Introduction
Obsessive-compulsive disorder (OCD) is characterized by repetitive, intrusive thoughts, images, or urges that cause anxiety and/or distress (obsessions) and anxiety and/or distress-relieving repetitive motor or mental acts (compulsions).1 The etiology of OCD is complex and involves both geneticReference Nestadt, Grados and Samuels2 and environmental factors.Reference Brander, Pérez-Vigil, Larsson and Mataix-Cols3-Reference Destrée, Albertella and Torres6 There is a greater prevalence of OCD in biological relatives of individuals diagnosed with OCD compared to controls,Reference Mataix-Cols, Boman and Monzani7 with the risk for OCD increasing with greater genetic relatedness, as indicated by a higher prevalence in close family members.Reference Mataix-Cols, Boman and Monzani7 Examination of monozygotic (MZ) and dizygotic (DZ) twins revealed a stronger concordance among MZ twins, suggesting a genetic influence of OCD.Reference Mataix-Cols, Boman and Monzani7
The etiology of OCD involves a genetic contribution of approximately 40–45%,Reference Mataix-Cols, Boman and Monzani7, Reference Taylor8 a finding that also emphasizes the importance of non-genetic environmental influences, including perinatal complications, reproductive cycle events, and infections, as well as trauma and stressful life events (SLEs).Reference Brander, Pérez-Vigil, Larsson and Mataix-Cols3, Reference Destrée, Albertella and Torres6, Reference Pauls, Abramovitch, Rauch and Geller9 Notably, non-shared environmental factors exerted a more substantial impact on OCD etiology than shared factors, such as parenting styles.Reference Cromer, Schmidt and Murphy10 While no single risk factor can be deemed causal for OCD,Reference Brander, Pérez-Vigil, Larsson and Mataix-Cols3 it is important to explore the intricate interplay between genetic and environmental elements, which may provide valuable insights for developing therapeutic and preventive interventions.
The concept of SLEs is probably broader and more heterogeneous than trauma. The DSM-511 defines a traumatic event as an “actual or threatened experience of death, serious injury, or sexual violence.” Although there is no universally accepted definition of SLEs (whether traumatic or not), they are usually described as events that induce either psychological or physiological distress, disrupt established goals, undermine control over a given situation, and require a subsequent period of adjustment or adaptation.Reference Adams, Kelmendi, Brake, Gruner, Badour and Pittenger12-Reference Dohrenwend14 The variability in SLEs’ definitions underscores the challenge of systematically categorizing and assessing SLEs and complicates research in this area. We speculate that OCD precipitated by SLEs may be commoner than OCD associated with traumatic events, given the greater prevalence of SLEs as compared to trauma.
The connection between trauma and OCD onset has been well-established in previous research,Reference Auxéméry15, Reference Fostick, Nacasch and Zohar16 with efforts made to delineate features associated with a putative posttraumatic OCD.Reference Cromer, Schmidt and Murphy10, Reference Fontenelle, Cocchi and Harrison17, Reference Araújo, Fontenelle and Berger18 Traumatic events were associated with more severe OCD symptoms in OCD patients, even after controlling for comorbidities and depressive symptoms.Reference Cromer, Schmidt and Murphy10 In the latter study, trauma was also significantly associated with checking and ordering compulsions.Reference Cromer, Schmidt and Murphy10 The association between trauma and more severe OCS was corroborated by Barzilay et al.Reference Barzilay, Patrick, Calkins, Moore, Gur and Gur19 in community youth. Fontenelle et al.Reference Fontenelle, Cocchi and Harrison17 compared individuals with “posttraumatic” versus “pre-traumatic” OCD and found the former to exhibit a later age at the onset of OCD, greater rates of self-mutilation, increased suicidality, and more frequent comorbidities with panic disorder with agoraphobia, and compulsive buying disorder.Reference Fontenelle, Cocchi and Harrison17 Thus, it seems that trauma is associated with a differentiated expression of OCD.
Despite the increasing understanding of the role of trauma in OCD, there is a paucity of studies investigating the relationship between SLEs more broadly and OCD.Reference Adams, Kelmendi, Brake, Gruner, Badour and Pittenger12 There is, however, extensive research exploring the links between SLEs and mood disorders, particularly depression.Reference You and Conner20-Reference Paykel22 Several studies suggest depression to be the most common comorbidity among treatment-seeking patients with OCD.Reference Zimmerman and Mattia23 Research has explored the influence of SLEs on depression’s onset,Reference Kendler, Karkowski and Prescott24 course,Reference Bos, Bouhuys, Geerts, van Os and Ormel25, Reference Paykel26 and gender distribution,Reference Shaik, Rajkumar, Menon and Gender27 and the impact of different typesReference Shaik, Rajkumar, Menon and Gender27–Reference Mitchell, Parker, Gladstone, Wilhelm and Austin29 and severities of SLEs on people with depression.Reference You and Conner20, Reference Mitchell, Parker, Gladstone, Wilhelm and Austin29 In a study examining the sociodemographic characteristics of depression preceded by exposure to SLEs, a higher risk for depression recurrence was identified among widowers lacking a support network, those with a history of suicidal behavior, and those receiving inadequate antidepressant treatment.Reference Serafini, Gonda, Canepa, Geoffroy, Pompili and Amore30 Despite being overrepresented among people with non-melancholic depression in one study,Reference Mitchell, Parker, Gladstone, Wilhelm and Austin29 depression after SLEs has also been associated with increased symptom severity and lower global functioning.Reference Muscatell, Slavich, Monroe and Gotlib31
Interest in the impact of SLEs on the phenotype of people with OCD has increased recently. More specifically, 2 studies addressed the links between SLEs and types of OCD symptoms. One reported an association between SLEs and symmetry symptoms in a sample at transdiagnostic risk for mental disorders, even after adjusting for comorbidities and general psychological distress.Reference Destrée, Albertella and Jobson32 Curiously, as described above, symmetry/ordering has also been associated with trauma.Reference Cromer, Schmidt and Murphy10 The other study identified a connection between SLEs in OCD and the presence of sensory phenomena, described as “just right” experiences, a sense of incompleteness, a subjective feeling of something being awry within the individual, and a discordant sense that one’s actions or experiences have not been properly completed.Reference Poletti, Gebhardt, Pelizza, Preti and Raballo33
Available evidence indicates that certain categories of SLEs, such as interpersonal abuse and family disruption,Reference Vidal-Ribas, Stringaris, Rück, Serlachius, Lichtenstein and Mataix-Cols34 may be associated with more severe OCD symptoms. This association remained significant even when controlling for other variables and specific OCD symptom dimensions, including aggression, sex/religion, and contamination.Reference Fontenelle, Destrée, Brierley, Thompson, Yücel and Albertella35 It is worth considering that the influence of SLEs on the development of OCD may not solely depend on the presence or type of SLEs but rather on the individual’s reactions and responses to these events.Reference Boals36, Reference Dykshoorn37 For instance, heightened thought suppression following a SLE is associated with elevated OCS prevalence.Reference McLaren and Crowe38 Additionally, research indicates that individuals who have experienced traumatic events often employ avoidance mechanisms as coping strategies.Reference Follette, Palm and Pearson39 Although it is unclear whether people exposed to SLEs use similar strategies, they may relieve distressing internal states only temporally and ultimately exacerbate unwanted distress.Reference Thompson, Arnkoff and Glass40
Based on the existing links between trauma and certain OCD cases, the role of SLEs in different mental disorders highly comorbid with OCD (eg, depression), the association between SLEs and certain types of OCD symptoms, and the impact of SLEs on the severity of OCD symptoms, we thought it would be justifiable to perform a broader review on the influence of SLEs of different OCD characteristics. More specifically, the present study aims to summarize the evidence on the difference between OCD whose onset was precipitated by SLEs (SLEs OCD) and OCD whose onset was unrelated to SLEs (“spontaneous” OCD or NSLEs). This systematic review sought to answer the following question: what are the sociodemographic and clinical features overrepresented in SLEs OCD?
Methods
This review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42020206894.Reference Huhne, Santos-ribeiro, Moreira-de-oliveira, De Menezes and Leonardo41 The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement.Reference Page, McKenzie and Bossuyt42
Criteria for considering studies for this review
Types of studies
We searched for cohort studies (retrospective and prospective) and case–control studies.
Types of participants
The target population was people diagnosed with OCD according to a formal/valid diagnostic tool.
Type of exposure
The exposure was the presence of SLEs identified with formal/valid tools.
Types of comparators
We compared the sociodemographic and clinical features of individuals whose OCD was precipitated by SLEs to the ones of individuals whose OCD was not precipitated by SLEs.
Types of outcome measures
The primary outcome of interest was OCD’s symptom dimension identified with formal/valid tools and OCD symptom severity measured with formal/valid tools. The secondary outcomes were gender, family history of OCD, age of OCD onset, comorbid disorders (measured with formal/valid tools), and the presence of depressive symptoms (measured with formal/valid tools).
Search methods for identification of studies
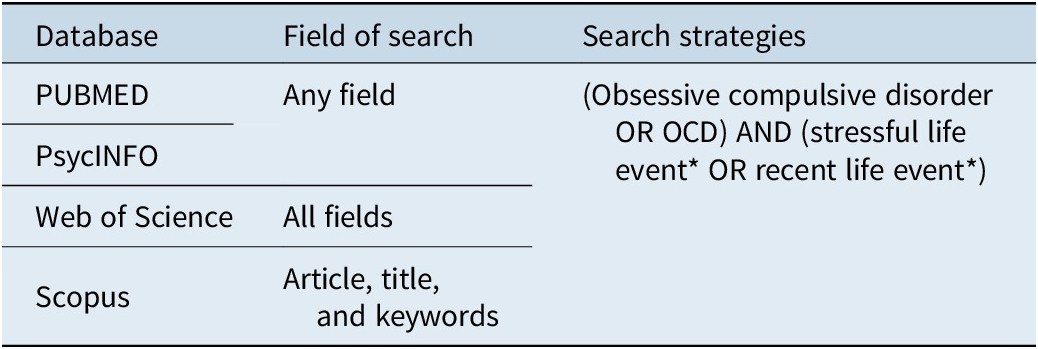
The search was conducted on November 27, 2023, in the databases PUBMED, Web of Science, PsycINFO, and Scopus. We did not constrain the search on a date or language. In addition, one independent reviewer (ME.M.-O.) performed a hand search of the selected studies’ reference lists to supplement the database search. The search strategy for each database is presented in Table 1.
Table 1. Online Search Features
Duplicate titles across databases were removed. Three independent reviewers (V.H. and S.S.-R. or V.H. and ME.M.-O.) assessed the articles. The screening was performed in 2 phases (title-abstract and then full text), and those articles that did not meet the inclusion criteria were excluded. Any disagreement was solved by a debate between the 3 researchers. In the absence of a consensus, a fourth researcher (L.F.F. or G.B.M.) made the decision. Unavailable reports were requested from the authors by email.
Data extraction and management
The data extraction was performed by 1 researcher (V.H.) and revised by 2 others (ME.M.-O., S.S.-R.). Any disagreement was resolved during our meetings. Qualitative and quantitative data were collected using an extraction template. We extracted information regarding the country of study, population, diagnostic method, demographics, age at onset, duration of symptoms, global and dimensional symptoms’ severities, and comorbidities. We also extracted information regarding study design, exposure data (SLEs scales), and information for risk of bias assessment. We have contacted the corresponding authors in case of doubts or the absence of critical information.
Assessment of risk of bias in included studies
We assessed the risk of bias through the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case–Control Studies.Reference Moola, Munn, Tufanaru, Aromataris, Sears, Sfetcu, Currie, Qureshi, Mattis, KMP-F and Aromataris43 All papers were evaluated by one researcher (V.H.) and revised by 2 others (ME.M.-O., S.S.-R.). Each aspect listed in the checklist was classified as “yes,” “no,” “unclear,” or “not applied” for each selected study. The study was only included in our review if less than 4 aspects were classified as “no.” In case of doubts or insufficient information on the report, corresponding authors were contacted for clarification. In case of disagreements, a fourth researcher (either L.F.F. or G.B.M.) made the decision.
Data analysis
All included studies were qualitatively summarized, but only 3 were able to be included in the meta-analysis. The meta-analysis data were plotted and managed in the software Comprehensive Meta-analysis (CMA) version 4.Reference Borenstein44 The effect size was calculated as the odds ratio to identify the relationship between SLEs and dichotomous sociodemographic and clinical characteristics, including gender, family history of OCD, and mood comorbidities. In contrast, the Hedges’ g statistic was used to calculate the effect size of continuous variables such as the severity of OCD (as measured by YBOCS), the age at OCD onset (in years), and the severity of depression (as measured by HAM-D). All analyses were conducted in accordance with a fixed-effect model and a 95% confidence interval.
The odds ratio results were interpreted according to Andrade,Reference Andrade45 which states that an OR < 1.00 means that exposure to the risk variable reduces the risk of the event, an OR > 1.00 means that the risk is increased, and an OR equal to 1.00 is not statistically significant. The Hedges’ g results were interpreted as small (0.2–0.5), medium (0.5–0.8), and large (>0.8). We used the I2 statistic interpreted following the guidelines by Higgins,Reference Higgins46 to assess the heterogeneity among the included studies. Thus, I2 values falling within the ranges of 0–25%, 25–50%, and 50–75% indicated low, moderate, and high levels of heterogeneity, respectively. Additionally, the Q statistic was reported, assuming a p-value of 0.05 as the threshold for significance for heterogeneity.
Results
Search results
The electronic search on databases yielded 4083 records. Duplicate titles across databases were removed, leaving 3735 records to be screened. Five reports were considered eligible according to the inclusion criteria. Supplementary Table S1 shows the PRISMA flow chart with information about the stages of the search and screening process.Reference Page, McKenzie and Bossuyt42
Description of studies
Five selected reports were published between 2011 and 2020. The selected studies were conducted in Spain (3), Japan (1), and Italy (1). All studies had a case–control design. In all studies, OCD symptom severity, depression symptoms, and SLEs were assessed with the Yale-Brown Obsessive Compulsive Scale (YBOCS),Reference Woody, Steketee and Chambless52 the Hamilton Depression Rating Scale (HAM-D),Reference Hamilton53 and the Paykel Life Events Scale,Reference Paykel, Prusoff and Uhlenhuth54 respectively. The Paykel Life Event Scale evaluates a wide range of SLEs spanning from those that might be considered more favorable, such as marriage, proposals, promotions, desired pregnancies, or the birth of a child, to those that are less desirable, including academic failures, financial hardships, or instances of spousal infidelity. Additionally, it encompasses events that could be deemed traumatic, such as experiences involving death or various forms of violence, such as jail sentence or incarceration. For the purposes of this study, only the occurrence of SLEs, irrespective of their nature—be it desirable, undesirable, or traumatic—was taken into account. The results are summarized in Table 2.
Table 2. Studies Included in the Systematic Review
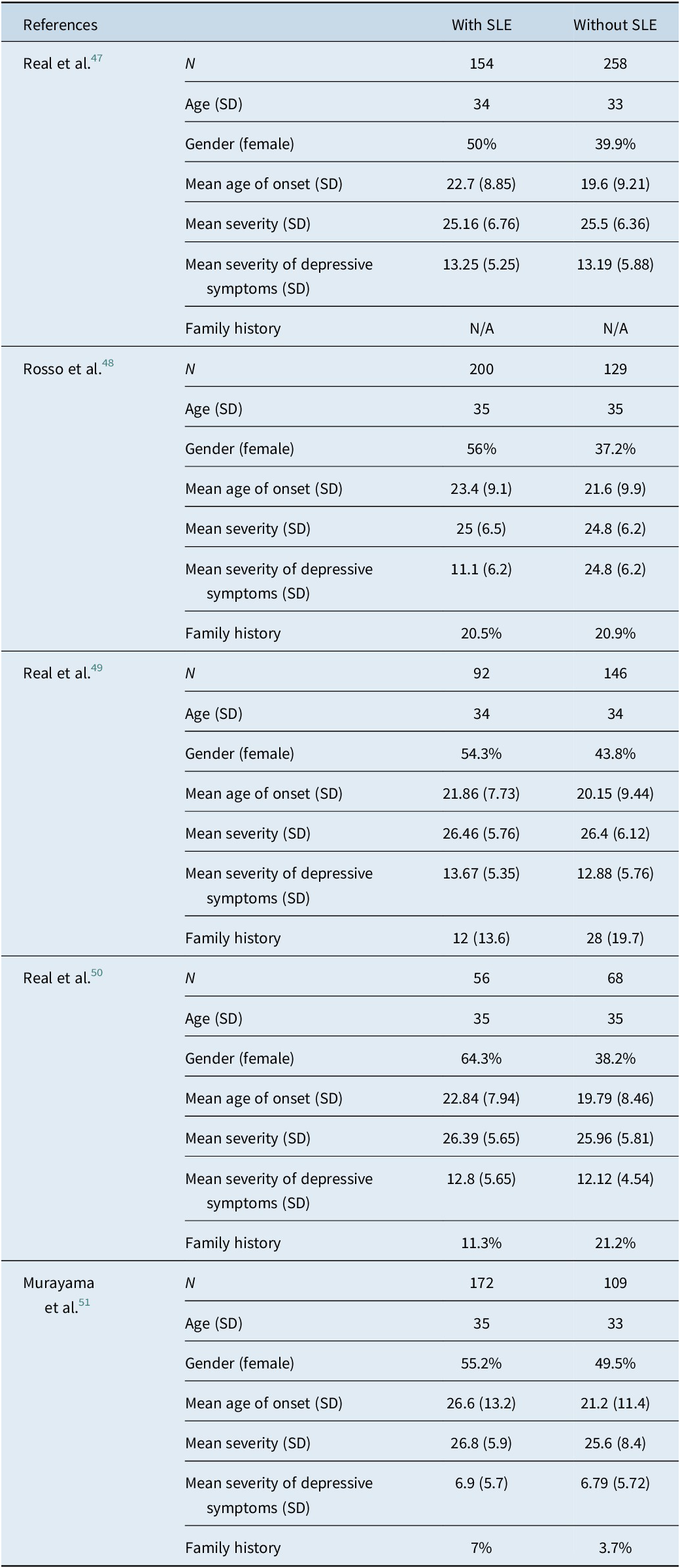
Real et al.Reference Real, Labad and Alonso47 investigated the presence of SLEs within the year preceding the onset of OCD in a large clinical sample (n = 412). They found the SLEs OCD to be associated with a later onset (odds ratio = 1.04; p = 0.001), obstetric complications (odds ratio = 5.54; p < 0.001), more contamination/cleaning-related symptoms (odds ratio = 1.99; p = 0.01), and less family history of OCD (odds ratio = 0.42; p = 0.014). While the study initially indicated a stronger association between SLEs OCD and female gender, this link lost statistical significance when they adjusted for the age of onset. Rosso et al.Reference Rosso, Albert, Asinari, Bogetto and Maina48 found SLEs OCD to be associated with female gender, acute onset, and somatic obsessions. The authors also found OCD associated with more severe SLEs, defined as the 20 top events on the Paykel Life Events Scale, to be associated with the symmetry-ordering symptom dimension.
Two additional studies from the same group provided additional information on the comparisons between SLEs OCD and NSLEs OCD, although their primary objective was of a biological nature. Real et al.Reference Real, Gratacòs and Labad49 investigated the involvement of the SLC1A1 gene in the treatment resistance of OCD patients. The study identified 3 Single Nucleotide Polymorphisms (SNPs) linked to pharmacological resistance. However, this association was only observed in OCD cases not associated with SLEs in the year before the disorder’s onset (spontaneous OCD). This study did not find any other significant difference between SLEs OCD and NSLEs OCD.
Real et al.Reference Real, Subirà and Alonso50 extended their investigation beyond the SLEs OCD’s clinical characteristics to explore neuroanatomical changes. Their findings were consistent with their earlier research from 2011, which identified female preponderance and later age at onset among SLEs OCD. Moreover, they found increased grey matter volume in individuals diagnosed with OCD compared to healthy controls. In SLEs OCD patients, they observed increased GM volume in the right anterior cerebellar hemisphere. In contrast, NSLEs OCD patients showed increased GM volume in the bilateral dorsal putamen and the central tegmental tract of the brainstem.
Murayama et al.Reference Murayama, Nakao and Ohno51 compared SLEs OCD with SLEs both 1 year and 1 month before the onset of OCD to NSLEs OCD (ie, individuals who had denied the occurrence of SLEs so close to the onset of the disorder). They observed that individuals who had suffered a SLE 1 year before the disorder’s onset exhibited a higher prevalence of contamination-related symptoms than the spontaneous OCD group.
Meta-analysis
Only 3 of the 5 selected studies were included in the metanalysis because of the probable sample overlap in the studies by Real et al. They recruited subjects during overlapping periods and in the same centers. Data from 1022 patients, who were divided into 2 groups: those with a history of SLE (n = 526) and patients without a history of SLE (n = 496), were compiled and analyzed. The results of these analyses are described in detail below.
Gender
The forest plot (Figure 1) depicts the odds ratio (OR) for female gender in individuals with SLEs OCD compared to those with NSLEs OCD. The pooled OR for the female gender in individuals with SLEs OCD was found to be positive (OR = 1.601, 95% CI: 1.241–2.066, p = 0.000), with a low heterogeneity across studies (I 2 = 25.357, Q = 2.679, p = 0.262). Thus, our review revealed a significant association between SLEs OCD and the female gender.
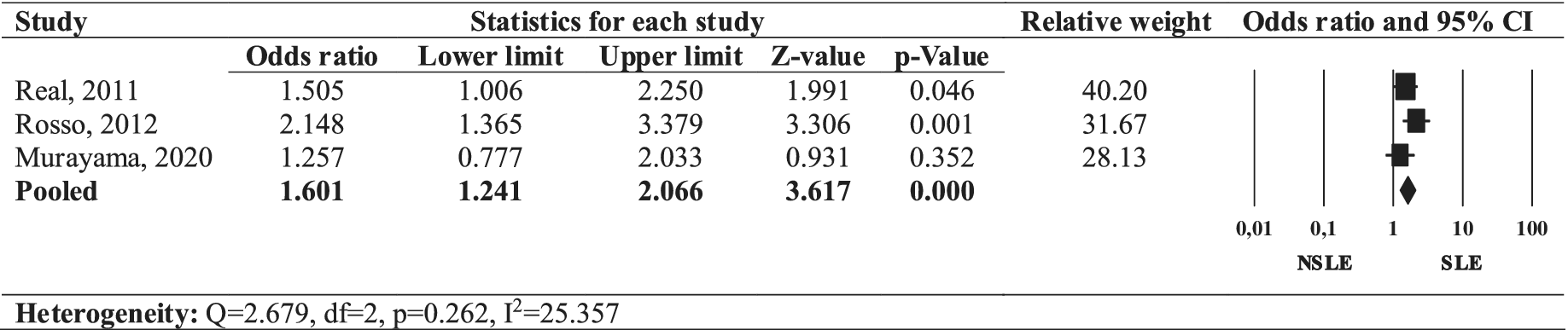
Figure 1. Forest plot of the odds ratio of female gender in SLEs OCD compared to NSLEs OCD.
Age of onset
The forest plot (Figure 2) illustrates the effect size of age of onset comparing SLEs OCD to NSLEs OCD. The pooled effect size of a later age of onset in individuals with SLEs OCD was positive (Hedges’ g = 0.316, 95% CI: 0.189–0.443, p = 0.000), with a low heterogeneity across studies (I 2 = 6.555, Q = 2.140, p = 0.343). Thus, SLEs OCD was associated with a later age of onset in our review.
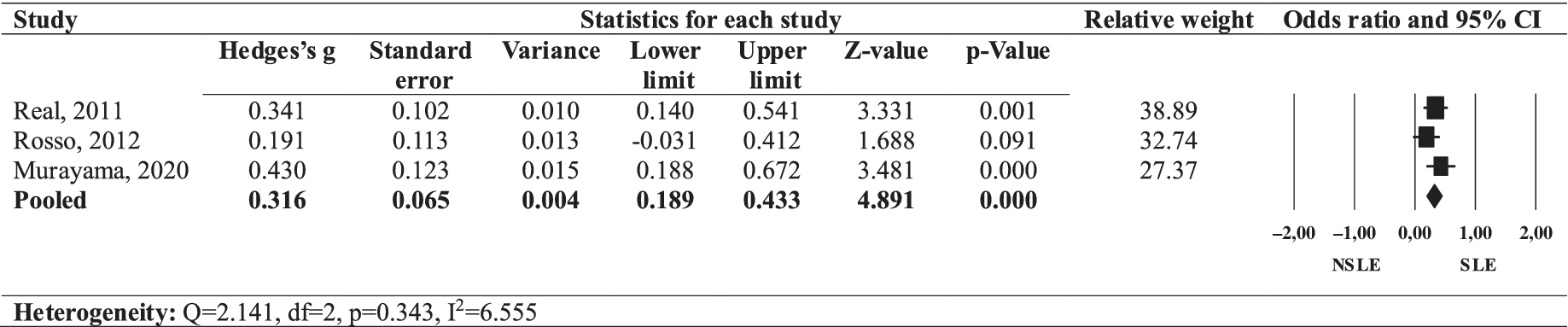
Figure 2. Forest plot of the effect size of onset age in SLEs OCD compared to NSLEs OCD.
Presence of a family history of OCD
The forest plot (Figure 3) depicts the OR for the presence of a family history of OCD in individuals with SLEs OCD compared to those with NSLEs OCD. The pooled OR for a family history of OCD in individuals with SLEs OCD was found to be non-significant (OR = 0.799, 95% CI: 0.534–1.194, p = 0.274), with a high heterogeneity across studies (I 2 = 67.443, Q = 6.143, p = 0.046). Thus, our review revealed a non-significant association between SLEs OCD and a familial history of OCD.
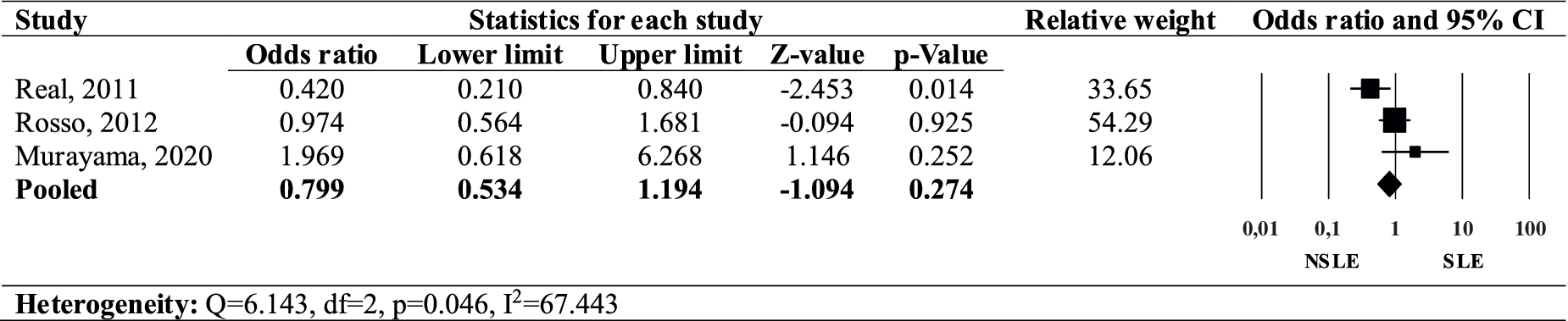
Figure 3. Forest plot of the odds ratio of family history of OCD in SLEs OCD compared to NSLEs OCD.
OCD symptom severity
The forest plot (Figure 4) illustrates the effect size of symptom severity comparing SLEs OCD to NSLEs OCD. The pooled effect size of a greater severity of OCD symptoms in individuals with SLEs OCD was non-significant (Hedges’ g = 0.081, 95% CI: −0.045-0.207, p = 0.209), with no heterogeneity across studies (I 2 = 0.000, Q = 0.793, p = 0.673). Thus, SLEs OCD was not associated with greater OCD symptom severity in our review.
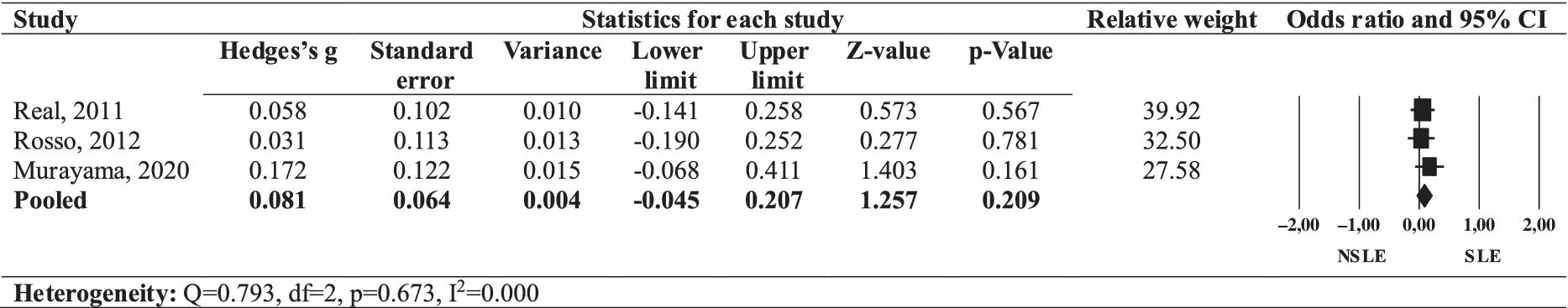
Figure 4. Forest plot of the effect size of OCD symptom severity in SLEs OCD compared to NSLEs OCD.
Comorbidities
The forest plot (Figure 5) presents the OR for mood disorder comorbidities in individuals with SLEs OCD compared to those with NSLEs OCD. The pooled OR for mood disorder comorbidities in individuals with SLEs OCD was positive (OR = 1.370, 95% CI: 1.022–1.838, p = 0.035), with a low heterogeneity across studies (I 2 = 18.787, Q = 2.463, p = 0.292). Thus, our review indicates that SLEs OCD was associated with increased rates of comorbid mood disorders.
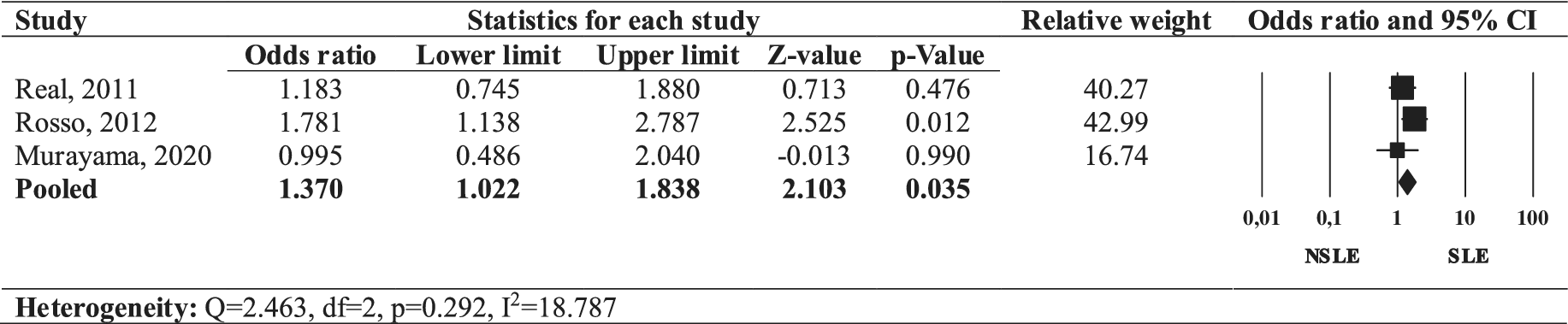
Figure 5. Forest plot of the odds ratio of mood comorbidities in SLEs OCD compared to NSLEs OCD.
Depressive symptoms
The forest plot (Figure 6) illustrates the effect size of the severity of depressive symptoms comparing SLEs OCD to NSLEs OCD. The pooled effect size of greater severity of depressive symptoms in individuals with SLEs OCD was non-significant (Hedges’ g = 0.025, 95% CI: −0.101-0.151, p = 0.696), with no heterogeneity across studies (I 2 = 0.000, Q = 0.064, p = 0.969). Thus, SLEs OCD was not associated with greater depressive symptom severity in our review.

Figure 6. Forest plot of the effect size of depressive symptom severity in SLEs OCD compared to NSLEs OCD.
Assessment of risk of bias in included studies
All the selected articles were assessed for quality and risk of bias according to the JBI Critical Appraisal Checklist for Case–Control Studies.Reference Moola, Munn, Tufanaru, Aromataris, Sears, Sfetcu, Currie, Qureshi, Mattis, KMP-F and Aromataris43 For the final evaluation of these studies by the JBI checklist, we also sought other sources for further information (Supplementary Material, articles listed in reference, and author contact). Supplementary Table S2 presents the assessment of these articles using the JBI checklist. No studies were excluded because of their risk of bias.
Discussion
Our review aimed to assess which sociodemographic and clinical features are more present in SLEs OCD in comparison to NSLEs OCD. Our findings revealed that SLEs OCD is associated with female gender, later onset, and a higher prevalence of comorbid mood disorders. Over the years, researchers have undertaken extensive efforts to categorize subtypes of OCDReference Miguel, Leckman and Rauch55 in order to better understand the disorder and try to identify its etiology, more specific risk factors, and the effectiveness of therapeutic interventions.Reference Taylor8, Reference Sharma, Sundar, Thennarasu and Reddy56-Reference Bhattacharyya and Khanna58 However, this endeavor is challenging due to the inherent heterogeneity of OCD, evident in its diverse clinical presentation, age of onset, and comorbidity patterns.Reference Taylor8, Reference Narayanaswamy, Viswanath, Veshnal Cherian, Bada Math, Kandavel and Janardhan Reddy59 The pursuit of OCD subtypes aligns with the broader aspiration of personalized care that allows tailoring treatments and interventions to the unique characteristics and underlying mechanisms of specific patient subgroups. Unfortunately, though, as became evident in our metanalysis, there is a paucity of studies focusing on the impact of SLEs in the course of OCD. Therefore, it is imperative to conduct more studies on this subject, especially including new types of SLEs, like the ones associated with COVID-19.
Consistent with previous studies, we found that SLEs are associated with a later onset of OCD. For instance, a previous cross-sectional investigation described “late-onset” OCD (onset developing at or after age 40) to be more prevalent among females and associated with chronic premorbid subclinical OCS, the co-occurrence of posttraumatic stress disorder (a proxy for trauma) after age 40, and a history of recent pregnancy in self or significant others.Reference Frydman, do Brasil and Torres57 Likewise, an Indian study suggested late-onset OCD to be characterized by more frequent environmental stressors (especially if they occurred later in life), female preponderance, and lack of family history of OCD.Reference Sharma, Sundar, Thennarasu and Reddy56 The latter study found that late-onset OCD without a positive family history was also more likely to be precipitated by SLEs.Reference Sharma, Sundar, Thennarasu and Reddy56
We also found an increased prevalence of females in OCD occurring after SLEs. This result is consistent with early-onset OCD being more commonly associated with the male gender in a number of studies.Reference Sharma, Sundar, Thennarasu and Reddy56, Reference Narayanaswamy, Viswanath, Veshnal Cherian, Bada Math, Kandavel and Janardhan Reddy59, Reference Fontenelle, Marques and Versiani60 Likewise, a recent survey by Benatti et al.Reference Benatti, Celebre and Girone61 in more than 200 Italian OCD patients reported females to exhibit a later age of onset when compared to male subjects. The increased prevalence of females in SLEs OCD may be attributed to higher perception of stress and suffering in women when experiencing determined events, such as loss of relationships (eg, problems with friends or relatives and death of loved ones).Reference Armstrong, Ronzitti, Hoff and Potenza62 Sex-related differences in the hypothalamus-pituitary–adrenal (HPA) axis stress response may influence how women and men respond to SLEsReference Armstrong, Ronzitti, Hoff and Potenza62 and explain why women display a higher vulnerability to developing stress-related disorders.Reference Armstrong, Ronzitti, Hoff and Potenza62
We identified an association between SLEs OCD and an elevated prevalence of comorbid mood disorders. This relationship may be attributed to the well-established connection between SLEs and depression,Reference Cohen, Murphy and Prather13, Reference Muscatell, Slavich, Monroe and Gotlib31, Reference Paykel63-Reference Kessler65 which might share genetic factors with OCD.Reference Bolhuis, Mcadams and Monzani66 Genetic markers associated with both OCD and depression include genes responsible for regulating serotonin and dopamine signaling pathways.Reference Murphy, Moya, Fox, Rubenstein, Wendland and Timpano67 Although the co-occurrence of OCD and depression may be ascribed to common genetic factors, the fact that both conditions may be associated with SLEs also suggests that shared environmental factors may explain their association.
In our comparative analysis between SLEs OCD cases and NSLEs OCD cases, variables like family history of OCD, OCD symptom severity, and the presence of depressive symptoms were not associated with SLEs. Although an early study suggested a higher prevalence of a positive family history of OCD in “very early” onset OCD, that is, OCD with an onset before age 5Reference Rosario-Campos, Leckman and Curi68 and our present review indicated SLEs OCD to be closely associated with later age at onset, our findings suggest that family history is not necessarily more etiologically important in NSLEs OCD than in SLE OCD. Similar findings were reported in another study comparing SLEs rates in familial versus non-familial OCD.Reference Albert, Maina, Ravizza and Bogetto69
Our findings that SLEs OCD was not associated with greater severity of OCS as compared to NSLEs OCD is at apparent odds with previous studiesReference Cromer, Schmidt and Murphy10, Reference Destrée, Albertella and Jobson32 which were able to demonstrate a relationship between trauma and the severity of OCS, particularly the ones involving symmetryReference Cromer, Schmidt and Murphy10, Reference Destrée, Albertella and Jobson32 and checking.Reference Cromer, Schmidt and Murphy10 This apparent divergence from the existing literature suggests that the SLEs’ impact on the severity of symptoms may be more circumscribed to certain dimensions rather than being more broadly impactful or that SLEs triggering or occurring before the onset of the OCD may only precipitate, but not necessarily aggravate, OCD. Accordingly, one study that reported an association between trauma and severity of OCS did not differentiate between SLEs occurring before or after the onset of OCD.Reference Cromer, Schmidt and Murphy10
The association between SLEs OCD and mood disorders (but not depressive symptoms) can be interpreted in different ways. Firstly, it is possible that SLEs may be more closely associated with clinically significant symptoms (ie, mood disorders)Reference Paykel21 rather than with milder depressive symptoms that may not be diagnostically relevant or even depressive symptoms secondary to other disorders such as OCD itself. Secondly, individuals with SLEs OCD lacking clinically significant depressive symptoms at the moment of the assessment may still have a history of mania or hypomania, thus qualifying for a diagnosis of a mood (bipolar) disorder instead. Bipolar disorder has also been associated with SLEs, which were reported to act as triggers to both first and subsequent mood episodes.Reference Aldinger and Schulze70-Reference Subramanian, Sarkar, Kattimani, Philip Rajkumar and Penchilaiya76
Despite shedding some light on the phenotype associated with SLEs OCD, we can only speculate on the development pathways linking SLEs and psychopathology in general and OCD in particular.Reference Adams, Kelmendi, Brake, Gruner, Badour and Pittenger12 Firstly, SLEs may directly and casually induce OCD symptoms in individuals with a more general (unspecific) genetic vulnerability. This pathway may be particularly relevant to individuals who develop OCD thematically related to the SLEs to which they have been exposed (eg, contamination obsessions after minor stressful accidents such as “stepping on dogs’ poop”).Reference Fontenelle, Albertella and Brierley77
Secondly, some individuals may harbor a specific genetic vulnerability for OCD, with SLEs merely acting as unspecific triggers for the onset of OCD. As compared to the first case, this pathway could be more relevant in OCD cases whose symptoms may be thematically unrelated to the SLEs (eg, counting compulsions after the end of a long-term relationship). Lastly, the third situation involves individuals who are already diagnosed with OCD; in this case, a SLE exacerbates the existing symptoms, for example, someone with preexisting OCD involving contamination themes who is exposed to contaminants.Reference Adams, Kelmendi, Brake, Gruner, Badour and Pittenger12
Our study has some significant strengths. For instance, we deliberately excluded studies that did not use validated scales to measure SLEs. For instance, studies that assessed OCD onset following single events, such as pregnancy or accidents, were omitted to maintain comprehensiveness and rigor in our review. Determining the grade of stress of an event without relying on a valid instrument could introduce bias in our findings. Early efforts to investigate the involvement of SLEs as precipitating factors in OCD relied on non-validated assessment tools, thus yielding highly fluctuating rates of SLEs occurring before OCD onset.Reference Fontenelle, Cocchi and Harrison17
Our study is subject to limitations. Firstly, it did not include studies that addressed childhood trauma in greater detail. Secondly, the reports included in our review only measured SLEs up to 1 year before OCD onset, while other studies indicate a higher prevalence of SLEs in OCD patients compared to healthy controls during their lifetime.Reference de Loof, Zandbergen, Lousberg, Pols and Griez78, Reference Sarkhel, Praharaj and Sinha79 Consequently, we may have overlooked the influence of lifelong SLEs on OCD characteristics. Admittedly, SLEs may shape an individual’s personality, potentially rendering them more susceptible to developing anxiety or other disorders.Reference Gothelf, Aharonovsky, Horesh, Carty and Apter80
Conclusion
In summary, our review highlights an association between SLEs and OCD, particularly in females, with OCD onset at a later age and a higher prevalence of comorbid mood disorders. Caution is advised, however, not to prematurely consider these distinctive clinical features as unequivocal proofs of the existence of a “valid” SLEs OCD subtype. As we were unable to find a difference between SLEs and NSLEs OCD in terms of family history of OCD, severity of OCD symptoms, and presence of depressive symptoms, there are probably more similarities rather than differences between these conditions.
In any case, more research should be pursued on the biological mechanisms (including, for instance, the involvement of the hypothalamic–pituitary–adrenal axis), pathophysiological models, and patterns of therapeutic response to conventional (ie, serotonin reuptake inhibitors or cognitive behavioral therapy) or other innovative treatments of SLEs OCD as compared to NSLEs. Nevertheless, we feel our research adds to the literature by showing the importance of assessing the presence of SLEs in individuals with OCD, as they may either precipitate or simply shape the clinical phenomenological expression of the disorder.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S1092852924000269.
Author contribution
All authors read and approved the submitted manuscript. All authors contributed to the formulation of overarching research goals and aims and to the design of methodology. V.H. substantially contributed to the analysis and interpretation of data and drafted the manuscript. S.S.-R. contributed to the analysis and interpretation of data, meta-analyzed the data, and critically revised the manuscript for important intellectual content. ME.M.-O. substantially contributed to the analysis and interpretation of data and critically revised the manuscript for important intellectual content. G.B.M. substantially contributed to the analysis and interpretation of data, critically revised the manuscript for important intellectual content, and approved the final version to be published. L.F.F. substantially contributed to the analysis and interpretation of data, critically revised the manuscript for important intellectual content, and approved the final version to be published.
Financial support
L.F.F. was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; grant # CNE E-26/200.950/2021, Rio de Janeiro, RJ, Brazil), and intramural grants from D’Or Institute for Research and Education (IDOR, Rio de Janeiro, RJ, Brazil).
Disclosure
The authors declare none.










