Carotenoids are lipophilic pigments synthesised by plants, algae and some micro-organisms. They are involved in various biological processes such as embryonic development( Reference Mora, Kuri-Melo and González-Gallardo 1 ), immunity( Reference Koutsos, Clifford and Calvert 2 ), reproduction( Reference Surai 3 ), and cell growth and differentiation( Reference Lampert, Holzchuh and Hessel 4 ), and they are known as antioxidants( Reference Surai 3 , Reference Müller, Fröhlich and Böh 5 ). Epidemiological studies have reported that carotenoids and their metabolites prevent several diseases, including cancers( Reference Wang, Ausman and Greenberg 6 ), CVD( Reference Voutilainen, Nurmi and Mursu 7 ) and age-related macular degeneration( Reference Krinsky, Landrum and Bone 8 ). Because animals do not synthesise carotenoids, they must obtain them from their diet. Considerable amounts of carotenoids accumulate in organs and tissues in response to feed intake( Reference Na, Song and Lee 9 – Reference Wang, Illingworth and Connor 11 ), and they have an important role in the determination of animal colour( Reference Bhosale and Bernstein 12 ). Several genetic polymorphisms have recently been identified that affect human and animal blood and tissue carotenoid status. In humans, several mutations or SNP associated with variations in carotenoid content affect the activity of β,β-carotene-15,15′-mono-oxygenase 1 (BCMO1), an enzyme responsible for the symmetrical cleavage of β-carotene into retinal( Reference Lindqvist, Sharvill and Sharvill 13 – Reference Borel, De Edelenyi and Vincent-Baudry 15 ). Polymorphisms such as SNP have also been identified in the BCDO2 gene that encodes β,β-carotene-9′, 10′-dioxygenase 2 responsible for the asymmetric cleavage of carotenoids into apocarotenoids( Reference Tian, Pitchford and Morris 16 , Reference Våge and Boman 17 ). They are also present in proteins involved in lipid metabolism, including membrane transporters such as scavenger receptor class B type I (SR-BI or SCARB1) and cluster determinant 36 (CD36), fatty acid-binding proteins and apolipoproteins( Reference Ortega, Castilla and Gómez-Coronado 18 – Reference Borel 22 ).
In the chicken, two fully linked SNP have also been identified within the proximal promoter of the BCMO1 gene at − 678 and − 621 upstream from the ATG codon( Reference Le Bihan-Duval, Nadaf and Berri 23 ). This polymorphism is associated with differential expression of the BCMO1 gene in the pectoralis major muscle, and is strongly related to variations in breast meat xanthophyll concentrations (lutein and zeaxanthin). The effects of the mutation have been further characterised by comparing chickens sharing a common genetic background but differing in this specific mutation( Reference Jlali, Graulet and Chauveau-Duriot 24 ). Reared on a classical maize-based diet, such birds showed marked differences in colour, lutein and zeaxanthin contents and BCMO1 mRNA levels in the pectoralis major muscle, but not in other tissues. By contrast, these birds showed no differences in vitamin A and E status and no differences in the activity of other genes involved in carotenoid transport (SCARB1 and CD36) and metabolism (BCDO2). The aim of the present study was to evaluate whether the BCMO1 polymorphism could affect the response to dietary β-carotene, the preferred substrate of the BCMO1 enzyme( Reference Lindqvist and Andersson 25 – Reference Kim and Oh 27 ), added to a wheat-based diet containing limited amounts of carotenoids. The activities of the BCMO1 and BCDO2 genes (involved in carotenoid conversion) and the SCARB1 and CD36 genes (involved in carotenoid transport) were determined from the site of uptake (duodenum) to the sites of accumulation (liver and skeletal muscle). The activity of intestine-specific homeobox (ISX), a major sensor of retinoid status involved in the feedback regulation of BCMO1 gene activity in the intestine, was also measured. The overall physiological consequences were evaluated through the record of the levels of carotenoids, fat-soluble vitamins A and E and cholesterol, and of performance traits (chicken growth, tissue yields and coloration).
Materials and methods
Reagents
Extraction reagent (RNA Now) for total RNA was obtained from Ozyme. DNase treatment was performed using Ambion's DNA-Free 1906 Kit obtained from CliniSciences, Moloney murine leukaemia virus RT (Superscript II) was purchased from Invitrogen and random primers were obtained from Promega. Real-time PCR premix (SYBR® Green I qPCR Master Mix Plus) was obtained from Eurogentec. All-E-β-carotene, retinol, lutein, α-tocopherol, δ-tocopherol, γ-tocopherol, tocopheryl acetate, retinyl acetate, retinyl myristate, retinyl palmitate and retinyl stearate were purchased from Sigma. Zeaxanthin, β-cryptoxanthin, echinenone, 9Z-β-carotene and 13Z-β-carotene were purchased from Carotenature. All solvents used were of ultra-performance liquid chromatography grade and obtained from VWR. Ultrapure water was prepared using a Milli-Q system (Millipore).
Animals, housing and diets
In the chicken, two fully linked SNP were identified within the proximal promoter of the BCMO1 gene at − 678 and − 621 upstream from the ATG codon( Reference Le Bihan-Duval, Nadaf and Berri 23 , 28 ). They corresponded to the substitution of two adenines (A/A) by two guanines (G/G) and defined two haplotypes, AN57A and GN57G, which segregate within chicken lines maintained at INRA. A total of 533 chicks were produced from nineteen males and seventy females whose status at the BCMO1 locus was known. Genotype was determined by high-resolution melting( Reference Le Bihan-Duval, Nadaf and Berri 23 ) analysis from genomic DNA extracted, according to Nadaf et al. ( Reference Nadaf, Gilbert and Pitel 29 ), from blood (breeders) or from two wing feathers removed at hatching (offspring). Because the two SNP are fully linked, genotyping took into account only the SNP positioned at − 621 upstream from the ATG codon. The following three genotypes were defined: homozygous AA and GG, and heterozygous AG.
From hatching to 3 weeks, all chickens were reared in a conventional poultry house( Reference Le Bihan-Duval, Nadaf and Berri 23 ) and received the same wheat-based starter diet (Table 1). From 3 to 9 weeks, only homozygous chickens were reared, representing 119 AA chickens, of which sixty-seven were females and fifty-two were males, and ninety-two GG chickens, of which sixty were females and thirty-two were males. Chickens were equally and randomly distributed into two groups within each genotype and sex that received a wheat-based diet with or without supplementation with 10 mg β-carotene/kg food (Table 1). The birds were individually weighed at hatching, and at 3, 6 and 9 weeks of age. The present study was conducted in accordance with the European Union Guidelines for Animal Care and under authorisation 37-112 delivered to C. B. by the French Ministry of Agriculture.
Table 1 Ingredients and analysed or calculated compositions of chicken starter (0–3 weeks) and growth (3 and 9 weeks) diets
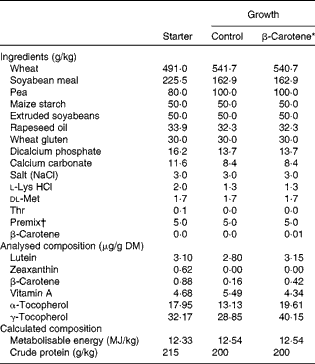
* β-Carotene is the control wheat-based growth diet supplemented with 10 mg β-carotene/kg food.
† Supplied per kg diet: 5·1 mg retinyl acetate; 0·1 mg cholecalciferol; 100 mg dl-β-tocopherol acetate; 5 mg menadione; 5 mg thiamin; 8 mg riboflavin; 7 mg pyridoxine; 0·02 mg cyanocobalamin; 100 mg niacin; 3 mg folic acid; 0·3 mg biotin; 25 mg calcium pantothenate; 550 mg choline; 80·8 mg manganese oxide; 90·1 mg zinc sulphate; 58·2 mg ferric sulphate; 20·0 mg copper sulphate; 2 mg calcium iodine; 0·2 mg sodium selenium; 0·6 mg cobalt carbonate; 50 mg antioxidant.
Sampling and phenotyping
At 9 weeks of age, chickens were weighed and slaughtered, and carcasses were processed as described in Berri et al. ( Reference Berri, Le Bihan-Duval and Debut 30 ). Blood samples were collected on heparin during bleeding and immediately chilled on ice. Blood samples were centrifuged at 3000 g for 10 min at 4°C, and serum was aliquoted and stored at − 20°C until analysed. Samples from the duodenal mucosa, liver and pectoralis major muscle were collected 15 min post-mortem from eight males and eight females of each genotype (AA v. GG) and diet (control v. β-carotene), and then immediately frozen in liquid N2 and stored at − 80°C until analysis.
All carcasses were processed 1 d after slaughter. Abdominal fat, breast muscles (pectoralis minor and pectoralis major) and liver were removed and weighed, and their respective yields were calculated and expressed as the percentage of live body weight at slaughter. Lipid contents of the liver and pectoralis major muscle were determined according to the method described by Folch et al. ( Reference Folch, Lees and Sloane Stanley 31 ). The colour of the pectoralis major muscle (internal part), duodenum (external face) and abdominal fat was measured by a Miniscan spectrocolorimeter (Hunterlab) according to the CIELAB trichromic system for lightness (L*), redness (a*) and yellowness (b*) values 15 min post-mortem for the duodenum and 24 h post-mortem for the other tissues. The absorbance spectrum of the plasma was measured between the wavelength of 400 and 700 nm by a spectrophotometer (Infinite M200; Tecan). Each absorbance spectrum was translated to make the value at 530 nm equal to zero( Reference Nozière, Grolier and Durand 32 , Reference Calderón, Chauveau-Duriot and Pradel 33 ). The colour index (CI), which corresponds to the absolute value of the integral of the translated spectrum between the wavelength of 450 and 530 nm, was measured by calculating the trapezoid area (TA) between the 450 and 530 nm wavelengths as follows:
The values were only taken into account when the CV between three repetitions was less than 5 %.
Quantitative PCR analysis
Total RNA was extracted from the duodenum, liver and pectoralis major muscle of eight females and eight males from each treatment group (genotype × diet), and quantitative PCR was performed using complementary DNA synthesised as described previously( Reference Sibut, Le Bihan-Duval and Tesseraud 34 ). Primers were designed (Table 2) and purchased from Eurogentec, quantitative PCR assays were run on a Roche LightCycler® 480 II using the LightCycler® 480 SYBR Green I Master (Roche Applied Science), according to the manufacturer's recommendations. Quantitative PCR conditions were set at 95°C for 5 min, followed by forty-five cycles of 10 s at 95°C, 20 s at 60°C or 62°C and 10 s at 72°C. The specificity of quantitative PCR was assessed by analysing the melting curves of the products and by verifying the size and sequence of the amplicons. Each PCR included negative control and unknown samples that were run in triplicate. The samples were normalised internally by using simultaneously the average cycle quantification of three reference genes (18S ribosomal RNA (18S), β-actin (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) whose stability in all the selected tissues was investigated using the geNorm application( Reference Vandesompele, De Preter and Pattyn 35 ). As recommended, the threshold for eliminating a gene was M≥ 1( Reference Hellemans, Mortier and De Paepe 36 ). Expression levels are expressed as normalised values( Reference Vandesompele, De Preter and Pattyn 35 ).
Table 2 Primers used for real-time PCR analysis
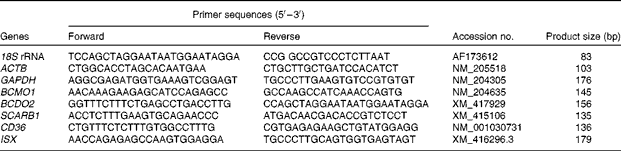
18S rRNA, 18S ribosomal RNA; ACTB, β-actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; BCMO1, β,β-carotene 15,15′-mono-oxygenase 1; BCDO2, β,β-carotene 9′,10′-dioxygenase 2; SCARB1, scavenger receptor class B type 1; CD36, cluster determinant 36. ISX, intestine-specific homeobox.
Biochemical analyses
Carotenoids and fat-soluble vitamins in plasma and tissues (duodenum, liver and pectoralis major muscle) were quantified by ultra-performance liquid chromatography (Waters), as described previously( Reference Chauveau-Duriot, Doreau and Nozière 37 ). After precipitation of proteins by ethanol, carotenoids and fat-soluble vitamins (A and E) were extracted from plasma and tissues with n-hexane and separated by ultra-performance liquid chromatography. The analytical conditions for ultra-performance liquid chromatography analysis were those recommended by Chauveau-Duriot et al. ( Reference Chauveau-Duriot, Doreau and Nozière 37 ). Under these conditions, carotenoids, and the entire vitamin A (retinol and retinyl esters) and vitamin E (α- and γ-tocopherol) forms were quantified at wavelengths of 450, 325 and 292 nm, respectively. Quantification of the compounds was performed using Empower Pro software (Waters), and their corresponding concentrations were calculated by using an external standard curve. The concentrations obtained were adjusted according to the percentage of the internal standard recovered.
Concentrations of total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, glucose and albumin were determined in plasma by enzymatic methods (Roche Diagnostics) using an automated assay (Hitachi Cobas C501; Roche Diagnostics). Plasma minerals (Ca, Fe, Mg and P) were determined according to the Association of Official Analytical Chemists method( 38 ) using a Vista-MPX ICP OES spectrophotometer (Varian Australia Private Limited).
Statistical analyses
Data were analysed using SAS 8.1 (SAS Institute, Inc.). Type I error was accepted as 5 %. The effects of BCMO1 genotype (AA v. GG), diet (control v. β-carotene) and their possible interactions were analysed using a two-way ANOVA (generalised linear model procedure) with type III sum of squares. Comparisons of means for each significant effect were made by Tukey's test using the least-squares mean statement of the generalised linear model procedure. Data are presented as least-squares means with their standard errors.
Results
Gene expression
The expression of BCMO1, BCDO2, SCARB1 and CD36 was detected in all the tissues tested (Tables 3 and 4). The expression of ISX was detected at much higher levels in the duodenum (about 60-fold) than in the liver, but was not detected in the pectoralis major muscle. The normalised expression of BCMO1 was affected by genotype and diet in a tissue-specific manner. In both diet conditions, there was a genotype effect in the pectoralis major muscle (AA≫GG, P< 0·0001). An opposite effect of the genotype was observed in the duodenum of chickens receiving the control diet (AA < GG, P= 0·0001) that was no longer observed when they were supplemented with the β-carotene diet. The addition of β-carotene to the diet lowered (P= 0·004) the mRNA levels of BCMO1 in the liver in both genotypes. The expression of SCARB1 was not affected by genotype, but was significantly decreased (P< 0·0001) when supplemented with the β-carotene diet, but only in the duodenum. The expression of ISX was also affected by diet but only in the GG genotype (β-carotene>control, P= 0·04). By contrast, mRNA levels of BCDO2 and CD36 were invariable between the genotypes and diets.
Table 3 Normalised mRNA levels of β,β-carotene 15,15′-mono-oxygenase 1 (BCMO1), β,β-carotene 9′,10′-dioxygenase 2 (BCDO2) and scavenger receptor class B type 1 (SCARB1) genes* in relation to genotype (G) and dietary β-carotene (D) in various tissues of 63-d-old chickens (Least-squares mean values with their standard errors; n 16)

CD36, cluster determinant 36; ISX, intestine-specific homeobox; ND, not detected.
* Data were normalised internally using three reference genes simultaneously (18S ribosomal RNA, β-actin and glyceraldehyde-3-phosphate dehydrogenase) whose stability in all the selected tissues was investigated using the geNorm application( Reference Nozière, Grolier and Durand 32 ).
Table 4 Intestine-specific homeobox (ISX) mRNA levels and retinol concentration in the duodenum and HDL-cholesterol concentration and colour index in the plasma of AA or GG chickens fed the control or the β-carotene-supplemented diet (Least-squares mean values with their standard errors; n 16)
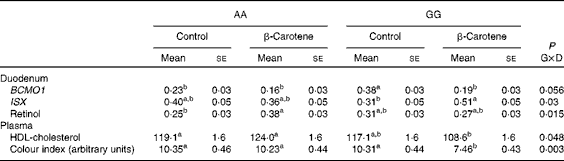
G, genotype; D, dietary β-carotene; BCMO1, β,β-carotene 15,15′-mono-oxygenase 1.
a,bLeast-squares mean values within a column with unlike superscript letters were significantly different (P <0·05).
Carotenoid and vitamin status
As for mRNA, lutein and zeaxanthin concentrations were affected by genotype and diet in a tissue-specific manner (Fig. 1). Concentrations of lutein and zeaxanthin were affected by genotype (AA < GG, P< 0·001) and diet in the pectoralis major muscle, but without any interaction: lutein and zeaxanthin concentrations were reduced (P= 0·02 and P< 0·0001, respectively) by supplementation of the β-carotene diet. Plasma lutein and zeaxanthin concentrations were lowered (P< 0·05) by supplementation of the β-carotene diet only in the GG genotype. The concentration of lutein was reduced by β-carotene supplementation in the duodenum (P= 0·02) and liver (P= 0·03) of GG chickens. Zeaxanthin concentration was absent in the liver and not regulated in the duodenum.
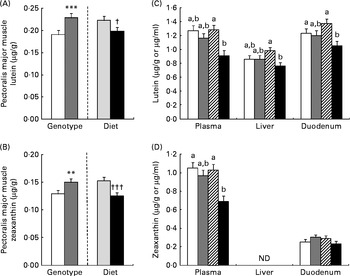
Fig. 1 Effects of genotype (G) or diet (D) on lutein (A) and zeaxanthin (B) concentrations in the pectoralis major muscle (□, AA; ![]() , GG;
, GG; ![]() , control; ■, β-carotene). Lutein (C) and zeaxanthin (D) concentrations in the plasma, liver and duodenum of AA or GG chickens fed the control or β-carotene-supplemented diet (□, AA control;
, control; ■, β-carotene). Lutein (C) and zeaxanthin (D) concentrations in the plasma, liver and duodenum of AA or GG chickens fed the control or β-carotene-supplemented diet (□, AA control; ![]() , AA β-carotene;
, AA β-carotene; ![]() , GG control; ■, GG β-carotene). Values are least-squares means (n 16 chickens per treatment), with their pooled standard errors represented by vertical bars. a,bLeast-squares mean values with unlike letters were significantly different within a tissue or plasma (P <0·05). Least-squares mean value was significantly different from that of the AA genotype: ** P <0·01, *** P <0·001. Least-squares mean value was significantly different from that of the control diet: † P <0·05, ††† P <0·001. For lutein concentrations, there was a significant effect for diet (P= 0·002 (plasma), P= 0·03 (liver) and P= 0·02 (duodenum)) and the genotype × diet interaction was significant (P= 0·004 (plasma), P= 0·02 (liver) and P= 0·04 (duodenum)). For plasma zeaxanthin concentrations, there were significant genotype (P= 0·02) and diet (P= 0·001) effects, and the genotype × diet interaction was significant (P= 0·03). ND, not determined.
, GG control; ■, GG β-carotene). Values are least-squares means (n 16 chickens per treatment), with their pooled standard errors represented by vertical bars. a,bLeast-squares mean values with unlike letters were significantly different within a tissue or plasma (P <0·05). Least-squares mean value was significantly different from that of the AA genotype: ** P <0·01, *** P <0·001. Least-squares mean value was significantly different from that of the control diet: † P <0·05, ††† P <0·001. For lutein concentrations, there was a significant effect for diet (P= 0·002 (plasma), P= 0·03 (liver) and P= 0·02 (duodenum)) and the genotype × diet interaction was significant (P= 0·004 (plasma), P= 0·02 (liver) and P= 0·04 (duodenum)). For plasma zeaxanthin concentrations, there were significant genotype (P= 0·02) and diet (P= 0·001) effects, and the genotype × diet interaction was significant (P= 0·03). ND, not determined.
Vitamin A and vitamin E status were affected by genotype or the incorporation of β-carotene in the diet, but the effects varied according to the tissue (Table 5). Lower concentrations of α- and γ-tocopherol were observed in the plasma (P= 0·001 and P= 0·003 for α and γ, respectively) of GG chickens. The concentration of α-tocopherol was also lower (P= 0·02) in the duodenum of GG chickens. The concentration of α-tocopherol was lowered in the plasma (P= 0·001) and in the tissues (P= 0·07 in the duodenum, P= 0·01 in the liver and P= 0·001 in the pectoralis major muscle) of chickens fed the β-carotene diet. By contrast, the concentration of vitamin A metabolites increased in response to the β-carotene diet. Several forms of retinyl esters (the storage forms of vitamin A) were detected only in the liver. Concentrations of total vitamin A were increased (P= 0·02) following β-carotene supplementation, essentially as a result of increased myristate and palmitate ester contents (P= 0·03 and P= 0·001, respectively). Retinol concentration was also increased (0·38 (se 0·03) v. 0·25 (se 0·03) μg/g; P< 0·05) in response to dietary β-carotene supplementation in the duodenum of AA chickens (Table 4).
Table 5 Concentrations* of vitamin A and E metabolites in relation to genotype (G) and dietary β-carotene (D) in several tissues and plasma of 63-d-old chickens (Least-squares mean values with their standard errors; n 8)

* Data are expressed in μg/g for tissues or μg/ml for plasma.
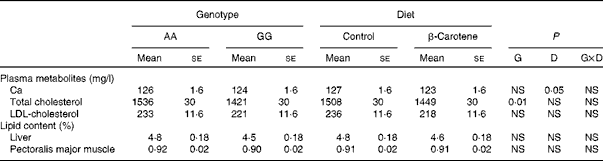
Plasma metabolites and tissue lipid contents
Of all the plasma metabolites measured, only cholesterol differed between the genotypes (Table 6). Total cholesterol concentration was higher (P= 0·01) in AA than in GG chickens. For HDL-cholesterol concentration, a significant effect of genotype was observed (P= 0·01) in a genotype × diet interaction manner (P= 0·05): HDL-cholesterol concentration was higher in AA than in GG chickens when fed the β-carotene diet (Table 4). A slight decrease (P< 0·05) in Ca concentration was also observed following dietary β-carotene supplementation. The lipid content of the liver and pectoralis major muscle was unaffected by genotype or diet.
Table 6 Concentrations of plasma metabolites and tissue lipid contents of 63-d-old chickens in relation to genotype (G) and dietary β-carotene (D) (Least-squares mean values with their standard errors; n 16)
Growth, body composition and colour parameters
Body weight at slaughter was higher (P= 0·02) in AA than in GG chickens, but not affected by diet (Table 7). Body composition was affected by both genotype and diet. Breast muscle yields were lower (P= 0·01) in AA than in GG chickens, and a trend towards higher values was observed following dietary β-carotene supplementation (P= 0·06). Dietary β-carotene increased leg yield (P= 0·002) and decreased abdominal fat yield (P= 0·03). The pectoralis major muscle was more yellow (P= 0·03) in GG than in AA chickens. The plasma CI was decreased by β-carotene supplementation in GG chickens (Table 4).
Table 7 Body weight and yields and colour traits of 63-d-old chickens in relation to genotype (G) and dietary β-carotene (D) (Least-squares mean values with their standard errors; n 92–120 per genotype and n 105–106 per diet except for plasma colour index)
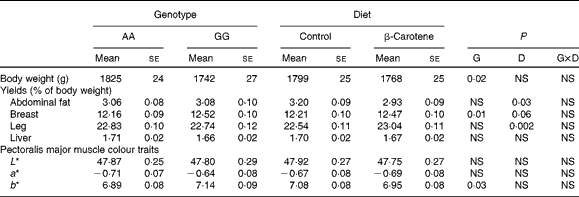
L*, lightness; a*, redness; b*, yellowness.
Discussion
By selecting chicken breeders of a pure line for their status at the BCMO1 locus, we were able to produce offspring homozygous (AA or GG) for this specific mutation, but sharing a common genetic background. This model is particularly pertinent to evaluate the consequences of variations in BCMO1 expression on chicken growth and metabolism in different nutritional conditions (presence or absence of β-carotene). The addition of β-carotene in the diet significantly altered the vitamin A status as evidenced by the increase in liver retinol accumulation, which is probably the result of BCMO1 gene activity. Numerous parameters were consistently affected in both genotypes. In the liver, two forms of retinyl ester (but not retinol) were increased in response to β-carotene supplementation in the diet, indicating an efficient conversion of dietary β-carotene in both genotypes. By contrast, concentrations of α-tocopherol in the plasma and all tissues, as well as concentrations of lutein (0·20 v. 0·22 μg/g) and zeaxanthin (0·12 v. 0·15 μg/g) in the pectoralis major muscle, were decreased by β-carotene supplementation. Similarly, Wang et al. ( Reference Wang, Illingworth and Connor 11 ) observed a significant reduction of lutein concentration in the plasma and several tissues including heart in leghorn chickens fed high supplements of dietary β-carotene. However, a discrepancy was found by these authors for zeaxanthin concentrations that were not different in the heart but in other tissues and plasma. Dietary β-carotene also reduced abdominal fat and slightly increased leg and breast yields without affecting overall chicken growth. The effects of β-carotene, a pro-vitamin A carotenoid, on body fat are consistent with previous results showing that dietary β-carotene reduces the lipid storage capacity of adipocytes in a BCMO1-dependent manner( Reference Lobo, Amengual and Li 39 , Reference Amengual, Gouranton and van Helden 40 ), suggesting a vitamin A-dependent mechanism. Interestingly, a reduction in Ca concentration was also observed in the plasma of chickens supplemented with the β-carotene diet. It has already been shown that vitamin A antagonises the Ca response to vitamin D in human subjects( Reference Johansson and Melhus 41 ). The activity of the BCMO1 gene was negatively regulated by dietary β-carotene in the liver, but not in the pectoralis major muscle. However, the negative effect of β-carotene on BCMO1 in the duodenum was only significant in the GG genotype. A decline in the mRNA levels of SCARB1 in the duodenum was also observed in response to dietary β-carotene supplementation. Several studies( Reference Bachmann, Desbarats and Pattison 42 – Reference Lobo, Hessel and Eichinger 44 ) have reported a negative control of BCMO1 and SCARB1 gene expression or enzyme activity by pro-vitamin A carotenoids, and/or vitamin A or its derivatives, such as retinoic acid, especially in the intestinal tract. This negative feedback is mediated by retinoid receptors (retinoic acid receptor and retinoid X receptor)( Reference Bachmann, Desbarats and Pattison 42 , Reference Takitani, Zhu and Inoue 45 ), and a common regulatory pathway for these two genes has previously been described in the intestine, involving the ISX response element( Reference Lobo, Hessel and Eichinger 44 , Reference Lietz, Lange and Rimbach 46 ). In the present study, the expression of ISX was up-regulated in response to dietary β-carotene supplementation in the duodenum of GG but not AA chickens. The concomitant increase in the expression of ISX and a decrease in the expression of BCMO1 in GG chickens suggest an efficient negative feedback that would allow maintaining stable duodenal retinol content. By contrast, this negative feedback appears as non-functional in the duodenum of AA chickens, which could explain the increased retinol accumulation following dietary β-carotene supplementation.
Significant interactions between genotype and diet were also observed for lutein concentrations in the plasma, liver and duodenum and for zeaxanthin concentrations in the plasma. These concentrations were similar between the genotypes in the control conditions, and significantly decreased by β-carotene supplementation only in the GG genotype. Competition between carotenoids has been reported, in which increased supply of β-carotene resulted in lower absorption of xanthophylls( Reference Wang, Illingworth and Connor 11 ). As vitamin E derivatives and cholesterol share the same transporters( Reference van Bennekum, Werder and Thuahnai 47 , Reference Reboul, Klein and Bietrix 48 ), we make the hypothesis that a similar mechanism could induce lower levels of all those metabolites in the plasma or tissues of chickens supplemented with β-carotene. Moreover, the increased β-carotene conversion may lower its competitive effect in AA chickens, while it may be maximal in GG chickens, and explain the decrease in plasma lutein and zeaxanthin concentrations, duodenum and liver lutein contents and circulating HDL-cholesterol levels following dietary β-carotene supplementation.
The effect of the genotypes on breast meat yellow colour (AA < GG), lutein and zeaxanthin contents (AA < GG) and BCMO1 mRNA levels (AA>GG) in the pectoralis major muscle, previously observed with chickens fed a maize-based diet( Reference Le Bihan-Duval, Nadaf and Berri 23 , Reference Jlali, Graulet and Chauveau-Duriot 24 ), was confirmed in the present study using a wheat-based diet, without any interaction with the dietary supplementation of β-carotene. In this tissue, lutein and zeaxanthin contents were lowered following β-carotene supplementation without any alteration in the gene expression of BCMO1. Thus, the differential activity of the BCMO1 gene between the AA and GG genotypes remains tightly linked with xanthophylls levels in this tissue. Although xanthophylls are not pro-vitamin A substrates for the BCMO1 enzyme( Reference Lindqvist and Andersson 25 , Reference Kim and Oh 27 ), the observations of the present study in chickens and the recent discovery of human genetic variants in the BCMO1 gene that are associated with variations in circulating or tissue levels of zeaxanthin and lutein( Reference Ferrucci, Perry and Matteini 14 , Reference Borel, De Edelenyi and Vincent-Baudry 15 ) argue in favour of a possible role of the BCMO1 enzyme in the metabolism of xanthophylls, which deserves further studies.
A significant effect of genotype, without interaction with diet, was recorded for a number of traits that did not differ in the previous study( Reference Jlali, Graulet and Chauveau-Duriot 24 ). We observed a significant effect of genotype on overall growth (AA>GG) and breast muscle yield (AA < GG). Plasma concentrations of the vitamin E isoforms α- and γ-tocopherol and duodenal content of α-tocopherol were significantly higher in AA than in GG chickens. The differences between the results of that study( Reference Jlali, Graulet and Chauveau-Duriot 24 ) and the present study could be explained by the increased statistical power due to the greater number of animals studied in the present study or might be the consequences of the reduced zeaxanthin content of the wheat-based diet used in the present study. As discussed above, competition phenomena are known to occur between carotenoids( Reference Wang, Illingworth and Connor 11 , Reference van den Berg and van Vliet 49 ) and between carotenoids and other nutrients( Reference Mamatha and Baskaran 50 ). In particular, β-carotene, vitamin E and cholesterol share similar transport mechanisms( Reference van Bennekum, Werder and Thuahnai 47 , Reference Reboul, Klein and Bietrix 48 ). The lower availability of β-carotene in the duodenum of AA chickens due to higher conversion by the BCMO1 enzyme may therefore result in the increased absorption of vitamin E and cholesterol.
By comparing genetic variants at the BCMO1 locus in chickens fed with or without dietary β-carotene supplementation, we identified a defect in the feedback regulation of BCMO1 gene expression by retinoids in the duodenum of AA chickens. The ISX-mediated negative feedback was observed in response to dietary β-carotene supplementation in GG chickens only. We make the hypothesis that the resulting decrease in β-carotene conversion by the BCMO1 enzyme could result in a lower uptake of xanthophylls, liposoluble vitamins and cholesterol due to competition in the duodenum of GG chickens. In the breast muscle, where ISX was not detectable, the gene expression of BCMO1 was insensitive to dietary β-carotene, while storage of xanthophylls was decreased. The relationship between BCMO1 mRNA levels and lutein and zeaxanthin contents in the pectoralis major muscle deserves further research for a complete explanation. From a basic perspective, the present data show that a SNP in the promoter of a metabolic gene (BCMO1) can lead to tissue-specific alterations of its expression and to diet × genotype interactions for a number of physiological parameters. Some are directly linked with the activity of the corresponding enzyme, such as retinol concentration in the duodenum, while others may rely on indirect effects such as the impact on lutein concentrations in the plasma, liver and duodenum. This illustrates the complexity of nutrigenetics. From an applied perspective, the present data show that the polymorphism at the BCMO1 locus is important to consider whether breast meat yellow colour and lutein content are traits of high added value, which could be the case for the production of yellow chickens. The present study also shows that dietary β-carotene is efficiently converted by broiler chickens into vitamin A, with favourable effects on carcass quality, such as decreased adiposity and increased breast muscle yields.
Acknowledgements
The authors thank M. Lessire for formulating the diets, P. Chartrin for measuring the tissue lipid contents, and the staff of the poultry breeding facilities (INRA, UE 1295 Pôle d'Expérimentation Avicole de Tours, F-37380 Nouzilly, France) and the avian research unit (INRA, UR83 Recherches Avicoles, F-37380 Nouzilly, France) for technical assistance.
The present study was funded by grants from INRA and DSM Nutritional Products, both contributing to the study design, data collection and redaction of the manuscript. M. J. was a PhD student supported by a grant from the Government of Tunisia.
The authors' contributions were as follows: C. B., M. J. D., E. L. B.-D., C. S. N. and M. J. contributed to the design of the study; C. B., M. J. D., M. J., E. G. and E. L. B.-D. contributed to tissue sampling and phenotypic data collection; M. J., E. G. and C. P. contributed to gene expression studies; E. G. carried out animal genotyping; B. G., M. J. and B. C.-D. performed the analyses on carotenoids and fat-soluble vitamins; C. S. N. contributed to plasma biochemical analyses; C. B. performed the statistical analyses; M. J. drafted the manuscript; C. B. and M. J. D. produced the final manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










