Introduction
Trilocha varians (Walker, 1855) is an insect of the family Bombycidae, that harms Ficus spp. Lepidoptera are holomorphic insects, so T. varians goes through four developmental stages common to holomorphic insects: eggs, larvae, pupae, and adults. T. varians is mainly distributed in South and Southeast Asian countries, such as Malaysia, Philippines, Vietnam, and Thailand. It is also widely distributed in southern China, especially in Taiwan and Hainan (Kedar et al., Reference Kedar, Malllaiah and Saini2014; Arya Reference Arya2019). It is a major pest of Ficus spp. and its larvae cause 80–90% of Ficus spp. leaves to fall off (Kedar et al., Reference Kedar, Malllaiah and Saini2014). Ficus spp. that have been reported to have been damaged by T. varians include Ficus elastica, Ficus benjamina, Ficus nitida, Ficus caraica, Ficus religiosa, Ficus infectoria, Ficus benghalensis, and Ficus septica (Arya, Reference Arya2019). Ficus spp. belongs to the family Moraceae with high ecological tolerance, high dispersal, and high germination rate, which is the reason why Ficus spp. as an exotic tree species in Macau, has become the main tree species for its parks (Zhang et al., Reference Zhang, Lai and Jim2017). Ficus spp. is a native species of southern China, mainly in Taiwan, Zhejiang, Fujian, Guangdong, Guangxi, Guizhou, Yunnan, and other provinces, with trees up to 15–25 m in height and about 50 cm in diameter at breast height (Flora Reipublicae Popularis Sinicae, http://www.cn-flora.ac.cn/). Ficus spp. has an upright trunk, distinct shape, and fast growth rate, which can quickly increase the coverage of urban greenery, and its large canopy can also provide effective shade for pedestrians, so it is often used as an ornamental tree and street tree in landscape planning (Chen, Reference Chen2020). At present, several papers have been published to describe the damage of Ficus spp. by T. varians. Basari et al. (Reference Basari, Mustafa, Yusrihan, Chin and Ibrahim2019) reported that Ficus microcarpa in Malaysia were attacked by T. varians larvae, resulting in 100% defoliation. Naeem-Ullah et al. (Reference Naeem-Ullah, Ramzan, Saeed, Iqbal, Umar, Sarwar, Ali, Saba, Abid, Khan and Ghramh2020) reported that F. microcarpa leaves in Pakistan were heavily eaten by T. varians, which severely affected the ornamental value of F. microcarpa. Daimon et al. (Reference Daimon, Yago, Hsu, Fujii, Nakajima, Kokusho, Abe, Katsuma and Shimada2012) also documented that the larvae of T. varians affect two species of Ficus, F. benjamina and F. microcarpa, in Taiwan, China, and Okinawa, Japan. Therefore, research on the potential distribution range of T. varians is beneficial to the management and prevention of Ficus spp. pests and the maintenance of the urban greening environment.
In its sixth assessment report in 2022, the Intergovernmental Panel on Climate Change (IPCC) states that the Earth's average temperature has increased by 1.5°C from pre-industrial temperatures. Climate change is manifested in many ways, including increased temperatures due to increased carbon dioxide concentrations, and extreme weather events such as droughts or storms (Jactel et al., Reference Jactel, Koricheva and Castagneyrol2019). Rising sea levels, insecurity of food and water, and increased rates of transmission of infectious diseases under climate change pose a threat to human health and safety (Barrett et al., Reference Barrett, Charles and Temte2015). Of course, global climate change not only threatens human survival but also affects other organisms. Climate change has already been reported to affect the body size of several contemporary species such as Apodemus speciosus and Apodemus argenteus, and may have an even greater impact on faunal communities in the future (Millien et al., Reference Millien, Lyons, Olson, Smith, Wilson and Yom-Tov2006). High temperatures increase insect mortality, and insect pheromone communication is disturbed by increased temperature and atmospheric gas concentrations (Boullis et al., Reference Boullis, Detrain, Francis and Verheggen2016). Climate change can also affect the distribution of insects. Climate change has been described to cause mountain butterflies to migrate to higher elevations (Rödder et al., Reference Rödder, Schmitt, Gros, Ulrich and Habel2021). Local extinctions of bumblebees have been reported in extreme temperatures, and the risk of extinction will increase if climate change continues (Sirois-Delisle and Kerr, Reference Sirois-Delisle and Kerr2018). Climate change may reduce the abundance of Ephemeroptera, Plecoptera, and Trichoptera insects in temperate alpine regions of Switzerland (Timoner et al., Reference Timoner, Fasel, AshrafVaghefi, Marle, Castella, Moser and Lehmann2021). In conclusion, climate change has far-reaching effects on the geographical distribution of insects, and it is necessary to predict the geographical distribution of T. varians under future climate change to help us better prevent their infestation of Ficus spp.
Species distribution models (SDMs) are useful for predicting the future spatial distribution of species under changing climate scenarios and have been widely used in conservation biology, evolutionary biology, and biogeography (Islam et al., Reference Islam, Rana, Islam, Hossain, Hossain and Hossain2021; Yang et al., Reference Yang, Huang, Jiang, Chen, Liu and Wang2022a, Reference Yang, Wen, Barzegar, Adamowski, Meng and Yin2022b). SDMs built using analytical methods such as climate change experiment, bioclimatic prediction system, generalised linear model, generalised additive model, ecological niche modelling, ecological niche factor analysis, genetic algorithm for rule-set prediction, and the maximum entropy algorithm (MaxEnt) have been widely used (Zhang et al., Reference Zhang, Liu, Li, Wang, Cheng, Yang and Kong2021). The MaxEnt is a method for modelling species distributions when only species distribution data are available, and has been shown to perform well in predicting distribution (Sutton and Martin, Reference Sutton and Martin2022; Zhao et al., Reference Zhao, Xiao, Shen and Li2022). Its accuracy and performance are higher than other models even with fewer distribution data (Aidoo et al., Reference Aidoo, Souza, Silva, Santana Júnior, Picanço, Osei-owusu, Setamou, Ekesi and Borgemeister2022). At the same time, the MaxEnt model also has the advantages of short running time, easy operation, good performance, and high accuracy (He et al., Reference He, Li, Li, Xu, Gao, Guo, Huo, Peng and Meng2021). Among the existing investigations, Xu et al. (Reference Xu, Zhuo, Li and Wang2022) predicted the suitable distribution area of Oryctes rhinoceros (L.) using the MaxEnt model. The results indicate that the MaxEnt model can be used for the research of suitable areas for insects. Similarly, Mugiyo et al. (Reference Mugiyo, Chimonyo, Kunz, Sibanda, Nhamo, Masemola, Modi and Mabhaudhi2022) used the MaxEnt model to map the spatial distribution of underutilised crop species under climate change.
Despite its impact on Ficus spp., no studies have yet predicted the current and potential future distribution of T. varians using the MaxEnt model. In our report, MaxEnt was used to simulate the current potential distribution area of T. varians and to predict the potential distribution area of T. varians for two periods in the 2050s and 2090s under the three conditions of SSP1-2.6, SSP2-4.5, and SSP5-8.5 (shared socioeconomic pathways). The key environmental variables and suitable ranges affecting the distribution of T. varians were also analysed in conjunction with additional environmental variables. This study can help us to take appropriate control measures in advance to reduce the loss of Ficus spp. and advance the process of rational ecological research.
Materials and methods
Species occurrence data
Adequate occurrence records are an important prerequisite for the construction of species’ ecological niche models (Li et al., Reference Li, Xu, Jin, Zhuo, Yang, Hu and Wang2020). Three sources of occurrence records were used in this study, namely the Global Biodiversity Information Facility (http://www.gbif.org/), National Specimen Information Infrastructure (http://www.nsii.org.cn/), and literature related to T. varians. The related literature is from China National Knowledge Infrastructure (https://www.cnki.net/), but the occurrence records in the literature lack specific latitude and longitude coordinates, so we determined the coordinates of the occurrence records by Google Earth (http://ditu.google.cn/). Finally, a total of 122 occurrence records were obtained and saved in the .csv format for predictive modelling.
Environmental variables
A total of 19 bioclimatic variables involved in this study were obtained from the Worldclim, and the data used in the modelling were based on meteorological records from 1970 to 2000. The IPCC, in its Fifth Assessment Report published in 2014, referred to the Representative Concentration Pathways (SSPs) being used in new climate model simulations. In this study, SSP1-2.6, SSP2-4.5, and SSP5-8.5 were selected to simulate the habitat suitability distribution of T. varians in the 2050s and 2090s, which represent three scenarios with different concentrations of greenhouse gas (GHG) emissions. Initial modelling was first performed using MaxEnt 3.4.4, and then the initial contribution of each environmental variable was calculated using the jackknife detection method, which eliminated mean temperature of coldest quarter (bio11), max temperature of warmest month (bio5), and precipitation of coldest quarter (bio19), the three variables with zero contribution. Then SPSS was used to calculate the Pearson correlation between the variables, and the variables with |p| > 0.8 were removed. The combined contribution rate and Pearson correlation finally identified four environmental variables for re-modelling, namely annual mean temperature (bio1), temperature seasonality (standard deviation × 100) (bio4), precipitation of driest month (bio14), and precipitation of driest quarter (bio17).
MaxEnt model building and evaluation
Re-modelling was performed based on the filtered dominant environment variables. The 25% of the occurrence records were randomly selected as the test set and the remaining 75% as the training set, and the computation was repeated ten times, with the replication run type set to ‘cross-validation’ and the maximum number of iterations set to 500. The receiver operating characteristic (ROC) curve is used to visualise the model performance, and the sum of the area under the curve (AUC value) is an important performance evaluation criterion to verify the accuracy of the model. The larger the AUC value, the greater the correlation between the environmental variables and the predicted geographical distribution of the species, and the better the prediction of the model (Zhang et al., Reference Zhang, Liu, Li, Wang, Cheng, Yang and Kong2021). An AUC value of 0.5 means that the model is not better than random; an AUC value of <0.5 means that the model is worse than random; an AUC value of 0.5–0.7 means that the model effect is weak; an AUC value of 0.7–0.9 means that the model effect is moderate; an AUC value of 0.9–1 means that the model effect is strong (Xu et al., Reference Xu, Li, Jin, Zhuo, Yang, Hu and Wang2020). Afterwards, response curves were established to analyse the response of T. varians to key environmental variables. According to the IPCC report, the probability of species presence (P) was divided into four classes, which were distinguished by different colours on the map: highly suitable (P ≥ 0.66, red), moderately suitable (0.33 ≤ P < 0.66, orange), weakly suitable (0.05 ≤ P < 0.33, blue), and unsuitable (P < 0.05, green).
Results
Model results validation
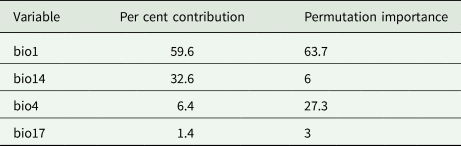
With an AUC value of 0.970 (fig. 1), the model performed strongly and was accurate enough to be used to predict the geographic distribution of T. varians. Environmental variables with Pearson's correlation |p| > 0.8 were screened (table 1) and the contribution of these environmental variables was calculated using a Jackknife (table 2). Bio1 had a contribution rate of 59.6%, bio14 32.6%, bio4 6.4%, and bio17 1.4%. The cumulative contribution rate reached 100%, so these four environmental variables were finally selected for distribution modelling.

Figure 1. Area under the ROC curve (AUC) for T. varians.
Table 1. Results of Pearson correlation coefficient

**The difference is extremely significant at the 0.01 level (P < 0.01).
Table 2. Contribution rate and permutation importance of each environment variables
The current distribution of T. varians
The suitable potential distribution area for T. varians is divided into four classes, with green representing unsuitable areas, blue representing low suitable areas, orange representing medium suitable areas, and red representing high suitable areas (fig. 2). Under the current climatic conditions (table 3), the suitable distribution area of T. varians is within the range of 92°13′E–122°08′E, 18°17′N–31°55′N, mainly concentrated in low latitude and low altitude areas. The area of the high suitability zone is 14.00 × 104 km2, accounting for 1.45% of the total area. It is mainly distributed in southern Guangdong, southwestern Guangxi, western Taiwan, Hong Kong, and the Hainan, and a small amount in southern Yunnan and southeastern Tibet. Most of these regions are located in the tropics and subtropics. Guangdong has the largest high suitability area of 5.43 × 104 km2, followed by Guangxi and Hainan with 2.89 × 104 and 2.77 × 104 km2. The area of a highly suitable area in Hainan accounts for 78.27% of the total area of the province, the area of a highly suitable area in Taiwan accounts for 44.03% of the total area of the province, the area of a highly suitable area in Guangdong accounts for 30.21% of the total area of the province, and the area of a highly suitable area in Guangxi accounts for 12.14% of the total area of the province. Among them, the high suitability area of Hong Kong accounts for 82.07% of the total area, which is the most suitable area for the survival of T. varians. The area of the middle suitable zone is 21.50 × 104 km2, accounting for 2.23% of the total area. It is mainly distributed in southern Guangdong and southern Guangxi, and a small amount is distributed in southern Yunnan and southern Fujian. The area of the low suitability zone is 71.95 × 104 km2, accounting for 7.47% of the total area. It is mainly distributed in Yunnan, Jiangxi, Fujian, southern Hunan, northern Guangxi, and northern Guangdong, and a small amount in Sichuan, Zhejiang, Tibet, and southern Guizhou. The rest are unsuitable areas, mainly distributed in northern China, such as Xinjiang, Inner Mongolia, Gansu, Hebei, Jilin, Heilongjiang, Liaoning, etc.

Figure 2. Current potential distribution of T. varians.
Table 3. Analysis of main suitable distributions of T. varians

Future distribution prediction of T. varians
In order to explore the impact of climate change on the distribution of T. varians, the potential distribution of three GHG concentration scenarios, SSP1-2.6, SSP2-4.5, and SSP5-8.5, was simulated by the MaxEnt, and a total of six predictions were obtained for two periods (the 2050s and 2090s) (fig. 3). The results show (table 4) that in 2050s, under SSP1-2.6, the area of the high suitability zone is 16.78 × 104 km2, the medium suitability zone is 17.56 × 104 km2, and the low suitability zone is 73.10 × 104 km2. Under SSP2-4.5, the area of the high suitability zone is 15.62 × 104 km2, the medium suitability zone is 19.79 × 104 km2, and the low suitability zone is 81.54 × 104 km2. Under SSP5-8.5, the area of the high suitability zone is 21.00 × 104 km2, the medium suitability zone is 18.77 × 104 km2, and the low suitability zone is 81.54 × 104 km2. Under SSP5-8.5, the area of the high suitability area is 21.00 × 104 km2, the area of the medium suitability area is 18.77 × 104 km2, and the area of the low suitability area is 74.53 × 104 km2. Compared with the current distribution, the area of high suitability zones all increased, by 19.86, 11.57, and 50.64%, respectively. The area of medium suitability zones all decreased, by 18.33, 8.00, and 12.70%, respectively. The area of low suitability zones all increased, by 1.60, 13.33, and 3.59%, respectively. In the 2090s, under SSP1-2.6, the area of the high suitability zone is 11.67 × 104 km2, medium suitability zone is 17.88 × 104 km2, and the low suitability zone is 74.28 × 104 km2. Under SSP2-4.5, the area of the high suitability zone is 17.07 × 104 km2, the medium suitability zone is 17.75 × 104 km2, and the area of the low suitability zone is 76.37 × 104 km2. Under SSP5-8.5, the area of the high suitability zone is 14.67 × 104 km2, the area of the medium suitability zone is 23.39 × 104 km2, and the area of the low suitability zone is 73.33 × 104 km2. Compared to the current distribution, the area of the high suitability zone decreased by 16.64% under SSP1-2.6, increased by 21.93% under SSP2-4.5, and increased by 4.79% under SSP5-8.5. The area of the medium suitability zone decreased by 16.84% under SSP1-2.6, decreased by 21.93% under SSP2-4.5, and increased by 8.80% under SSP5-8.5. The area of low suitability zones all increased, by 3.24, 6.14, and 1.92%, respectively. Although the area of the suitable zone for T. varians increased and decreased under different conditions, the T. varians did not undergo significant migration.

Figure 3. Future distribution of T. varians.
Table 4. Predicted suitable areas for T. varians under current and future climatic conditions

Separate comparisons of changes in the area of high suitability areas showed that provinces with high suitability areas did not change in different periods and scenarios, only the area changed to different degrees (table 5). Yunnan had a substantial increase in the area of high suitability area under the SSP5-8.5 scenario in both periods, with an increase of 43.96 and 87.91%, respectively. In Fujian province, the area of high suitability area under the SSP5-8.5 scenario in the 2050s period increased substantially except for a large increase in the area of high suitability area under all other conditions. Guangxi showed an increase in all conditions except for a 60.21% decrease in the area of high suitability area under the SSP1-2.6 scenario in the 2090s period. Taiwan, on the other hand, was the opposite of Guangxi, with only an increase of 11.95% under the SSP1-2.6 scenario in the 2090s. Guangdong decreased by 3.5% under the scenario of SSP5-8.5 in the 2090s, and the area of high suitability area increased under all other conditions. In Hong Kong, the area of high suitability area changed the most under the SSP5-8.5 scenario in the 2090s period, decreasing by 77.78%. Hainan had less change under all scenarios. The areas of high suitability areas in Tibet all showed an increase in the 2050s period and a decrease in the 2090s period. In terms of the total area of high suitability areas in China, the area increased under all scenarios except for the SSP5-8.5 scenario, which decreased by 16.64% in the 2090s.
Table 5. Current and future changes in the size of high suitable areas for T. varians

Analysis of environmental variables
In order to identify key environmental variables that influence the potential distribution of T. varians, the regularised training gains of environmental variables were analysed by a jackknife. Among them (fig. 4), bio1 had the largest regularised training gain of more than 2.4, bio14 and bio17 had regularised training gains of less than 1.7, and bio4 had regularised training gains of less than 1.8. Meanwhile, these four environmental variables have a cumulative contribution of 100% and a cumulative permutation importance of 100%. Therefore, the most critical environmental variable affecting the geographical distribution of T. varians is the annual mean temperature (bio1), the temperature seasonality (standard deviation × 100) (bio4), the precipitation of driest month (bio14), and the precipitation of driest quarter (bio17). In other words, the temperature factor and precipitation factor had the most significant effect on the geographical distribution of T. varians. The optimum range of environmental variables affecting the geographical distribution of T. varians over a range was analysed by response curves (presence probability: P > 0.66). The results showed (fig. 5) that the optimal range of annual mean temperature (bio1) was >20.50°C, peaking at 22.58°C; the temperature seasonality (standard deviation × 100) (bio4) was <554.17, peaking at 234.67; the precipitation of driest month (bio14) was 19.46–26.17 and >58.79 mm, peaking at 22.73 mm; the precipitation of driest quarter (bio17) was 72.38–96.65 and >264.98 mm, peaking at 86.36 mm.

Figure 4. Importance of environment variables to T. varians.

Figure 5. Response curve of environmental variables to occurrence probability of T. varians.
Discussion
In this study, it was found that the distribution of T. varians was mainly influenced by four environmental factors: annual mean temperature (bio1), temperature seasonality (standard deviation × 100) (bio4), precipitation of driest month (bio14), and precipitation of driest quarter (bio17). The high suitability area of T. varians is mainly in the Pearl River Basin, and the suitability area will increase in the future, but the centre of mass did not undergo significant migration. Temperature and precipitation were found to be important environmental factors affecting the distribution of lepidopteran pests in previous articles of lepidopteran-related studies, e.g. Spodoptera exigua was affected by precipitation of the coldest quarter (bio19), annual precipitation (bio12) and mean temperature of the coldest quarter (bio11) (Falsafi et al., Reference Falsafi, Alipanah, Ostovan, Hesami and Zahiri2022); Zeuzera pyrina was affected by precipitation of the coldest quarter (bio19), precipitation seasonality (bio15) and precipitation of driest quarter (bio17) (Fekrat and Farashi, Reference Fekrat and Farashi2022); the distribution of Parapediasia teterrella was influenced by annual mean temperature (bio1), mean temperature of the coldest quarter (bio11), and precipitation of the coldest quarter (bio19) (Jie et al., Reference Jie, Yang and Li2020). Temperature and precipitation were equally key environmental variables in this study. The suitable distribution area for T. varians is in the Pearl River Basin (102°14′E–115°53′E; 21°31′N–26°49′N), which has a tropical monsoon climate and a subtropical monsoon climate (Gu et al., Reference Gu, Qiang, Vijay and Peijun2016; Zhang et al., Reference Zhang, Liu, Li, Wang, Cheng, Yang and Kong2021). The tropical monsoon climate is characterised by year-round hot temperatures above 20°C and annual precipitation of 1500–2000 mm. (Wu et al., Reference Wu, Wang, Hu, Tian and Tian2008). The average annual temperature in the subtropical monsoon climate zone ranges from 15 to 22°C, and annual precipitation ranges from 800 to 1600 mm (Wu et al., Reference Wu, Wang, Hu, Tian and Tian2008). In the existing studies, 24–26°C and 60.5% relative humidity are usually chosen for selection and cultivation of T. varians, indicating that it is suitable for survival under these conditions (Basari et al., Reference Basari, Mustafa, Yusrihan, Chin and Ibrahim2019; Ramzan, Reference Ramzan2020). The results of this paper show that the optimal range of annual average temperature (bio1) suitable for T. varians is greater than 20.50°C, the precipitation of driest month (bio14) >58.79 mm, and the precipitation of driest quarter (bio17) >264.98 mm. Temperature and precipitation in subtropical and tropical monsoon climates also fall within this range. Meanwhile, the results demonstrated that T. varians is completely unsuitable in northeast, north, and northwest China. This may be related to the local temperature and precipitation, for example, northwest China has a continental arid climate where the temperature can reach below −30°C in the coldest months and the annual precipitation is 100–200 mm (Yang et al., Reference Yang, Huang, Jiang, Chen, Liu and Wang2022a, Reference Yang, Wen, Barzegar, Adamowski, Meng and Yin2022b). Comparison of the topographic map of China provided by the Chinese government website with the predictions of our survey revealed that T. varians is mainly distributed at low elevations and low latitudes, such as Guangdong, Guangxi, and Hainan. These places are all below 200 m in elevation, below 30°N in latitude, with abundant precipitation and high mean annual temperatures (Wu et al., Reference Wu, Wang, Hu, Tian and Tian2008). This indicates that T. varians does not have good environmental adaptability and can only survive in areas with suitable precipitation and temperature.
Under different future climatic conditions, some of the high, medium, and low suitability zones for T. varians increased and some decreased. The total suitable zone showed an increase, but not significant. T. varians is mainly distributed in the Pearl River Basin. It is reported that the monthly mean temperature in the Pearl River Basin will increase by 0.25–0.34°C per decade under RCP4.5 and by 0.42–0.60°C per decade under SSP5-8.5, and the mean annual precipitation will also increase under both RCPs (Duan et al., Reference Duan, Huang, Li, Zhou, Ren and Tian2020). However, under RCP4.5, drought events lasting 3–4 months will increase by 4.3% and those lasting more than 5 months will increase by 3.4%. Under RCP8.5, more medium- and long-term drought events with higher severity will occur (Zhou et al., Reference Zhou, Chen and Xiao2021). In CMIP6, new scenario shared economy paths (SSPs) were developed that integrate CMIP5 representative centralised paths (RCPs), so the results can be used to some extent as a reference to explain the findings of this paper (Hirsch et al., Reference Hirsch, Guillod, Seneviratne, Beyerle, Boysen, Brovkin, Davin, Doelman, Kim, Mitchell, Nitta, Shiogama, Sparrow, Stehfest, Vuuren and Wilson2018). The results of our study showed that the area of most of the high and low suitability zones for T. varians increased under future climatic conditions, which may be related to the increase of temperature and precipitation in the future. At the same time, the medium suitability zones mostly decreased, which may be related to the increase in drought duration.
The increase in the area of high, medium, and low suitability areas for T. varians in most future scenarios suggests that future environmental conditions will be more favourable for T. varians to survive and reproduce. Logan et al. (Reference Logan, Regniere, Gray and Munson2007) modelled future climate change and found that warming favours the reproduction and survival of Lymantria dispar, while its colonisation of poplar will increase from 33% in 1991 to 100% in 2071. Hodkinson (Reference Hodkinson1997) found that the host range of Cacopsylla groenlandica expanded from one species of willow to four species of willow due to climate warming. Therefore, it is hypothesised that changes in the distribution area of T. varians may result in increased damage to Ficus spp. or more Ficus spp. species being infested.
The MaxEnt model is highly accurate for species distribution prediction but still differs from reality. In the current work, we chose environmental variables from meteorological records from 1970 to 2000, but global climate change has been dramatic in recent years. The IPCC mentioned in the second part of its Sixth Assessment Report that global warming will exceed 1.5 or 2°C during the 21st century, and that there are large uncertainties in the conversion of emission scenarios into concentration pathways due to uncertainties in climate-carbon cycle feedbacks. The average precipitation will also increase, but with seasonal and regional variability, with an increase in synoptic variability (Zhuo et al., Reference Zhuo, Xu, Pu, Wang and Ye2020). Therefore, the environmental variables used in this study may differ significantly from those under future climate change. Also, the factors affecting the distribution of species are multiple. Human activities affect the distribution of species, and the distribution of plants fed on by phytophagous insects also affects the distribution of insects (Qin et al., Reference Qin, Jin, Batsaikhan, Nyamjav, Li, Jia, Xue, Sun, Wu, Indree, Shi and Xiao2020; Yang et al., Reference Yang, Huang, Jiang, Chen, Liu and Wang2022a, Reference Yang, Wen, Barzegar, Adamowski, Meng and Yin2022b). In this study, we only considered the effects of 19 environmental variables on the distribution of T. varians. We did not explore whether there is a link between the distribution area of Ficus spp. and the potential distribution of T. varians. However, our report can still explain to some extent the suitable distribution area of T. varians and provide reference value for controlling it.
Conclusions
In this work, the currently suitable areas for T. varians were analysed using the MaxEnt model, and key environmental variables affecting the distribution of T. varians were identified. The potential distribution of T. varians was predicted under three concentration pathways, SSP1-2.6, SSP2-4.5, and SSP5-8.5, for the periods of 2050s and 2090s, respectively. The research concluded that the current high, medium, and low suitability zones of T. varians predicted by the MaxEnt model accounted for 1.45, 2.23, and 7.47% of the total land area of China, respectively, and were mainly distributed in Guangdong, Guangxi, Hainan, Taiwan, and Hong Kong. The annual mean temperature (bio1), the temperature seasonality (standard deviation × 100) (bio4), the precipitation of driest month (bio14), and the precipitation of driest quarter (bio17) are the key environmental variables affecting the geographical distribution of T. varians. Under future climate change, the geographic distribution of T. varians did not shift significantly and remained concentrated in Guangxi, Guangdong, Hainan, Taiwan, and Hong Kong.
Availability of data and material
The data supporting the results are available in a public repository at: GBIF.org (5 June 2022) GBIF Occurrence Download https://doi.org/10.15468/dl.7dsrm9 and Qianqian Qian (2024): Locations of Trilocha varians figshare dataset. https://doi.org/10.6084/m9.figshare.25133168.v1.
Author contributions
Qianqian Qian and Zhihang Zhuo: conceptualisation; Danping Xu: methodology; Zhihang Zhuo: software; Wenkai Liao: investigation; Qianqian Qian: writing – original draft; Zhihang Zhuo: writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Financial support
This research was funded by Sichuan Province Science and Technology Support Program (2022NSFSC0986), China West Normal University Support Program (20A007, 20E051, 21E040, and 22kA011).
Competing interests
None.













