Article contents
Health technology assessment–based development of a Spanish breast cancer patient decision aid
Published online by Cambridge University Press: 07 October 2011
Abstract
Objectives: The aim of this study was to develop a breast cancer Patient Decision Aid (PDA), using a Health Technology Assessment (HTA) process, to assist patients in their choice of therapeutic options, and to promote shared decision making among patients, healthcare professionals, and other interested parties.
Methods: A systematic review (SR) was conducted of existing breast cancer patient Decision Aids encountered in the main scientific journal databases and on institutional Web sites that create PDAs, together with a Qualitative Research (QR) study, using semi-structured interviews and focus group with stakeholders (patients, family members, and health professionals), with the aim of developing a PDA for breast cancer.
Results: The SR shows that PDAs in breast cancer not only increase patient knowledge of the illness, leading to more realistic expectations of treatment outcomes, but also reduce passivity in the decision-making process and facilitate the appropriate choice of treatment options in accordance with patient medical and personal preferences. The analysis of QR shows that both breast cancer patients and healthcare professionals agree that surgery, adjuvant treatments, and breast reconstruction represent the most important decisions to be made. Worry, anxiety, optimism, and trust in healthcare professionals were determined as factors that most affected patients subjective experiences of the illness. This HTA was used as the basis for developing a PDA software program.
Conclusions: The SR and QR used in the development of this PDA for breast cancer allowed patients to access information, gain additional knowledge of their illness, make shared treatment decisions, and gave healthcare professionals a deeper insight into patient experiences of the disease.
Keywords
- Type
- THEME: PATIENTS AND PUBLIC IN HTA
- Information
- International Journal of Technology Assessment in Health Care , Volume 27 , Issue 4 , October 2011 , pp. 363 - 368
- Copyright
- Copyright © Cambridge University Press 2011
References
REFERENCES
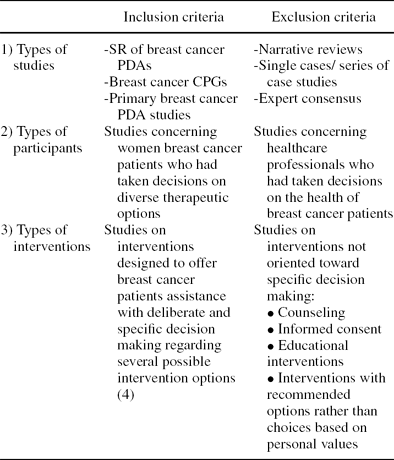
Table 1. Inclusion and Exclusion Criteria for Breast Cancer PDA Studies
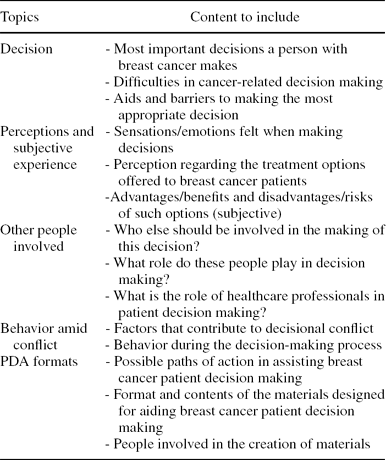
Table 2. Script Used for Semi-structured Interviews and Focus Group With Health Professionals, Patients, and Family Members

Table 3. Breast Cancer Patient Profile Items

Table 4. Results of the SR of Breast Cancer PDAs
- 10
- Cited by



All too often, there is minimal or inappropriate evidence of the benefit/risk of the different cancer treatments involved, with the added uncertainty that outcomes tend to vary from one individual to another (Reference Edwards and Elwyn2). Patient subjective perceptions of how they feel, their judgment of how long treatments should last, or their knowledge of the pros and cons of chosen treatments also vary significantly. Approximately, 50 percent of newly diagnosed breast cancer patients feel clinically significant levels of anxiety, distress, or depression. In this context, Breast cancer Patient Decision Aids (PDA) are useful for increasing patient knowledge and confidence in the decision-making process, while serving to reduce decisional conflicts (Reference Edwards and Elwyn2).
Given the lack of appropriate aid tools within this area, the Madrid based Lain Entralgo Agency's Health Technology Assessment Unit (UETS) has identified the need to develop a national PDA. In Spain, a generally paternalistic attitude among physicians toward breast cancer patients still exists. However, our objective has been to create a PDA based on both a review of scientific evidence and patient opinions and experience, using a systematic and rigorous HTA process. The systematic approach used has enabled this research group to create a PDA of international relevance, given that it is appropriate for use in all Spanish speaking countries
The objective of this research was to create a tool to facilitate shared patient/physician decision making. The resulting PDA allows patients, through face to face collaboration with healthcare professionals, to adopt a more active role in the choice of treatment options, in accordance with medical and personal preferences.
METHODS
A systematic review (SR) of existing breast cancer patient PDAs, as well as a series of qualitative research (QR) interviews of patients, family members, and healthcare professionals was conducted to create a specifically designed PDA for breast cancer patients, in accordance with IPDAS (International Patient Decision Aid Standards) methodology (Reference Elwyn, O'Connor and Stacey3).
Systematic Review
The PDAs included in the study, published in both English and Spanish between 1999 and December 2009, were found in MEDLINE, EMBASE, CINAHL, PsycINFO, Cochrane Library, INAHTA, and CRD databases.
Clinical Practice Guidelines (CPG) for breast cancer patients were also reviewed from varying sources, such as the National Guideline Clearinghouse (USA), the Canadian Medical Association, the National Library for Health (UK), the National CPG Program of Spain (GuiaSalud), New Zealand Guidelines Group (NZGG), and the National Health, and Medical Research Council (NHMRC) of Australia.
Health Technology Assessment Agency publications and the Web sites of international institutions working with PDAs, such as the Ottawa Hospital Research Institute (OHRI), the Foundation for Informed Medical Decision Making (USA), and the “informedhealthchoice” group (Cardiff University) were also reviewed.
The inclusion and exclusion criteria are shown in Table 1. The evidence presented in the selected studies was classified in accordance with SIGN (Scottish Intercollegiate Guidelines Network) (18) recommendations, the Oxman et al. checklist for systematic reviews (Reference Oxman, Cook and Guyatt15), the Guyatt et al. checklist for clinical trials and observational studies (Reference Guyatt, Sackett and Cook5), and the QARI (Critical Evaluation Instrument) checklist for qualitative studies (Reference Pearson16). CPGs were assessed with the AGREE instrument (Appraisal of Guidelines Research and Evaluation) (21). Finally, the Quality assessment of the PDAs found was analyzed in accordance with IPDAS quality criteria (Reference Elwyn, O'Connor and Stacey3).
Table 1. Inclusion and Exclusion Criteria for Breast Cancer PDA Studies
SR, systematic review; PDA, patient decision aid; CPG, clinical practice guideline.
Qualitative Research
Semi-structured interviews and focus group discussion methods were used to assess the psychosocial needs of female breast cancer patients. Information on patients requests and their personal experiences of the illness was gathered, while the specific needs arising in the therapeutic options decision-making process were identified through group discussions.
Semi-structured interviews with healthcare professionals, breast cancer patients, and family members were conducted and audio recorded by a UETS sociologist and a psycho-oncologist, in accordance with the indications stated in the Jacobsen and O'Connor manual (Reference Jacobsen and O'Connor6). The focus group consisted of breast cancer patients and discussion sessions were synchronized in time with the interviews. The discussion groups were open and provided with a moderator and observer. To structure the total time available, a script to elicit responses to relevant topics was written using relevant aspects from the systematic review. The content of the script is shown in Table 2.
Table 2. Script Used for Semi-structured Interviews and Focus Group With Health Professionals, Patients, and Family Members
Patients and professionals were selected for interview using similar sampling criteria to the Gorden's four questions-criterions for the selection of contexts and cases (Reference Valles22). Three target-groups were established: healthcare professionals from Spanish hospitals (gynecologist, oncologist, oncology radiotherapy nurse, oncologist surgeon, plastic surgeon, and psycho-oncologist), patients (women with breast cancer, 20 to 85 years old, undergoing any type of treatment and at any stage of the disease), and patient family members. Recruitment was made possible thanks to the personal and social networks of the researchers not involved in the project, including patient organizations. Snowball sampling strategy was used (Reference Salganik and Heckathorn17) and patient interviews were planned in accordance with the typology shown in Table 3. Criteria identical to that used during the interview stage was applied to define the profiles of the women who participated in the focus group.
Table 3. Breast Cancer Patient Profile Items
Before interviews and focus group discussions, informed consent was requested to ensure that the information collected could be used for research purposes. A transcription of the interviews and focus group discussions was analyzed, inserting the most representative verbatim within a basic categorization (Topics, Table 2). Simultaneously, the qualitative assessment of the data collected was processed with qualitative analysis software (NVIVO8).
Design of the PDA
A Patient Decision Aid Tool for women breast cancer patients was created through an HTA process, involving the SR and QR described. The information gathered was included in the content of the PDA, together with the results from QR on the needs expressed by members of the focus group during the therapeutic option decision-making process. The most appropriate PDA format was determined, in accordance with IPDAS (Reference Elwyn, O'Connor and Stacey3) and the Ottawa Decision Support Framework (Reference O'Connor and Jacobsen13) criteria.
RESULTS
Systematic Review
Subsequent to the elimination of duplicated studies, a total of 512 were identified as relevant for our purposes, from a total of 564. A further reading of the abstracts, resulted in the selection of 263 studies, from which 10 were finally chosen for this work. Of this final total, two were SRs, two were CPGs, and six were primary studies (three RCTs, one quasi-experimental study, one descriptive study, and one qualitative study) (1;Reference Lacey7–Reference Morris, Drake, Saarimaki, Bennett and O'Connor9;Reference O'Connor, Bennett and Stacey11;14;Reference Stacey19;Reference Street, Voigt, Geyer, Manning and Swanson20;Reference Whelan, Sawka and Levine23;Reference Whelan, Levine and Willan24). The results gathered, in accordance with IPDAS group evaluation criteria (Reference Elwyn, O'Connor and Stacey3;Reference O'Connor, Bennett and Stacey11), are shown in Table 4.
Table 4. Results of the SR of Breast Cancer PDAs
PDAs, patient decision aids; RR, relative risk; CI, confidence interval; P, p-value; MD, mean deviation.
Qualitative Research
The healthcare professionals interviewed included: one gynecologist, one oncologist, one oncology radiotherapy nurse, one oncologist surgeon, two plastic surgeons, and one psycho-oncologist. Two patients and two family members were also interviewed individually. The focus group included six patients. The interviews and focus group were conducted at UETS facilities, with the exception of five interviews with healthcare professionals, carried out in their respective hospitals. Results of the QR were grouped into the following subject sections (See Method Topics, Table 2).
Decisions. Clinicians, patients, and family members consider surgery, adjuvant treatments, and breast reconstruction as the most important decisions to be made. Patients and family members judged the main difficulties in decision making to be: the absence of a trusted professional from whom to receive information, a lack of support from family members, myths regarding treatment side effects, and little assistance in decision making. Healthcare professionals believe that difficulties arise due to a lack of decision-making skills, little trust in the professional, age (“the younger the patient the more decisional conflict”), uncertainty regarding the most important aspects, changes in body image, and social pressure (“especially from close family”). Both professionals and family members considered the following aspects as helpful for decision making: patient awareness of their own involvement in the decision-making process, support (from psychologists, physicians, spouses and patient associations), reliable general information, and probability projections associated with each therapeutic option.
Perceptions: Subjective Experience and Expectations. Healthcare professionals believe that patients have an inherent desire to trust physicians, as a means to overcoming “the fears” surrounding their illness. Patients themselves expressed both negative (feelings of imminent death, loneliness, worry, and anxiety) and positive emotions (self-encouragement, confidence in their chosen choice of action, optimism).
Other People Involved. Patients and healthcare professionals taking part in our qualitative research stated that although spouses should play a supportive role in the decision-making process, any final decision should be made by patients themselves. Close family members believe that they and the family doctor should be involved in the process.
Decision-Making Behavior: Decisional Conflict. In an attempt to reduce decisional conflict, patients show a tendency to seek a second medical opinion and alternative therapies. Clinicians believe that patients should practice relaxation techniques or participate in discussion groups to gain support from other patients facing the same illness.
Ideal PDA Format and Contents. Patients consider that PDAs in hard copy format provide immediate availability, while multimedia formats, such as a computer application were considered as ideal and effective at offering detailed information on treatment phases in interactive modules. Information on body image and chemotherapy side effects was also important to patients. Family members specified information on benefits/risks expressed in terms of probability as important and clinicians highlighted the need for a simple, picture-based, and interactive format.
Patient Decision Aids for Breast Cancer: The Tool
The PDA includes clinical information about invasive and noninvasive breast cancer treatment. It is not aimed at patients with lobular carcinoma in situ, nor inflammatory breast cancer. Results of the QR led to the selection of software with support material on paper as the most appropriate format, allowing the patient immediate access to the contents. The software was designed and developed by a UETS psycho-oncologist and a multimedia content specialist, under the technical direction of the UETS. The PDA offers an interrelated treatment sequence that leads to a particular situation created specifically for the user. It includes visual aids that explain the probability of benefits/risks, information regarding body image, experiences of other people in similar situations, decisional balance sheets with which to reflect on the pros and cons of each option and, statistical and audio-animated graphics.
The PDA tool is available on the Spanish Network of HTA Agencies website (http://aunets.isciii.es/web/guest/acceso_apoyo_metodo).
DISCUSSION
In contrast to the shared decision-making approach taken in English speaking countries (Reference Edwards and Elwyn2;Reference O'Connor, Drake, Fiset, Graham, Laupacis and Tugwell12), doctor–patient relationships in this country have been traditionally centered on a more paternalistic approach. However, as is evident from this PDA, this new paradigm is slowly becoming accepted within our healthcare system
The objective of this study was to describe how HTA processes, involving a Systematic Review of evidence and Qualitative Research study, have been used to develop a PDA for female breast cancer patients in Spain. After an assessment of the different “technologies” used in breast cancer treatments, our findings were applied to the development of this tool; the first of its kind in Spanish National Healthcare System within the aforementioned clinical context and an instrument through which Spanish speaking patients and healthcare professionals may manage the uncertainty involved in treatment choices. This tool serves as an aid to gaining a greater understanding of the disease and a deeper awareness of other patient experiences. Moreover, the fact that the systematic approach to obtaining patient preferences and values was effective and the fact that the tool may be used in other Spanish speaking countries endow this PDA with a truly international relevance. Its design has been focused on providing a response to patient needs identified through the HTA process. The methodology used was effective in capturing many spontaneous thoughts, given that patients could freely express their ideas in an encouraging atmosphere of easy going group discussion, based on the principles of sociological pairing (Reference Valles22). For this reason, our sampling has tried to represent all of the psychosocial profiles necessary. With the interviews of both patients and professionals, we have included a dual view of breast cancer; from those who treat it and those who suffer from the disease.
The results would have been even more detailed if we had been able to work with a larger number of patients recruited from different geographical locations. This was not possible due to limited time and resources. Regardless, the qualitative research has obtained answers to all of the questions posed in the script developed from the previous systematic review. The tool is based mainly on experience of the disease and not on organizational differences in the area of assistance.
A validation process of the PDA involving patient participation has yet to be performed, so expectations may not have been incorporated sufficiently. However, a pilot phase with this involvement is planned before the implementation phase to ensure that any pertinent modifications may be incorporated.
The tool's content assists healthcare professionals and patients in the understanding of how diagnosis, clinical history, and personal values and preferences affect decision making regarding treatment options. The tool will also be of great utility value for family members, carers or any other party with an interest in breast cancer.
The emotional impact of a breast cancer diagnosis in combination with the large quantity of contrasting information can be overwhelming for patients, making this PDA essential. With regard to the psychological aspects of the disease, there is no “one size fits all” strategy for patients when it comes to facing cancer. For this reason, personal values and preferences are explored within the tool. The PDA contains decisional balance instruments to assist patients in their choice of treatment options, in accordance with individual preferences.
This tool disregards therapeutic options that present little or insufficient evidence and focuses on the most widely used treatments for which robust published evidence can be found in the main databases. Treatments not endorsed by evidence could confuse patients in the decision-making process.
In conclusion, the present PDA for breast cancer, developed within the framework established by the HTA Agencies, has been created following a systematic and rigorous HTA process. It has succeeded in improving the quality of decisions for specific situations and has encouraged a shared decision-making approach in which both patients and healthcare professionals take on a participative role. This work supports and facilitates objective patient involvement within the HTA process; a need that has already been recognized at an international level by the network of agencies. In addition to incorporating quantitative evidence, it incorporates evidence eliciting patient perspectives obtained through social science research, including primary qualitative research conducted by our team at the UETS (Reference Facey, Boivin and Gracia4).
CONTACT INFORMATION
Fatima Izquierdo, Masters in Psycho-Oncology ([email protected]), Researcher, Javier Gracia, MD, MPH ([email protected]), Researcher, Mercedes Guerra ([email protected]), Academic Librarian, Documentalist; Juan Antonio Blasco, MD, MPH, MHTA ([email protected]), Health Technology Assessment Unit (UETS) Director, Elena Andradas, MD, MPH, MHTA, ([email protected]), Agencia Lain Entralgo, Unidad de Evaluación de Tecnologías Sanitarias, C/Gran Via 27, Madrid 28013, Spain
CONFLICT OF INTEREST
All authors report they have no potential conflicts of interest.