SFA have been linked to the development of metabolic disorders, including diabetes( Reference Forouhi, Koulman and Sharp 1 ), hyperlipidaemia( Reference Siri-Tarino, Sun and Hu 2 ) and hypertension( Reference Wang, Manson and Forman 3 ). The underlying mechanisms include activating NF-κB to alter gene expression, serving as signal molecules and modulating membrane fluidity( Reference Calder 4 , Reference Rocha, Caldas and Oliveira 5 ). The effect of straight chain SFA on metabolic process depends on the carbon chain length. Excess dietary palmitic acid (C16 : 0) impairs insulin sensitivity and increases blood pressure and lipids( Reference Mayneris-Perxachs, Guerendiain and Castellote 6 , Reference Kim, Lim and Lee 7 ). In contrast, very-long-chain SFA (VLCSFA) with 20–24 carbon atoms may play a favourable role in metabolic disorders. Several epidemiological studies reported that higher circulating levels of VLCSFA were inversely associated with blood TAG( Reference Lemaitre, Fretts and Sitlani 8 ), insulin resistance( Reference Fernandez-Real, Broch and Vendrell 9 ), incident diabetes( Reference Forouhi, Koulman and Sharp 1 , Reference Lemaitre, Fretts and Sitlani 8 ), arterial fibrillation( Reference Fretts, Mozaffarian and Siscovick 10 ), CHD( Reference Malik, Chiuve and Campos 11 ), cardio-embolic stroke( Reference Chung, Cho and Do 12 ) and CVD mortality( Reference Fretts, Mozaffarian and Siscovick 13 ).
Circulating VLCSFA are from minor dietary constituents and are also derived by endogenous synthesis from C16 : 0 or C18 : 0 as well as inter conversions among one another mediated by β-oxidation and elongation( Reference Kihara 14 ). VLCSFA are found at significant quantities in a limited range of foods, including peanuts, lotus nuts and rapeseed/rapeseed oil( Reference Yang, Wang and Pan 15 ). In vivo, VLCSFA are major elements of ceramides and sphingomyelins, and the lower level of ceramides have been suggested to increase hepatocyte apoptosis and proliferation( Reference Pewzner-Jung, Brenner and Braun 16 ), obesity risk( Reference Samad, Badeanlou and Shah 17 ) and inflammatory response( Reference Samad, Badeanlou and Shah 17 ) among many other structural roles in, for instance, the brain and skin( Reference Mielke, Bandaru and Haughey 18 , Reference Choi and Maibach 19 ). Further studies have reported that some functions of ceramides are chain-length dependent( Reference Grosch, Schiffmann and Geisslinger 20 ). For example, based on cell culture experiments, C24 : 0 promotes proliferation, whereas C16 : 0 often has antiproliferative and pro-apoptotic effect( Reference Grosch, Schiffmann and Geisslinger 20 ). Previous epidemiological studies showed that VLCSFA were inversely correlated with phospholipid C16 : 0 levels( Reference Lemaitre, Fretts and Sitlani 8 , Reference Fretts, Mozaffarian and Siscovick 10 ), which suggested that VLCSFA and C16 : 0 might have competitive effect in some situation with opposite biological functions.
To our knowledge, no study to date has assessed the relation of VLCSFA to the metabolic syndrome (MetS) in a Chinese population. We aimed to explore the associations of plasma VLCSFA with prevalence of the MetS at baseline and risk of incident MetS of all initially metabolically healthy participants in a 2-year follow-up study. Further, we additionally evaluated the associations between VLCSFA with five MetS components.
Methods
Study population
The study population was from an investigation conducted by Chinese Centre for Disease Control and Prevention from April 2010 to December 2012 in Shunyi district of Beijing. Eligible participants had to live in the area for more than 1 year and had no plan to move within 1 year, no serious diseases of heart, lung, liver and kidney and no pregnancy. A total of 2008 participants aged between 35 and 59 years were recruited in Shunyi. All participants were interviewed in-person to collect information on demographic characteristics, history of diseases, medical conditions, current cigarette smoking status (at least one cigarette a day), alcohol drinking status (at least once a week during the past year), physical activity status (at least once a week, excluding walking and riding), agricultural working (typical agricultural workers), health education (conducted by local Centres for Disease Control) and other lifestyles. Height, weight, blood pressure and waist circumference (WC) were measured twice. Fasting venous blood was obtained for biochemical testing, including total cholesterol, HDL-cholesterol, TAG and plasma glucose. Plasma glucose was tested within 3 h and plasma lipids on the day of sampling. All biochemical testing was performed with an automatic biochemical analyser at Centre for Disease Control. Plasma was separated at 1500 g /min centrifugation for 15 min and stored at −80°C. The above information and biosamples were collected a second time after 2 years.
Among the 1915 participants with available baseline fatty acid data, we excluded 185 participants with CHD, stroke, chronic respiratory diseases and malignant tumour at baseline; one participant with no MetS information was also excluded. Finally, 1729 participants were included in the current analyses. Among them, 81 % had the 2-year follow-up data.
The study protocol was approved by the Ethical Review Committee of the National Centre for Chronic and Non-communicable Disease Control and Prevention, Chinese Centre for Disease Control and Prevention. All participants provided informed written consent. The trial was registered at chictr.org.cn as ChiCTR-EOC-17012759.
Ascertainment of the metabolic syndrome
The MetS was defined according to the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian Americans( Reference Grundy, Cleeman and Daniels 21 ). Participants with any three of the five following items were identified as having the MetS: (1) WC ≥90 cm in men or ≥80 cm in women, (2) TAG ≥1·7 mmol/l, (3) HDL-cholesterol ≤1·03 mmol/l in men or ≤1·30 mmol/l in women, (4) blood pressure ≥130/85 mmHg or current use of antihypertensive medications and (5) fasting plasma glucose ≥5·6 mmol/l or use of oral antidiabetic agents or insulin.
Plasma fatty acids measurement
GC/flame ionisation detector (FID) was used to measure plasma fatty acids profile( Reference Sun, Ma and Campos 22 , Reference Han, Rozen and Boyle 23 ). Briefly, 100μl of plasma with 1,2-dihenarachidoyl-sn-glycero-3-phosphocholine as internal standard was extracted in dichloromethane/methanol and the extracted lipids were reacted with methanol and sulphuric acid to yield fatty acid methyl ester (FAME). After methylation, FAME were extracted with n-hexane and redissolved in isooctane. Analysis was performed using an Agilent 6890 GC/FID equipped with a Supelco SP-2560 capillary column (100 m×0·25 mm inside diameter×0·20μm thickness; Agilent Technologies Inc.). Data were expressed as weight percentage of total fatty acids. Samples were organised in batches of up to 20, which included two samples from a standard pool for quality control (QC). The CV of QC were 10·8 % for C20 : 0 (arachidic acid), 12·4 % for C22 : 0 (behenic acid) and 14·6 % for C24 : 0 (lignoceric acid).
Statistical analysis
Wilcoxon rank-sum test for continuous variables and the χ 2 test for categorical variables were used to examine the differences of basic characteristics between groups with the MetS and without the MetS. Spearman correlation was used to evaluate correlations of VLCSFA and other plasma fatty acids. Logistic regression analysis was applied to examine the associations of VLCSFA and risk of the MetS. VLCSFA were classified into four groups based on the quartiles of VLCSFA in participants without the MetS at baseline. Several models were fitted to examine the association of each VLCSFA and risk of the MetS: (1) model adjusted for age, sex and agricultural work (main model); (2) model adjusted for age, sex, agricultural work, education, smoking, alcohol drinking and physical activity (fully adjusted model) and (3) models additionally adjusted for the potential mediators based on fully adjusted model, including C16 : 0, C18 : 0, C18 : 3n-3, C22 : 6n-3, n-6 PUFA and MUFA (exploration models). These selected fatty acids were significantly correlated with VLCSFA with the Spearman coefficients more than 0·25. As the fully adjusted models did not change the results, main model was used in the following analyses. The associations of VLCSFA and risk of incident MetS during 2-year follow-up were conducted only in participants without the MetS at baseline. Non-linear OR of the MetS was estimated by applying a restricted cubic spline regression model with three knots at the 5th, 50th and 95th percentiles( Reference Desquilbet and Mariotti 24 ). Multinomial logistic regression was used to estimate the associations of VLCSFA and risk of the MetS according to the number of MetS components at baseline. As the sample size in each group was limited, the VLCSFA levels were classified into high or low group based on the median of VLCSFA in subjects without the MetS. Low VLCSFA levels group were assigned as the reference. When the associations between VLCSFA and individual components of the MetS were evaluated, each of the other four components was adjusted in the models separately. To explore modification effects of some a priori risk factors on the association between VLCSFA and MetS risk, stratified analyses were conducted by age, sex, physical activity, agricultural working, smoking status and current alcohol drinking. Continuous variables were classified into high v. low levels based on the median levels in controls. The OR were expressed as the risk of the MetS for 1 sd increase of VLCSFA. Likelihood ratio tests were conducted to examine interactions. All analyses were conducted with R software and SAS 9.3 software. All P values were two-sided.
Results
A total of 1729 participants were included in this study; 565(32·7 %) of these were identified as the MetS at baseline. Baseline characteristics of participants with or without the MetS were presented in Table 1. The two groups were similar in sex, smoking status, alcohol drinking and physical activity. Compared with participants without the MetS, the MetS participants were older and did less agricultural work. The MetS group had lower level of plasma C18 : 0, C20 : 0, C22 : 0, C24 : 0, C22 : 6n-3, C18 : 2n-6, C20 : 4n-6 and n-6 PUFA, but had higher C16 : 0, MUFA, C18 : 3n-3, C20 : 5n-3 and n-3 PUFA. Women and individuals without diabetes had higher levels VLCSFA (online Supplementary Tables S1a and S1b). In addition, higher VLCSFA were associated with lower BMI, lower TAG and higher HDL. Participants with higher levels of C20 : 0 or C22 : 0 tended to be non-smoker and non-alcohol drinker, do more agricultural work, have no hypertension and have lower WC and cholesterol (online Supplementary Table S1a). Furthermore, older ages were inversely related to higher C20 : 0, but directly to higher C24 : 0 (online Supplementary Tables S1a and S1b). Participants with higher C24 : 0 were more likely to have hypertension (online Supplementary Table S1b).
Table 1 Basic characteristics of subjects with and without the metabolic syndrome (MetS) at baseline (Mean values and standard deviations; frequencies and percentages)

WC, waist circumference; TC, total cholesterol; VLCSFA, very-long-chain SFA.
* Continuous variables were expressed as means and standard deviations and categorical variables were expressed as frequency (percentage among cases or controls).
† P values were calculated from Wilcoxon rank sum test for continuous variables and χ 2 test for categorical variables.
‡ Includes C14 : 1n-5, C16 : 1n-7, C16 : 1n-9, C18 : 1n-7, C18 : 1n-9, C20 : 1n-9, C22 : 1n-9 and C24 : 1n-9 in this study.
§ Includes C18 : 3n-3, C20 : 3n-3, C20 : 5n-3, C22 : 3n-3, C22 : 5n-3 and C22 : 6n-3.
|| Includes C18 : 2n-6, C18 : 3n-6, C20 : 2n-6, C20 : 3n-6, C20 : 4n-6, C22 : 2n-6, C22 : 4n-6 and C22 : 5n-6 in this study.
All the three VLCSFA were significantly correlated with each another (r s 0·75, 0·23, 0·21 for C20 : 0 with C22 : 0, C24 : 0 and C22 : 0 with C24 : 0, online Supplementary Table S2). In the fully adjusted model, compared with the lowest quartile, the highest level of VLCSFA was associated with increased risk of the MetS; the multivariate-adjusted OR were 0·18 (95 % CI 0·13, 0·25) for C20 : 0, 0·26 (95 % CI 0·18, 0·35) for C22 : 0, 0·19 (95 % CI 0·13, 0·26) for C24 : 0 and 0·16 (95 % CI 0·11, 0·22) for total VLCSFA (Table 2). OR of the MetS were non-linear in C20 : 0, C22 : 0, C24 : 0 and total VLCSFA (Fig. 1, P non-linearity<0·0001 for C20 : 0 and total VLCSFA, P non-linearity=0·002 for C22 : 0, P non-linearity=0·021 for C24 : 0). The VLCSFA were significantly correlated with C16 : 0, C18 : 0, MUFA, C18 : 3n-3, C22 : 6n-3 and n-6 PUFA with absolute r s more than 0·25 (online Supplementary Table S2). Separately adjusted for these fatty acids did not affect the associations between VLCSFA and MetS risk (online Supplementary Table S3). In addition, the VLCSFA levels were inversely associated with the number of the MetS components (all the P trend<0·0001, online Supplementary Table S4). In the multinomial logistic regression analysis, the associations of VLCSFA and MetS risk were stronger with the increase of the number of the MetS components (Table 3).
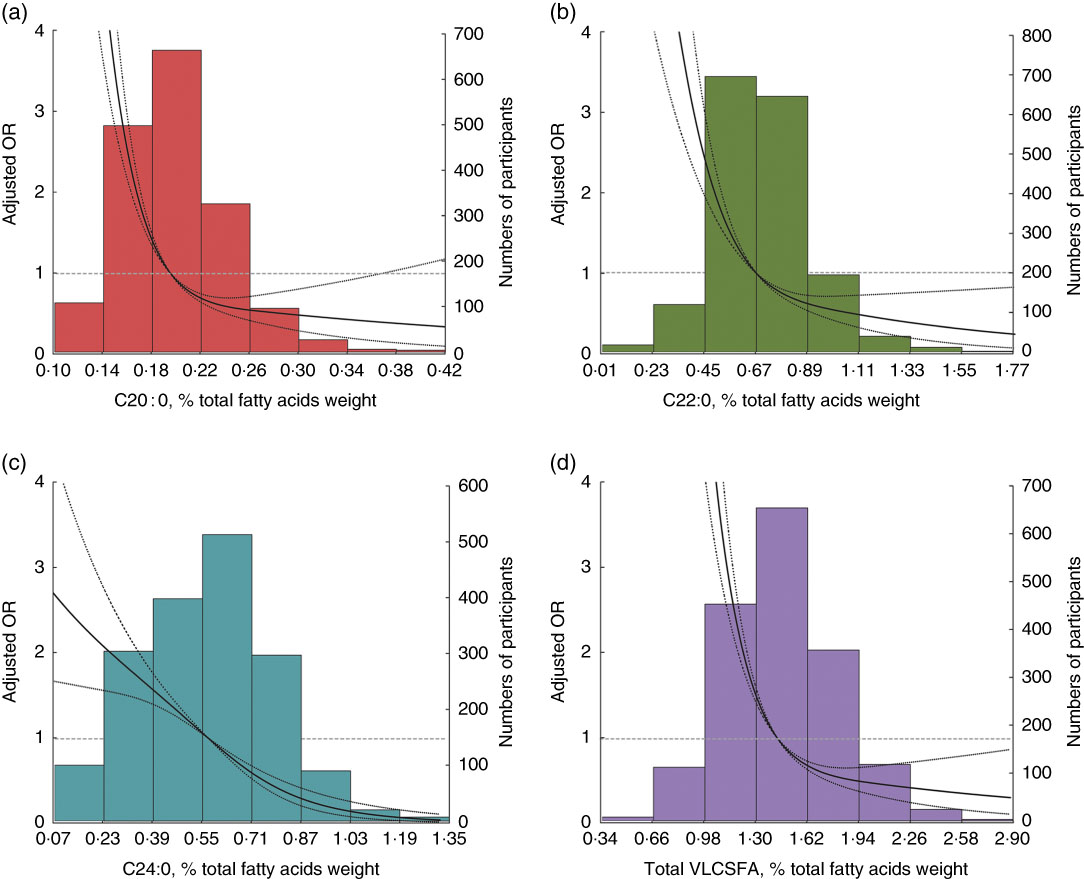
Fig. 1 Risk of the metabolic syndrome by very-long-chain SFA (VLCSFA) levels at baseline. Lines represent OR (95 % CI) based on restricted cubic splines for VLCSFA levels with knots at the 5th, 50th and 95th percentiles. OR were estimated using a logistic regression model after adjustment for age, sex and agricultural work; bars represent the numbers of participants. (a) C20 : 0 (P non-linearity<0·001), (b) C22 : 0 (P non-linearity=0·002), (c) C24 : 0 (P non-linearity=0·021) and (d) total VLCSFA (P non-linearity<0·0001).
Table 2 The associations of very-long-chain SFA (VLCSFA) and prevalence of the metabolic syndrome (MetS) at baseline* (Odds ratios and 95 % confidence intervals)
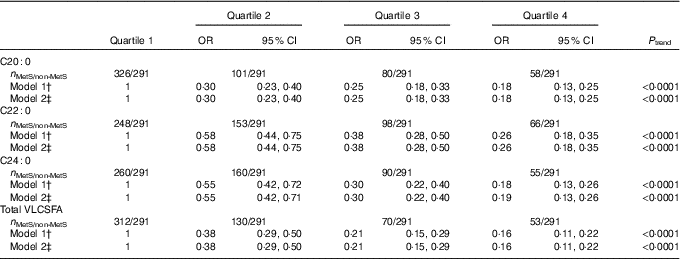
* Logistic regression was used to estimate the OR and CI. VLCSFA were classified into four group based on the quartiles of VLCSFA in subjects without the metabolic syndrome: 0·18, 0·20 and 0·23 for C20 : 0; 0·59, 0·70 and 0·84 for C22 : 0; 0·46, 0·61 and 0·73 for C24 : 0; 1·32, 1·51 and 1·72 for total VLCSFA.
† Model 1: adjusted for sex, age and agricultural work.
‡ Model 2: additionally adjusted for education, smoking, alcohol drinking and physical activity.
Table 3 Associations of very-long-chain SFA (VLCSFA) and risk of the metabolic syndrome (MS) according to the number of MS components at baseline* (Odds ratios and 95 % confidence intervals)
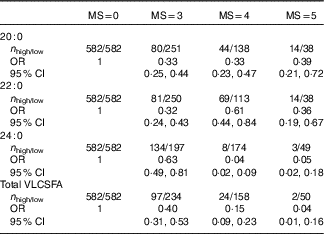
* Multinomial logistic regression was used to estimate the OR and CI. All the OR was adjusted for sex, age and agricultural work. As the sample size in each case group was limited, VLCSFA were classified into high/low group based on the median of VLCSFA in subjects without the metabolic syndrome. Low group was the reference.
Among 899 subjects without the MetS at baseline, we identified 212 incident cases of the MetS during the 2-year follow-up. The level of C20 : 0, C22 : 0 and total VLCSFA was significantly associated with decreased incident MetS risk; comparing with the lowest quartile, the multivariate-adjusted OR for the highest quartile were 0·21 (95 % CI 0·12, 0·35) for C20 : 0, 0·20 (95 % CI 0·11, 0·34) for C22 : 0, 0·75 (95 % CI 0·44, 1·26) for C24 : 0 and 0·28 (95 % CI 0·17, 0·46) for total VLCSFA (Table 4).
Table 4 Associations of very-long-chain SFA (VLCSFA) and risk of incident metabolic syndrome (MetS) after 2 years* (Odds ratios and 95 % confidence intervals)
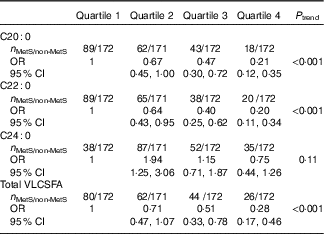
* Only subjects without the MetS at baseline were included in the analysis. VLCSFA were classified into four group based on the quartiles of VLCSFA in subjects without the MetS: 0·18, 0·21 and 0·23 for C20 : 0; 0·60, 0·73 and 0·86 for C22 : 0; 0·46, 0·63 and 0·75 for C24 : 0; 1·32, 1·51 and 1·72 for total VLCSFA. Models were adjusted for sex, age and agricultural work.
The associations between VLCSFA levels and each of five individual MetS components were analysed. Among the five MetS components, the associations were strongest for VLCSFA and elevated TAG (Q4 v. Q1: OR was 0·11 (95 % CI 0·07, 0·17) for C20 : 0, 0·10 (95 % CI 0·06, 0·16) for C22 : 0, 0·07 (95 % CI 0·04, 0·12) for C24 : 0 and 0·04 (95 % CI 0·02, 0·07) for total VLCSFA, P trend<0·0001, Fig. 2, online Supplementary Table S5).
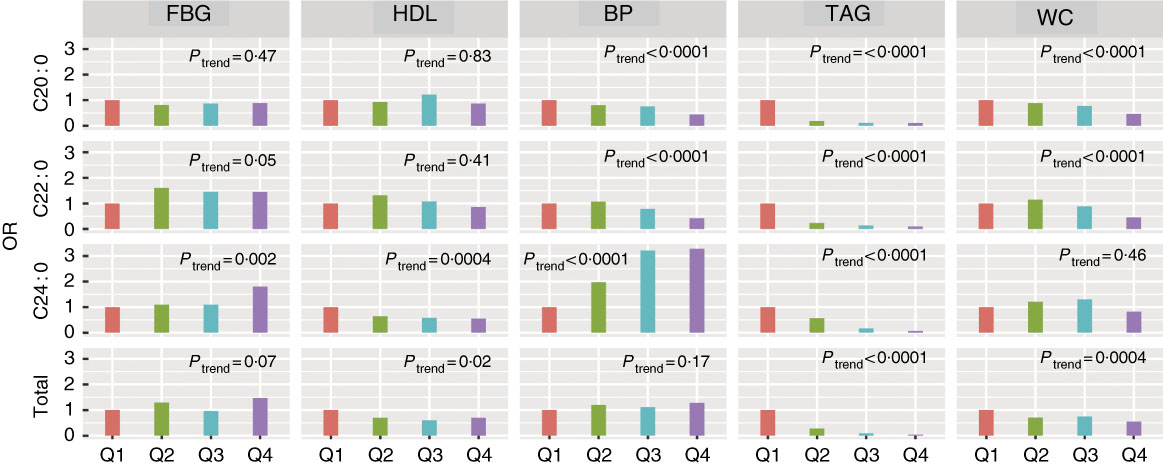
Fig. 2 Associations of very-long-chain SFA and risk of the metabolic syndrome components at baseline. Logistic regression was used to estimate the OR and CI. All the OR were adjusted for age, sex and agricultural work. The five components were adjusted in the models, except for itself. The five components were classified into high/low group according to the cut-off in the metabolic syndrome definition. BP, blood pressure; FBG, fasting blood glucose; WC, waist circumference.
We also explored effect modification on the associations of VLCSFA and MetS risk by several a priori risk factors, including age, sex, smoking status (yes and no), current alcohol drinking (yes and no), physical activity and agricultural working (Fig. 3). The inverse associations between C20 : 0 and MetS risk were stronger in women than in men (OR=0·37 (95 % CI 0·31, 0·44) for women and OR=0·57 (95 % CI 0·46, 0·69) for men; P for interaction=0·002) and in non-alcohol drinkers than in current alcohol drinkers (OR=0·38 (95 % CI 0·32, 0·45) for non-alcohol drinkers and OR=0·61 (95 % CI 0·49, 0·75) for current alcohol drinkers; P for interaction=0·0008) (Fig. 3).
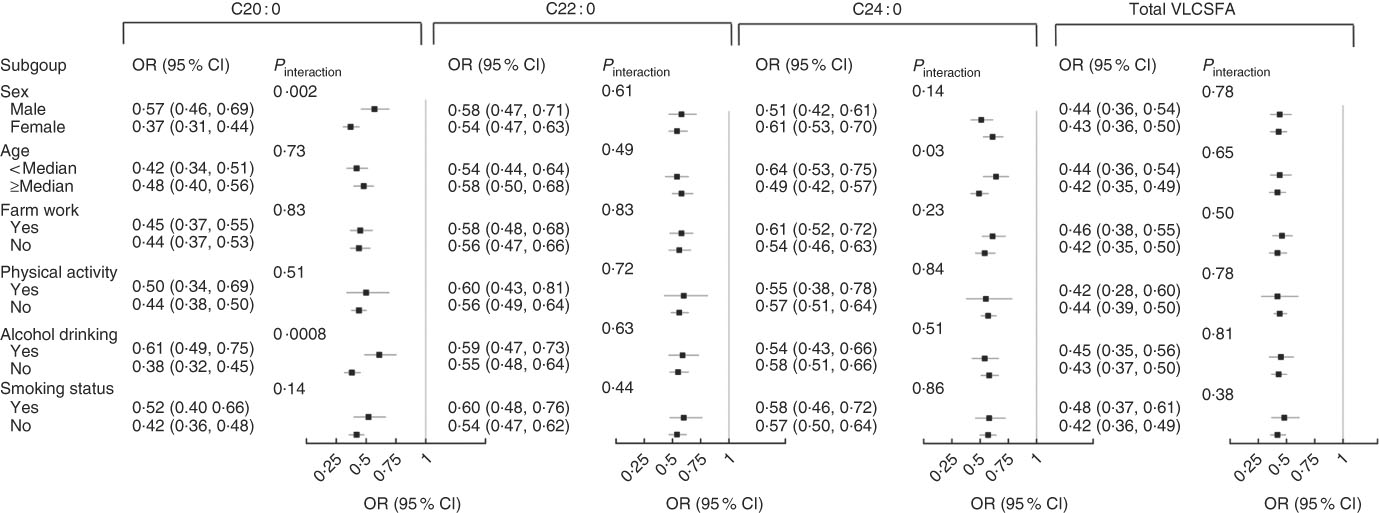
Fig. 3 Stratified analysis of the associations of very-long-chain SFA (VLCSFA) and risk of the metabolic syndrome at baseline. All the OR were adjusted for age, sex and agricultural work. The OR meant the risk of the metabolic syndrome for 1 sd increase of VLCSFA.
Discussion
In a population of 1729 Chinese adults, we observed that plasma VLCSFA levels were significantly and inversely associated with prevalence and incidence of the MetS. Further adjustment for each of C16 : 0, C18 : 0, C18 : 3n-3, C22 : 6n-3, n-6 PUFA or MUFA did not negate the associations. The associations of VLCSFA and MetS risk became stronger with the increase of the number of the MetS components. Among the five individual MetS components, higher plasma VLCSFA were strongly associated with plasma TAG.
Our observations are generally in line with several previous studies that have reported the relationship between blood VLCSFA and various metabolic conditions in other populations( Reference Forouhi, Koulman and Sharp 1 , Reference Lemaitre, Fretts and Sitlani 8 , Reference Fretts, Mozaffarian and Siscovick 10 – Reference Fretts, Mozaffarian and Siscovick 13 , Reference Alhazmi, Stojanovski and Garg 25 ). Three prospective studies published recently showed that higher levels of three VLCSFA (C20 : 0, C22 : 0 and C24 : 0) were associated with lower risk of incident diabetes( Reference Forouhi, Koulman and Sharp 1 , Reference Lemaitre, Fretts and Sitlani 8 , Reference Alhazmi, Stojanovski and Garg 25 ). A study with 3179 participants from the Cardiovascular Health Study (CHS) reported that higher plasma phospholipid VLCSFA were cross-sectionally associated with lower blood TAG and WC( Reference Lemaitre, Fretts and Sitlani 8 ). In the Nurses’ Health Study and Health Professionals Follow-Up Study, plasma VLCSFA were positively correlated with HDL( Reference Malik, Chiuve and Campos 11 ). Diabetes, WC, circulating TAG and HDL are components of the MetS. Consistently, we found inverse associations between plasma VLCSFA and MetS risk; TAG showed the strongest associations with VLCSFA. In addition, we found significant inverse associations of C20 : 0 and C22 : 0 with WC, and direct associations of C24 : 0 with HDL. However, we observed that C22 : 0 and C24 : 0 were directly associated with fasting blood glucose, which was different from the results of above studies. If the covariate of TAG was excluded from the analyses model in our study, the associations of C22 : 0 and C24 : 0 with fasting blood glucose were reversed. In the CHS study, VLCSFA were associated with lower risk of diabetes, but if blood TAG was adjusted in the model, the associations were not significant( Reference Lemaitre, Fretts and Sitlani 8 ). This suggested that the associations of VLCSFA with blood glucose might be mediated by lower TAG. Mechanistic studies are needed to confirm these results. Other recently published studies reported inverse associations of circulating levels of VLCSFA with atrial fibrillation( Reference Fretts, Mozaffarian and Siscovick 10 ), cardioembolic stroke( Reference Chung, Cho and Do 12 ), CHD( Reference Malik, Chiuve and Campos 11 ) and total mortality( Reference Fretts, Mozaffarian and Siscovick 13 ). The MetS was a major intermediate risk factor of these diseases and may explain these associations( Reference Hata, Doi and Ninomiya 26 – Reference Janczura, Bochenek and Nowobilski 28 ).
The biological functions of VLCSFA in vivo remain unclear. VLCSFA are major components of ceramides and sphingomyelins, which are key intermediates linking saturated fats and inflammatory cytokines to regulate cell function. Insulin resistance is the key feature of the MetS. VLCSFA are major components of ceramides and sphingomyelins. A large body of evidence from non-human experimental studies has supported a role of ceramides in insulin signalling and β-cell function as well as the development of diabetes( Reference Chavez and Summers 29 ). Specifically, C16-ceramide has been suggested to induce β-cell apoptosis, impair insulin signalling and reduce insulin synthesis, which can lead to insulin resistance( Reference Galadari, Rahman and Pallichankandy 30 , Reference Summers 31 ). In contrast, ceramides with VLCSFA appear to be protective. VLCSFA affect liver homoeostasis, myelin maintenance and anti-inflammatory response through inhibition of endogenous C16-ceramide synthesis( Reference Kihara 14 , Reference Pewzner-Jung, Brenner and Braun 16 ), but the biological pathways have not been fully understood and need to be investigated in future mechanistic studies. In addition, very-long-chain acyl-CoA synthetase may be up-regulated by PPARδ and evidence showed that PPARδ played a role in fat metabolism to prevent obesity( Reference Wang, Lee and Tiep 32 ).
The sources of circulating VLCSFA include both exogenous diet and endogenous synthesis( Reference Kihara 14 ). The VLCSFA are found in limited foods, including peanuts, lotus nuts and rapeseed/rapeseed oil( Reference Yang, Wang and Pan 15 ). Several studies have shown that peanut intake increases blood C22 : 0 and C24 : 0( Reference Lemaitre, Fretts and Sitlani 8 ). Interestingly, peanut butter consumption was significantly associated with a more favourable plasma lipid profile, including lower LDL-cholesterol, and total cholesterol in the Nurses’ Health Study( Reference Li, Brennan and Wedick 33 ). VLCSFA can be synthesised from C18 : 0 by elongases, especially elongation of very long chain fatty acids protein 1 (ELOVL1), which selectively elongates SFA with more than eighteen carbons( Reference Kihara 14 ). Indeed, our data show that the fatty acids related to each other by a single round of elongation were highly correlated, suggesting that higher dietary intake resulted in higher levels of the elongation product: 18 : 0→20 : 0 and 20 : 0→22 : 0 (the Spearman coefficients were 0·48 for C18 : 0 and C20 : 0, and 0·75 for C20 : 0 and C22 : 0). The ELOVL1 gene coding for the first committed step in elongation is regulated in concert with ceramide synthase 2 (CERS2), a key enzyme for C24 sphingolipid synthesis( Reference Ohno, Suto and Yamanaka 34 ). This regulation may ensure that the production of C24-CoA by elongation is coordinated with its utilisation. How the dietary intake and endogenous metabolism contribute to circulating levels of VLCSFA and subsequently affect the metabolic disorders requires further study.
We also explored the modifying effects by several a priori factors on the relation of VLCSFA to MetS risk. We found a stronger association between C20 : 0 and MetS risk in subjects without alcohol drinking than those with current alcohol drinking. This phenomenon was consistent with the finding in the CHS study and our study that C20 : 0 was inversely correlated with the amount of alcohol drinks and current alcohol drinking status (yes/no)( Reference Lemaitre, Fretts and Sitlani 8 ). Heavy alcohol drinking is a risk factor for the MetS( Reference Baik and Shin 35 ) and may neutralise the beneficial effects of VLCSFA on the MetS. We also observed moderate interaction effects of sex on the association of C20 : 0 with the MetS. In the CHS study and our study, women tended to have higher C20 : 0( Reference Lemaitre, Fretts and Sitlani 8 ), which may explain our findings that the association of C20 : 0 and MetS risk was stronger in women than in men.
Our study is the first study to explore the associations of plasma VLCSFA and MetS risk in Chinese population. Our study has some limitations. First, the study design was initially cross-sectional, but we confirmed the cross-sectional results among baseline non-MetS subjects with 2-year follow-up data. Second, the half-life of plasma fatty acids was short and it may not reflect long-term dietary pattern. The fatty acids of erythrocyte or fat adipose tissue were needed to confirm the associations of VLCSFA with MetS risk. Third, we analysed statistically plasma fatty acids in units of weight percentage as is reported in most studies( Reference Lemaitre, Fretts and Sitlani 8 , Reference Malik, Chiuve and Campos 11 ), which is most useful in assessing whether fatty acid imbalance is related to metabolic status. Specific fatty acid concentration (e.g. mg/ml plasma) is more appropriate to assess whether the mass of a specific circulating fatty acid is outside of a range that is needed to support the metabolism( 36 ). Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr in press). In addition, with the advancement of lipidomics technology, a detailed identification and quantification of VLCSFA in different lipid classes or tissues is of crucially helpful in understanding their roles in metabolic disorders. Unfortunately, we did not measure the fatty acids in different lipid classes (TAG, sphingomyelins, etc.). Fourth, no information on the lipid-lowering medication use was available. However, the hyperlipidaemia awareness rates (8·3 %), treatment rates (7·0 %), and detection rates (26·2 %) were very low in China, especially in rural areas( Reference Chang and Wang 37 ), which suggests a possible mild lipid-lowering medication influence. Last but not least, compared with other fatty acids such as n-6 PUFA, C16 : 0 and MUFA, the proportion of VLSCFA was very low. However, after adjusting for each of these fatty acids separately, the association of VLCSFA and risk of the MetS remained statistically significant. Besides, we also adjusted for other potential confounders including lifestyle factors in the statistics model, but residual confounding by non-available or unknown factors is also possible, such as menopausal status of women or dietary VLCSFA.
In summary, we confirmed that higher levels of plasma VLCSFA are significantly associated with lower risk of the MetS in Chinese adults. The association of VLCSFA with blood TAG was stronger than other four MetS components. Further studies are warranted to explore the underlying mechanisms.
Acknowledgements
We thank Di Du from the Fudan University and Shuchun Lin from the Shanghai Institutes for Biological Sciences for their help in statistic consultation.
This work was supported by the National Key Research and Development Plan (Y. G., grant no. 2016YFD0400200) and the 100 talented plan of Chinese Academy of Sciences (Y. G.). This research received no specific grant from any funding agency, commercial or not-for-profit sectors. All funders had no role in the design, analysis or writing of this article.
Y. G. and J. Z. formulated the research questions and designed the study. J. Z., X. L., X. L., Q. C., Y. Z. and Z. L. carried it out. J. Z., X. L. and H. Z. analysed the data. J. Z., T. J. B., Y. S. and Y. G. wrote the article.
The authors declare that there are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114518002106










