Introduction
Substance use is an established risk factor for earlier and more severe psychotic outcomes (Andrade, Reference Andrade2016; Helle et al., Reference Helle, Ringen, Melle, Larsen, Gjestad, Johnsen and Løberg2016). Initiation of use typically occurs during adolescence, when the developing brain is especially vulnerable to the deleterious effects of substances (Degenhardt, Stockings, Patton, Hall, & Lynskey, Reference Degenhardt, Stockings, Patton, Hall and Lynskey2016; Gururajan, Manning, Klug, & van den Buuse, Reference Gururajan, Manning, Klug and van den Buuse2012). Exposure to substances may be particularly detrimental for young people who present risk factors for psychosis (Kelleher et al., Reference Kelleher, Connor, Clarke, Devlin, Harley and Cannon2012), with substance use interventions delivered to youth at high-risk of psychosis highlighted as a potential avenue for the prevention of psychotic disorders (Carney, Cotter, Firth, Bradshaw, & Yung, Reference Carney, Cotter, Firth, Bradshaw and Yung2017).
Previous reviews examining the relationship between substance use and psychosis have focused predominantly on cannabis and clinical psychosis outcomes. Among these, a systematic review indicated that cannabis use prior to the age of 18 years increased risk of an earlier onset of psychosis only among cases with more severe use and pre-existing vulnerability – that is, a family history of psychosis (Bagot, Milin, & Kaminer, Reference Bagot, Milin and Kaminer2015). Subsequent meta-analyses have described a dose–response relationship of increasing likelihood of psychosis with increasing use of cannabis (Marconi, Di Forti, Lewis, Murray, & Vassos, Reference Marconi, Di Forti, Lewis, Murray and Vassos2016), and reported that adolescent cannabis use increased the risk for psychosis and predicted an earlier onset of the disorder, with family history of psychosis, earlier age of onset and frequency of cannabis use, and concurrent use of other substances all strengthening the association (Kiburi, Molebatsi, Ntlantsana, & Lynskey, Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021). Another meta-analysis identified the age at onset of psychosis for cannabis users as 2.7 years younger than for non-users, and for those with broadly defined substance use, the age at onset of psychosis was 2.0 years younger (Large, Sharma, Compton, Slade, & Nielssen, Reference Large, Sharma, Compton, Slade and Nielssen2011).
Concurring effects have been described among young people at clinical high-risk (CHR) of psychosis who are putatively in the prodromal phase of illness that immediately precedes the onset of frank psychosis. In a meta-analysis in which the majority of CHR individuals experienced attenuated or brief intermittent psychotic symptoms (Carney et al., Reference Carney, Cotter, Firth, Bradshaw and Yung2017), compared to non-CHR controls, CHR individuals had higher rates of cannabis use (27% v. 17%) and cannabis use disorders, and CHR cannabis users experienced more severe psychotic symptoms than CHR non-users. In another meta-analysis, current (but not lifetime) cannabis use disorder increased risk of psychosis among CHR youth (Kraan et al., Reference Kraan, Velthorst, Koenders, Zwaart, Ising, van den Berg and van der Gaag2016).
In terms of substance use more broadly, a meta-analysis examining relationships between a range of environmental risk factors and subclinical psychotic experiences in child and adult samples identified the use of cannabis, alcohol, as well as other substances, as risk factors for later psychotic experiences (Linscott & van Os, Reference Linscott and van Os2013). Meta-analyses have also described a significant association between CHR state and tobacco use (Fusar-Poli et al., Reference Fusar-Poli, Tantardini, De Simone, Ramella-Cravaro, Oliver, Kingdon and McGuire2017), with 33% of CHR individuals smoking tobacco relative to 14% of non-CHR controls (Carney, Cotter, Bradshaw, Firth, & Yung, Reference Carney, Cotter, Bradshaw, Firth and Yung2016).
It remains unclear whether substance use and psychotic experiences relate to each other as causal, triggering, or maintaining factors. However, robust data from randomised and controlled laboratory studies suggest that exposure to substances such as cannabis causes disruptions to brain development that elicit negative psychiatric outcomes (Sherif, Radhakrishnan, D'Souza, & Ranganathan, Reference Sherif, Radhakrishnan, D'Souza and Ranganathan2016). Intravenous delta-9-tetrahydrocannabinol administration (Δ-9-THC; the active ingredient of cannabis that causes the psychoactive effects) has been found to have a dose-dependent effect on psychotic-like symptoms in healthy volunteers (D'Souza et al., Reference D'Souza, Perry, MacDougall, Ammerman, Cooper, Wu and Krystal2004). Studies of rodents have found psychotic-like signs in adult rodents after adolescent cannabinoid exposure, but not after adult cannabinoid exposure (Rubino & Parolaro, Reference Rubino and Parolaro2014), suggesting adolescence constitutes a more vulnerable exposure window.
The aim of this systematic review was to assess the current evidence relating to both the prevalence of any substance use (including tobacco, alcohol, cannabis, and other substances) in children and adolescents who report experiencing subclinical psychotic symptoms (or psychotic-like experiences; PLEs), and the prevalence of PLEs in those who report using substances. We further sought to compare these prevalence rates to those in comparison (control) groups. An upper age limit of 17 years was chosen in order to restrict the analyses to studies focused on the period prior to the typical age of onset of the psychosis prodrome during later adolescence or young adulthood (Ruhrmann, Schultze-Lutter, & Klosterkötter, Reference Ruhrmann, Schultze-Lutter and Klosterkötter2010; Tandon, Nasrallah, & Keshavan, Reference Tandon, Nasrallah and Keshavan2009; Yung et al., Reference Yung, Nelson, Baker, Buckby, Baksheev and Cosgrave2009). We restricted our analyses to this pre-prodrome period to identify prospects for earlier intervention. Among CHR individuals, more than one-in-five (22%) transition to psychotic illness within 3 years (Fusar-Poli et al., Reference Fusar-Poli, Salazar de Pablo, Correll, Meyer-Lindenberg, Millan, Borgwardt and Arango2020), and many experience persistent psychopathology, psychosocial impairment, and poor quality of life (Simon et al., Reference Simon, Borgwardt, Riecher-Rössler, Velthorst, de Haan and Fusar-Poli2013). These outcomes highlight the need for earlier detection and intervention to prevent prodromal symptoms and their associated adverse outcomes (Laurens & Cullen, Reference Laurens and Cullen2016).
Method
The review was registered with PROSPERO (CRD42018106597) and conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009). All exclusion/inclusion decision-making, data extraction, and data analyses were performed in duplicate by two authors (SLM, and ML or KRL), with any disagreements resolved by discussion between authors.
Study eligibility and search strategy
The review incorporated cross-sectional, cohort, twin, and case-control studies. Inclusion criteria were: (1) studies of participants aged ⩽17 years; and (2) studies that reported assessment of both PLEs and substance use in at least 75% of participants, as measured by self-/informant-report questionnaires, interviews, or case notes. Exclusion criteria were: (1) studies of participants with a diagnosis of psychotic illness and (2) a lack of primary data (e.g. reviews). Searches were conducted in MEDLINE, PsycINFO, and CINAHL to identify articles published in English until July 2022 (updating the initial scoping search conducted in April 2018). Search terms are detailed in online Supplementary materials (S1). Articles were screened for eligibility in three stages: (1) by title and abstract; (2) by full-text review; and (3) by manual search of the reference lists of the eligible articles to locate studies not identified by database search.
Data extraction
The following information was extracted from the included studies: (1) study characteristics, including study design and setting; (2) sample characteristics, including sample size, mean age, and gender (% male); (3) substance use characteristics, including, where available, the type of substance used, if assessed when intoxicated, and the assessment method and tool used; (4) PLE characteristics, including type of PLE, the assessment method, and tool used; and (5) counts of adolescents with and without PLEs/substance use or, if no counts were reported, measures of association were extracted.
Summary measures and synthesis of results
Studies were categorised into four meta-analyses: prevalence of PLEs in youth with substance use (meta-analysis 1a); comparison of the prevalence of PLEs in youth with v. without substance use (meta-analysis 1b); prevalence of substance use in youth with PLEs (meta-analysis 2a); and comparison of the prevalence of substance use in youth with v. without PLEs (meta-analysis 2b). Studies were allocated to each meta-analysis according to the data available; studies reporting raw data for cases only were allocated to meta-analyses 1a and/or 2a, studies reporting raw data for both cases and controls were allocated to meta-analyses 1a, 1b, 2a, and/or 2b, while studies reporting only effect sizes were allocated to meta-analyses 1b and/or 2b. Given sufficient data, additional meta-analyses were conducted on correlations to assess dose-dependence between substance use and PLEs. To ensure independence of the main analyses, if studies reported data on multiple time frames, PLEs, or substance use types in the same participants, data from the most commonly reported PLE or substance across studies only was used.
Meta-analyses were completed in Comprehensive Meta-Analysis Version 3 [CMA-3; Borenstein, Hedge, Higgins, & Rothstein, Reference Borenstein, Hedge, Higgins and Rothstein2009] using random effects models. For meta-analyses 1a and 2a (prevalence studies), pooled data were compiled as event rates, and for meta-analyses 1b and 2b (association studies), odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. We pooled count data, ORs and their CIs, with means and their standard deviations (s.d.s) for meta-analyses 1b and 2b. Where studies reported only risk ratios for a PLE outcome, these were treated as ORs (Zhang & Yu, Reference Zhang and Yu1998) in studies of adolescents, as the prevalence of PLEs is <10% in this population (13–18 years; 7.5%; Kelleher et al., Reference Kelleher, Connor, Clarke, Devlin, Harley and Cannon2012). This was not done in studies of children, as PLEs are more common in that age group (9–12 years; 17%; Kelleher et al., Reference Kelleher, Connor, Clarke, Devlin, Harley and Cannon2012). The reverse was true for substance use outcome, as adolescence is the peak period during which substance use occurs (Degenhardt et al., Reference Degenhardt, Stockings, Patton, Hall and Lynskey2016).
Effect sizes for ORs were defined as small if OR < 2.0, medium if OR between 2.0 and 5.0, large if OR > 5.0, and very large if OR > 10.0. Differences in percentages were defined as small if ~7, medium if ~18, large if ~30, and very large if ⩾45, and effect sizes for correlations were weak if r ~ 0.10, medium if r ~ 0.30, and strong if r ⩾ 0.50 (Rosenthal, Reference Rosenthal1996). Heterogeneity was measured with the Q test and I 2, where the I 2 statistic indexes the percentage of the variability in effect estimates that is due to heterogeneity rather than to sampling error. Outliers and influencers were assessed using the one-study removed analysis (Viechtbauer & Cheung, Reference Viechtbauer and Cheung2010). Funnel-plot analyses assessed risk of publication bias. Where Egger's test indicated possible publication bias, Duval and Tweedie's trim and fill test was reported, which provides an adjusted effect size for a symmetric funnel plot (Borenstein et al., Reference Borenstein, Hedge, Higgins and Rothstein2009).
Given sufficient studies, planned subgroup and meta-regression analyses were conducted to assess causes of heterogeneity. Potential moderators included: study quality, gender distribution, age at assessment, method of assessment, frequency of substance use, type of substance use, type of PLE, whether the PLE occurred while intoxicated, and whether the ORs were adjusted. For subgroup analyses, we did not assume a common variance within subgroups, so results may be imprecise in analyses that included fewer than five studies in each subgroup (Borenstein et al., Reference Borenstein, Hedge, Higgins and Rothstein2009). Meta-regressions were conducted with a restricted maximum likelihood model, as recommended for small-to-medium-sized meta-analyses, and the Knapp Hartung distribution, recommended for random effects models (Borenstein et al., Reference Borenstein, Hedge, Higgins and Rothstein2009). When statistical pooling was not possible, relevant studies were retained for narrative reporting.
Quality assessments
A standardised critical appraisal instrument [the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist; www.strobe-statement.org] was used to assess included study reporting quality. A percentage score was calculated for each study to represent the total number of STROBE items reported. Percentage scores were then averaged across studies in each meta-analysis to give an indication of overall risk of study bias, with averaged scores ⩽25% rated as high risk of study bias, and ⩾75% rated as low risk of study bias.
The quality of the pooled evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. According to GRADE, pooled results from observational studies are considered inherently of low quality due to possible confounding factors which should be evenly distributed across groups in randomised studies. Quality of results can be upgraded if overall risk of study bias is low, if samples are large, if pooled effect sizes are large or dose dependent, if there is no residual confounding, or if the evidence is direct, consistent, or precise (Guyatt et al., Reference Guyatt, Oxman, Sultan, Glasziou, Akl, Alonso-Coello and Schünemann2011). Indirectness refers to approximated measures, comparisons, or samples; inconsistency to significant heterogeneity among studies results; and imprecision to large CIs across the pooled effect size (CIs > 0.25 in either direction; Schunemann, Reference Schunemann2008). As GRADE guidelines for measuring precision do not apply to prevalence data, we considered pooled event rates as imprecise if CIs were larger than 10% in either direction.
Results
Study selection
As detailed in the flow diagram illustrated in Fig. 1, the searches yielded 2223 references, of which 666 were excluded as duplicates, 1243 were excluded following review of title and abstract, and a further 259 were excluded following full text review. The remaining 55 studies met inclusion criteria (Addington et al., Reference Addington, Liu, Goldstein, Wang, Kennedy, Bray and MacQueen2019; Albertella & Norberg, Reference Albertella and Norberg2012; Auther et al., Reference Auther, McLaughlin, Carrión, Nagachandran, Correll and Cornblatt2012; Barkhuizen, Taylor, Freeman, & Ronald, Reference Barkhuizen, Taylor, Freeman and Ronald2019; Bassett, Schunk, & Crouch, Reference Bassett, Schunk and Crouch1996; Bechtold, Hipwell, Lewis, Loeber, & Pardini, Reference Bechtold, Hipwell, Lewis, Loeber and Pardini2016; Besli, Ikiz, Yildirim, & Saltik, Reference Besli, Ikiz, Yildirim and Saltik2015; Bourque, Afzali, O'Leary-Barrett, & Conrod, Reference Bourque, Afzali, O'Leary-Barrett and Conrod2017a; Bourque et al., Reference Bourque, Spechler, Potvin, Whelan, Banaschewski, Bokde and Conrod2017b; Brink et al., Reference Brink, van Driel, el Bouhaddani, Wardenaar, van Domburgh, Schaefer and Veling2020; Colins, Vermeiren, Noom, & Broekaert, Reference Colins, Vermeiren, Noom and Broekaert2013; Colins et al., Reference Colins, Vermeiren, Vreugdenhil, Schuyten, Broekaer and Krabbendam2009; Cruz & Domínguez, Reference Cruz and Domínguez2011; DaBreo-Otero, Reference DaBreo-Otero2021; Dolphin, Dooley, & Fitzgerald, Reference Dolphin, Dooley and Fitzgerald2015; Drobinin et al., Reference Drobinin, Van Gestel, Zwicker, MacKenzie, Cumby, Patterson and Uher2020; Evans & Raistrick, Reference Evans and Raistrick1987; Fonseca-Pedrero, Lucas-Molina, Pérez-Albéniz, Inchausti, & Ortuño-Sierra, Reference Fonseca-Pedrero, Lucas-Molina, Pérez-Albéniz, Inchausti and Ortuño-Sierra2020; Forrester, Reference Forrester2012; Friedman, Utada, Glickman, & Morrissey, Reference Friedman, Utada, Glickman and Morrissey1987; Garland & Howard, Reference Garland and Howard2010; Goulter, McMahon, & Dodge, Reference Goulter, McMahon and Dodge2019; Harley et al., Reference Harley, Kelleher, Clarke, Lynch, Arseneault, Connor and Cannon2010; Hartsell, Reference Hartsell2021; Hides et al., Reference Hides, Lubman, Buckby, Yuen, Cosgrave, Baker and Yung2009; Jones, Calkins, Scott, Bach, & Gur, Reference Jones, Calkins, Scott, Bach and Gur2017; Jones, Gage, & Heron, Reference Jones, Gage and Heron2018; Konings, Henquet, Maharajh, Hutchinson, & Van Os, Reference Konings, Henquet, Maharajh, Hutchinson and Van Os2008; Lansing, Plante, Fennema-Notestine, Golshan, & Beck, Reference Lansing, Plante, Fennema-Notestine, Golshan and Beck2018; Levy & Weitzman, Reference Levy and Weitzman2019; Lindgren et al., Reference Lindgren, Manninen, Laajasalo, Mustonen, Kalska, Suvisaari and Therman2010; Mackie, Castellanos-Ryan, & Conrod, Reference Mackie, Castellanos-Ryan and Conrod2011; Mackie et al., Reference Mackie, Wilson, Freeman, Craft, Escamilla De La Torre and Lynskey2021; McGorry et al., Reference McGorry, McFarlane, Patton, Bell, Hibbert, Jackson and Bowes1995; McMahon et al., Reference McMahon, Corcoran, Keeley, Clarke, Coughlan, Wasserman and Cannon2021; Miettunen et al., Reference Miettunen, Törmänen, Murray, Jones, Mäki, Ebeling and Veijola2008; Mundy, Robertson, Robertson, & Greenblatt, Reference Mundy, Robertson, Robertson and Greenblatt1990; Opaleye et al., Reference Opaleye, Noto, Sanchez, Gonçalves de Moura, Galduróz and Carlini2009; Rimvall et al., Reference Rimvall, van Os, Rask, Olsen, Skovgaard, Clemmensen and Jeppesen2020; Schifano, Forza, & Gallimberti, Reference Schifano, Forza and Gallimberti1994; Scott et al., Reference Scott, Martin, Bor, Sawyer, Clark and McGrath2009; Shakoor et al., Reference Shakoor, Zavos, McGuire, Cardno, Freeman and Ronald2015; Shervette, Schydlower, Lampe, & Fearnow, Reference Shervette, Schydlower, Lampe and Fearnow1979; Shrier, Harris, Kurland, & Knight, Reference Shrier, Harris, Kurland and Knight2003; Stain et al., Reference Stain, Bucci, Baker, Carr, Emsley, Halpin and Startup2016; Stainton et al., Reference Stainton, Chisholm, Woodall, Hallett, Reniers, Lin and Wood2021; Sunderland et al., Reference Sunderland, Forbes, Mewton, Baillie, Carragher, Lynch and Slade2021; Tekulve, Alexander, & Tormoehlen, Reference Tekulve, Alexander and Tormoehlen2014; van Gastel et al., Reference van Gastel, Wigman, Monshouwer, Kahn, van Os, Boks and Vollebergh2012; Vaughn, Reference Vaughn2006; Wang et al., Reference Wang, Chen, Chen, Liu, Wu, Chen and Fan2022; Watts et al., Reference Watts, Wood, Jackson, Lisdahl, Heitzeg, Gonzalez and Sher2021; Whitt, Garland, & Howard, Reference Whitt, Garland and Howard2012; Yilmaz Kafali et al., Reference Yilmaz Kafali, Turan, Akpınar, Mutlu, Özkaya Parlakay, Çöp and Toulopoulou2022; Zammit, Owen, Evans, Heron, & Lewis, Reference Zammit, Owen, Evans, Heron and Lewis2011). Table 1 summarises the characteristics of the included studies, and online Supplementary Table S2 details each STROBE item rating.

Fig. 1. Flow diagram showing the process of inclusion/exclusion through the different phases of the meta-analysis.
Table 1. Descriptive summary of the included studies

ADI, Adolescent Diagnostic Interview; APSS, Adolescent Psychotic Like Symptoms Screener; ASSIST, Alcohol, Smoking and Substance Involvement Screening Test; AUDIT, Alcohol Use Disorders Identification Test; BSI, Brief Symptom Inventory; CAPE, Community Assessment of Psychotic Experiences; CAARMS, Comprehensive Assessment of At Risk Mental States; CBCL, Child Behaviour Checklist; CIDI, Composite International Diagnostic Interview; DISC-IV, Diagnostic Interview Schedule for Children, version 5; HAIS, Homeless Adolescent Interview Schedule; K DISC-IV, Diagnostic Interview Schedule for Children, Fourth Edition; K-SADS-PL, The Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime; MAYSI-II, Massachusetts Youth Screening Instrument Second Version, Inventory; MMM, Marijuana Motives Measure; OTI, Opiate Treatment Index; PLE, psychotic-like experience; PLEQ-C = Psychotic-Like Experiences Questionnaire for Children; PLIKSi, Psychosis-like Symptom Interview; PLIKS-Q = Psychotic-Like Symptoms Questionnaire; POSIT, Problem Oriented Screening Instrument for Teenagers; PQ, Prodromal Questionnaire; PQ-B, Prodromal Questionnaire – Brief Version; PROD-screen, screen for prodromal symptoms of psychosis; RCT, randomised controlled trial; SADS-E, The Kiddie Schedule for Affective Disorders and Schizophrenia Epidemiological; SCI-PSY, Clinical Interview for Psychotic Spectrum; SCL-90, Symptom Checklist-90-Revised; SIDS, Structured Interview for Prodromal Syndromes; SOPS, Scale of Prodromal Symptoms; SPEQ, Specific Psychotic Experiences Questionnaire; SPI-CY, Schizophrenia Proneness Instrument – Child and Youth version; SUQ, Substance Use Questionnaire; VSSI, Volatile Solvent Screening, YSR, Youth Self Report; YRBQ, Youth Risk Behaviour Survey.
An overall summary of the results and GRADE quality assessments associated with each primary meta-analysis is provided in Table 2.
Table 2. Primary meta-analyses results and GRADE quality assessments
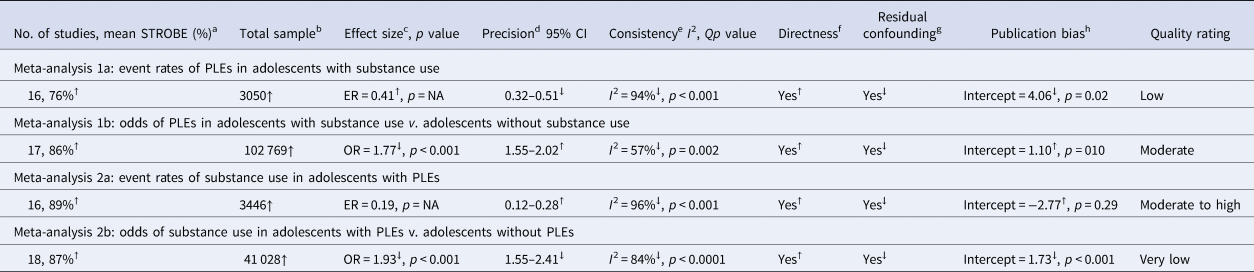
GRADE, Grading of Recommendations, Assessment, Development and Evaluation; No., number; ER, event rate; NA, not applicable to ER data; OR, odds ratio; PLEs, psychotic-like experiences; ↑, upgraded; ↓, downgraded.
All studies were observational and therefore were assumed to be of low quality (GRADE recommendation).
a Upgraded if STROBE average was ⩾75%; downgraded if STROBE average was ⩽25%.
b Upgraded if samples were large (⩾300); downgraded if samples were small (<100).
c Upgraded if effect sizes were large (ER > 0.30, OR > 5.0); downgraded if effect sizes were small (ER < 0.07, OR < 2.0).
d Upgraded if CIs were precise (ER < 0.10, OR < 0.25 in either direction from the effect size); downgraded if imprecise (ER > 0.10, OR > 0.25 in either direction from the effect size).
e Upgraded if I 2 was small and p > 0.05; downgraded if I 2 was large and p < 0.05.
f Upgraded if measures, samples, and comparisons (for ORs) were direct; downgraded if they were not direct.
g Upgraded if there was no residual confounding; downgraded with probable residual confounding.
h Upgraded if Eggers test p > 0.05; downgraded if Egger's test p < 0.05.
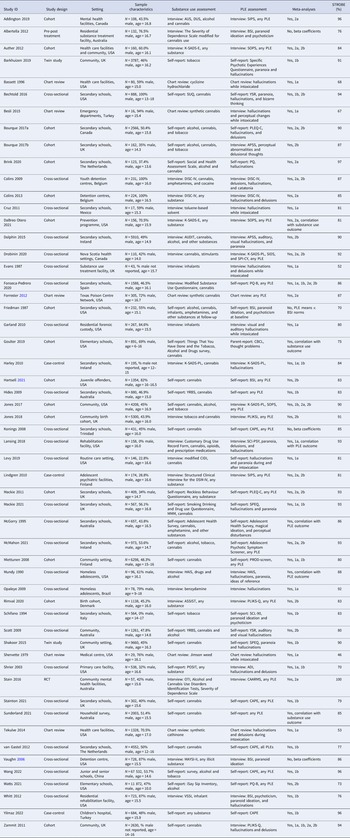
Meta-analysis 1a: rates of PLEs among adolescents with substance use
Meta-analysis of 16 studies (Fig. 2, panel A) incorporated a total of 3050 individuals who reported using substances. The random effects model indicated that 41% of substance users reported PLEs (event rate = 0.41, 95% CI 0.32–0.51). The one-study removed analysis to assess the effects of outliers found no differences in the event rate with each study removed (rate range = 0.38–0.43). The averaged STROBE quality rating indicated a low risk of study bias (76%). The overall quality of the pooled evidence was rated ‘low’, with an imprecise CI, and substantial heterogeneity (Table 2). As Egger's test indicated possible publication bias, the adjusted event rate using Duval and Tweedie's trim and fill test was 0.27.

Fig. 2. Forest plots of (panel A) prevalence rates of PLEs among adolescents with substance use and (panel B) the odds of PLEs in adolescents with v. without substance use.
Subgroup analyses are presented in online Supplementary Table S3. These indicate no moderating effects of study design (cross-sectional, cohort, or chart review), substance use or PLE assessment method (interview, chart review, or self-report), type of substance used (cannabis or inhalants), whether the participant was intoxicated at the time of experiencing PLEs, or PLE type (any hallucinations, visual hallucinations, paranoia/delusions). Meta-regression analyses revealed no moderating effects of gender (% male), age at assessment, or study quality score.
Meta-analysis 1b: odds of PLEs among adolescents with v. without substance use
Meta-analysis of 17 studies (Fig. 2, panel B) was conducted on a total of 102 769 individuals with and without substance use. The random effects model revealed a small-to-medium-sized effect, with adolescent substance users nearly twice as likely to report PLEs than their non-substance using counterparts (OR 1.77, 95% CI 1.55–2.02, p < 0.001). The one-study removed analysis found no differences in effect size with each study removed (OR range = 1.69–1.83, all p < 0.001). The averaged STROBE quality rating indicated a low risk of study bias (86%). The overall quality of the pooled evidence was rated ‘moderate’, with lower, but still significant heterogeneity, a precise CI, and no evidence of publication bias (Table 2).
Subgroup analyses (online Supplementary Table S4) identified no moderating effects of study design (cross-sectional, cohort, case-control, or twin study), substance use or PLE assessment method (self-report or interview), type of substance (cannabis lifetime or weekly use, alcohol, or tobacco), or type of PLE (hallucinations or paranoia/delusions). Insufficient studies reported adjusted ORs to assess this potential moderator. Meta-regression analyses also revealed no moderating effects of gender distribution or study quality. Age at assessment showed a significant medium-sized effect, with studies of younger samples having greater odds of PLEs in substance users v. non-users than studies with older samples (coefficient = −0.32, p = 0.009). Data from three studies (Lansing et al., Reference Lansing, Plante, Fennema-Notestine, Golshan and Beck2018; McGorry et al., Reference McGorry, McFarlane, Patton, Bell, Hibbert, Jackson and Bowes1995; Mundy et al., Reference Mundy, Robertson, Robertson and Greenblatt1990) were able to be pooled in a correlation meta-analysis assessing dose-dependence between the level of any substance use and the number/severity of any PLE as the outcome. This analysis (online Supplementary Fig. S5) contained 911 adolescents and found a weak but significant correlation between increased substance use and increased PLEs (correlation = 0.22, 95% CI 0.15–0.29, p < 0.001), with low heterogeneity (I 2 = 9%).
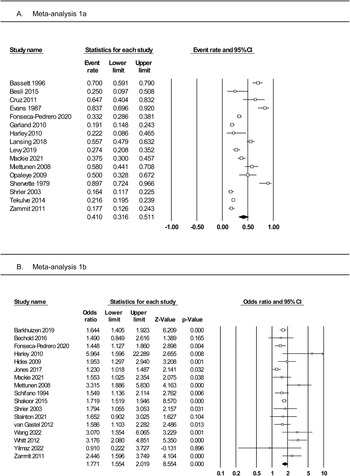
Meta-analysis 2a: rates of substance use among adolescents with PLEs
Meta-analysis of 16 studies (Fig. 3, panel A) was conducted on a total of 3446 individuals who reported PLEs. The random effects model indicated a small effect, with 19% of individuals with PLEs reporting using substances (rate = 0.19, 95% CI 0.12–0.28). The one-study removed analysis found no differences in event rate with each study removed (rate range = 0.16–0.21). The averaged STROBE quality rating indicated a low risk of study bias (89%). The overall quality of the pooled evidence was rated ‘moderate to high’ (Table 2).

Fig. 3. Forest plots of (panel A) prevalence rates of substance use among adolescents with PLEs and (panel B) the odds of substance use in adolescents with v. without PLEs.
Subgroup analyses (online Supplementary Table S6) identified significant moderating effects of PLE measure (interview = 0.26, self-report = 0.11) and substance type (alcohol = 0.44, tobacco = 0.24, lifetime cannabis = 0.19, weekly cannabis = 0.04, amphetamines = 0.07, cocaine = 0.03). There were no moderating effects of study design, substance use measure, gender distribution, age at assessment, or study quality. There were insufficient studies reporting PLE type to assess this potential moderator.
Meta-analysis 2b: odds of substance use among adolescents with and without PLEs
Meta-analysis of 18 studies (Fig. 3, panel B) was conducted on a total of 41 028 individuals with and without PLEs. The random effects model indicated a medium effect size, with adolescents who reported PLEs twice as likely to use substances compared to those not reporting PLEs (OR 1.93, 95% CI 1.55–2.41, p < 0.0001). The one-study removed analysis found no differences in effect size with each study removed (OR range = 1.83–2.03). The averaged STROBE quality rating indicated a low risk of study bias (87%). The quality of the pooled evidence was rated as very low (Table 2). As Egger's test indicated possible publication bias, the adjusted OR using Duval and Tweedie's trim and fill test was 1.41.
Subgroup analyses (online Supplementary Table S7) found no moderating effects of study design (cross-sectional or cohort), method of assessment of PLEs or substance use (interview or self-report), type of substance (cannabis, alcohol, tobacco, amphetamine, or cocaine), or whether ORs were adjusted. Although the analyses comparing alcohol and cocaine use between adolescents with and without PLEs were not significant, alcohol, cannabis, tobacco, and amphetamine all showed significantly increased rates of use in youth with PLEs compared to youth without PLEs. Meta-regressions revealed no moderating effects of gender, age at assessment, or study quality. There were insufficient studies reporting PLE type to assess this potential moderator. Data from three studies (DaBreo-Otero, Reference DaBreo-Otero2021; Goulter et al., Reference Goulter, McMahon and Dodge2019; Sunderland et al., Reference Sunderland, Forbes, Mewton, Baillie, Carragher, Lynch and Slade2021) were pooled in a correlation meta-analysis assessing dose-dependence between the number/severity of any PLE and the level of any substance use as the outcome. This analysis (online Supplementary Fig. S8) contained 2995 children and adolescents and found a weak but significant correlation between increased PLEs and increased substance use (correlation = 0.17, 95% CI 0.05–0.33, p = 0.04), with high heterogeneity (I 2 = 93%).
Studies not included in the meta-analyses
Due to their reporting beta coefficients (Roth, Le, Oh, Van Iddekinge, & Bobko, Reference Roth, Le, Oh, Van Iddekinge and Bobko2018) three studies were not able to be included in the correlation meta-analyses: Albertella and Norberg (Reference Albertella and Norberg2012) reported a significant reduction in the amount of cannabis used by adolescents reporting subclinical symptoms of psychoticism and paranoid ideation on the Brief Symptom Inventory (BSI) following a 3-month residential programme [the Program for Adolescent Life Management (PALM])]. They reported no significant associations between pre-treatment psychoticism and paranoid ideation and pre-treatment cannabis use. Vaughn (Reference Vaughn2006) found that poly-substance use was related to higher mean levels of paranoid ideation and Konings et al. (Reference Konings, Henquet, Maharajh, Hutchinson and Van Os2008) found that cannabis use prior to the age of 14 years, but not in the whole sample or in the sample aged over 14 years, predicted later PLEs.
Friedman et al. (Reference Friedman, Utada, Glickman and Morrissey1987) used adolescent non-patient BSI norms as controls so could not be combined with other controls. They reported higher mean scores on psychoticism and paranoid ideation subscales of the BSI among a sample of substance using high school students. The strength of association between substance use and psychopathology in general increased over time (from 15.1 to 16.8 years).
Discussion
This systematic review synthesised the available evidence regarding the prevalence and association of PLEs and substance use among children and adolescents aged 17 years or younger. Results indicate that around two-in-five young people who used substances experienced PLEs (meta-analysis 1a), and around one-in-five young people who experienced PLEs reported using substances (meta-analysis 2a). Those who used substances were twice as likely to experience PLEs than those who did not use substances (meta-analysis 1b), similar to the rate of substance use in those with v. without PLEs (meta-analysis 2b).
Most of the included studies assessed cannabis, but alcohol, tobacco, and amphetamine use were each also associated with PLEs. These findings are consistent with prior reviews not restricted to child and adolescent samples, where 48.7% of CHR individuals reported lifetime cannabis use and 25.8% reported current cannabis use (Farris, Shakeel, & Addington, Reference Farris, Shakeel and Addington2020), and exposure to any substance at least doubled the risk for psychotic experiences in general population samples (Linscott & van Os, Reference Linscott and van Os2013). We also observed a dose-dependent association between increased substance use (frequency or amount) and increased PLEs (number or severity). This has also been found among adult samples assessed for cannabis use preceding the onset of psychosis (Marconi et al., Reference Marconi, Di Forti, Lewis, Murray and Vassos2016), but not for PLEs when adjusted for multiple covariates (Degenhardt et al., Reference Degenhardt, Saha, Lim, Aguilar-Gaxiola, Al-Hamzawi, Alonso and McGrath2018).
While heterogeneity was observed in all analyses, all but one of the subgroup analyses and meta-regressions identified no moderating effects of study design, study quality, gender, age at assessment, measure used to assess PLEs or substance use, PLE or substance type, or whether participants were intoxicated at the time of the PLE assessment. Several of these subgroup analyses were constrained by few studies, particularly those assessing PLE and substance type. A moderating effect was found in one analysis, where younger mean age at assessment was associated with increased PLEs in those with v. without substance use. This effect was also found in studies that assessed PLEs in early onset (defined variably as before 14 or 16 years of age) compared to late onset (14 or 16 years and older) cannabis use (Jones et al., Reference Jones, Calkins, Scott, Bach and Gur2017; Konings et al., Reference Konings, Henquet, Maharajh, Hutchinson and Van Os2008; Stefanis et al., Reference Stefanis, Delespaul, Henquet, Bakoula, Stefanis and Van Os2004), indicating earlier and prolonged use increases the likelihood of PLEs. The only other moderating effect was identified in the analysis comparing rates of substance use in adolescents with and without PLEs. When PLEs were measured by interviewer rating, higher rates of substance use were observed than in studies using self-reported PLEs (rate = 0.26 v. 0.11). In that analysis, alcohol, tobacco, and cannabis were the most commonly used substances, and alcohol, cannabis, tobacco, and amphetamine use were each significantly greater in the subgroup analysis comparing young people with v. without PLEs). We also observed a dose-dependent association between increased PLEs and increased substance use, which has similarly been identified in adult samples assessing severity of PLEs and increased subsequent substance use (Degenhardt et al., Reference Degenhardt, Saha, Lim, Aguilar-Gaxiola, Al-Hamzawi, Alonso and McGrath2018).
Our findings show that the rate of PLEs among substance-using youth (41%) was notably higher than the rate of PLEs identified previously in meta-analysis on samples of the general youth population (aged 13–18 years; 7.5%) (Kelleher et al., Reference Kelleher, Connor, Clarke, Devlin, Harley and Cannon2012). Our findings also reveal an elevated rate of substance use in adolescents experiencing PLEs (19%). Globally, between the ages of 15 and 19 years, 4.8% of males and 2.2% of females have consumed alcohol, while 2.4% of males and 1.6% of females have used illicit substances (Degenhardt et al., Reference Degenhardt, Stockings, Patton, Hall and Lynskey2016). These relationships might be explained, in part, by a self-medication mechanism, whereby young people who experience PLEs, who may also be experiencing depression or anxiety symptoms (Varghese et al., Reference Varghese, Scott, Welham, Bor, Najman, O'Callaghan and McGrath2011), may be at greater risk of using substances in order to cope with the potential symptom-related distress (Smit, Bolier, & Cuijpers, Reference Smit, Bolier and Cuijpers2004). However, evidence to support this hypothesis is limited, and it is possible that substance misuse and PLEs share similar risk factors, such as genetic predisposition (Degenhardt & Hall, Reference Degenhardt and Hall2006). Another explanation might be that dopamine dysregulation underlies the association, as antipsychotics block dopamine receptors while agonists elicit positive symptomatology (Dean & Murray, Reference Dean and Murray2005). Repeated exposure to substances that increase dopamine levels could produce a progressing and lasting response, particularly in those with a genetic predisposition (Dean & Murray, Reference Dean and Murray2005). Psychotic symptoms have been shown to be elicited by progressively smaller, repeated doses of cocaine (Bartlett, Hallin, Chapman, & Angrist, Reference Bartlett, Hallin, Chapman and Angrist1997).
Our findings have notable clinical and policy implications. Psychotic experiences in childhood and adolescence have been associated with a four-fold increase in risk of psychotic disorders (Healy et al., Reference Healy, Dooley, Coughlan, Kelleher, Brannigan, Clarke and Cannon2019). The pre-prodromal phase of illness represents an opportunity for early intervention to potentially prevent and/or delay the onset of psychosis, while more benign and more effective treatments are possible (Laurens & Cullen, Reference Laurens and Cullen2016). Substance use cessation treatment should be a focus in this early stage and included in early intervention programmes for psychotic illnesses. Considering the normative rate of substance use among youth, as well as current trends towards marijuana legalisation in many jurisdictions, increased efforts are needed to educate young people and the broader public about the serious mental health risks linked to substance use. Together, findings from the current meta-analyses suggest that delivery of universal substance use prevention programmes to youth aged 17 years and younger may help to avert PLEs, and that targeted interventions for young people with PLEs may help to discourage their engagement in substance use. These hypotheses need to be explicitly tested.
While this systematic review extends previous evidence of associations between substance use and prodromal symptoms and psychotic disorders, limitations should be noted. The analyses drew predominantly on cross-sectional data, even in the cohort studies, as many outcomes were reported >17 years of age in those studies. Therefore, the capacity to determine the direction of effects was limited. Despite assessing multiple moderators, the high levels of heterogeneity observed suggest other sources of between-study differences not investigated here. Some of the subgroup analyses were also hampered by the small number of studies, and some subgroups, such as current cannabis use and count/frequency of PLEs, were unable to be assessed due to lack of data. Access to individual participant data may allow greater assessment of between-study differences in future meta-analyses.
In summary, our findings support the notion that adolescents with PLEs have increased rates of substance use, and young substance users have increased rates of PLEs. These individuals may represent a subclinical group at risk of transitioning to CHR and psychosis, and efforts in developing early detection and intervention might prevent or postpone onset of adult psychopathology across both psychotic and addictive domains. Further rigorous longitudinal studies are needed to clarify the temporal relationship between psychosis and substance use, especially given increasing permissiveness towards recreational cannabis use.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722003440
Acknowledgements
We thank Virginia Jones and Tamara Nevin (at Australian Catholic University, Brisbane) for early assistance with the selection and refinement of search terms.
Financial support
KRL was supported by an Australian Research Council Future Fellowship (FT170100294).
Conflict of interest
None.