases of rubella could be clinically unapparent rubella cases, unaffordable cost of laboratory testing and practice of seeking medical help from local practitioners rather than from tertiary-care centres. As adolescents may not seek clinical intervention even if they develop fever with rash, the complete absence of rubella in the 13–17 years age group may reflect age-related differences in accessing healthcare rather than the absence of disease as seen through this study. About 15% of women of childbearing age are susceptible to adult-onset rubella and hence, for potential congenital transmission.
This study provides preliminary data on rubella activity in South India and has some limitations. First, it is a hospital (tertiary care)-based study using secondary data and the population studied may not exactly reflect that community. Second, prospective studies at the community level and community-based surveillance is required to estimate the actual burden of disease. Extrapolating hospital-based data, as in our study, to the general population should be done with caution.
The initial vaccination strategies adopted to eliminate rubella in the US and UK had flaws; in the USA, pregnant women were still susceptible to rubella occurring in children and adults and in UK, circulation of rubella virus continued in the unvaccinated population (males, children and girls who refused vaccination). Vaccination strategies were revised on realizing that any reduction in CRS must target not only children of both sexes but also seronegative women of childbearing age. Further susceptibility could be attributed to migration of people from non-endemic rubella areas and/or areas of limited rubella vaccine use, and adverse publicity given to the vaccine.
Rubella epidemics were known to occur every 5–9 years prior to introduction of the vaccine, giving girls at least two opportunities to acquire immunity before they reach childbearing age. However, the periodicity of these epidemics depends on an adequate build-up of the number of susceptible individuals in the community. If the susceptible build-up is delayed, the inter-epidemic gap increases and more susceptible women reach childbearing age vulnerable to the devastating effects of the virus during pregnancy. Limited availability of this private-sector vaccine to the underprivileged also affects transmission dynamics and increases susceptibility in women of childbearing age. This partial coverage also increases the inter-epidemic gap.
Important recommendations of the WHO for CRS prevention are:
• Prevention of CRS can only be achieved by immunization of adolescent girls and/or women of childbearing age.
• Elimination of rubella and CRS can be achieved by universal immunization of infants and seronegative women of childbearing age [Reference Hinman12].
In countries like India, where vaccine coverage may be sub-optimal, immunization of adolescent girls (and boys) and adult women may be effective for prevention of CRS. Unlike childhood vaccination, adolescent and adult immunization has been proved to be better at reducing the number of women of childbearing age at risk of acquiring rubella infection [Reference Hinman12, Reference Anderson and May13]. Such a shift in strategy can only be done with an overview of the susceptibility profile of the target population, the acceptability of the shift in society and the economic feasibility of such a large-scale operation. However, problems in implementing vaccination programmes in different parts of India may not permit a single vaccination programme for the entire country. The estimated measles vaccine coverage in Tamil Nadu is about 98% and it would be good to ‘piggyback’ rubella vaccine on this successful programme with a switch from monovalent measles to MR or MMR vaccine.
Devising and implementing new immunization strategies in a culturally, socially, economically diverse country such as India would require active participation of physicians, laboratory scientists, epidemiologists, public health advisors and immunization advocacy groups.
DECLARATION OF INTEREST
None.



