Vitamin C (VC), an essential micronutrient for maintaining homeostasis in humans and some mammals, has an important role in antioxidant activity to protect against oxidative stress and is also known to be a coenzyme for biosynthesis of collagen, l-carnitine and norepinephrine( Reference Li and Schellhorn1 , Reference Ang, Pullar and Currie2 ). The current RDA of VC for adults is 75–90 mg/d in the USA, while it is 100 mg/d in Germany, Switzerland and Japan( Reference Levine, Wang and Padayatty3 ). The dietary intake of VC at five to twenty times the RDA has been reported to reduce not only serum lipid peroxide in clinical trials( Reference Huang, Appel and Croft4 , Reference Nakhostin-Roohi, Babaei and Rahmani-Nia5 ) but also hydrogen peroxide (H2O2)-induced DNA damage in lymphocytes isolated from healthy subjects( Reference Panayiotidis and Collins6 ).
Glucocorticoids (GC) are steroid hormones with an important role in regulating various physiological processes such as energy metabolism, neurological function and immune function. GC are also stress hormones that activate the hypothalamic–pituitary–adrenal axis when secreted in response to physical or psychological stress( Reference Cain and Cidlowski7 , Reference Kadmiel and Cidlowski8 ). Excess of GC has been shown to cause atrophy of lymphoid tissues such as spleen and thymus and to increase the death of lymphocytes in these tissues( Reference Vishwas, Mukherjee and Haldar9 , Reference Yi, Zhu and Wu10 ), resulting in the inhibition of immune functions such as cytokine production, lymphocyte proliferation and cytotoxicity, natural killer (NK) activity and antigen-presenting capacity( Reference Elenkov11 , Reference Moser, De Smedt and Sornasse12 ). The immunosuppressive effects of GC are considered to increase the risk of infection and cancer( Reference Cain and Cidlowski7 , Reference Jensen, Thomsen and Engebjerg13 ). Reduction in antioxidant activity or increased oxidative stress are known to contribute to lymphocyte damage and immune dysfunction( Reference Kesarwani, Murali and Al-Khami14 ). Dexamethasone (DEX) is a GC analogue that has been reported to increase the production of reactive oxygen species (ROS) along with reduction in antioxidants such as reduced glutathione (GSH) and superoxide dismutase (SOD) in lymphoid tissue, including spleen and thymus( Reference Yi, Zhu and Wu10 , Reference Hu, Shuai and Chen15 ). Suppression of antioxidant activity for removal of ROS can lead to oxidative damage of proteins, lipids and DNA, resulting in apoptotic death of splenocytes and T cells( Reference Yi, Zhu and Wu10 , Reference Matsushita, Freigang and Schneider16 , Reference Potula, Hawkins and Cenna17 ). In addition, intracellular deficiency of GSH and SOD decreases proliferation and cytokine production by T cells or antigen-presenting cells (APC)( Reference Massilamany, Gangaplara and Kim18 – Reference Khan, Rahim and Boddupalli20 ). It has been reported that VC prevents GC-induced lymphocyte death by maintaining the GSH level( Reference Pavlovic, Pavlovic and Kocic21 , Reference Pavlovic, Pavlovic and Kamenov22 ), but the in vivo effects of high-dose VC intake on the lymphocyte count and immune function after GC administration are not clearly understood.
Senescence marker protein 30 (SMP30) is a 34-kDa protein that was initially identified in the rat liver, and its expression has been reported to decrease with age in both sexes( Reference Fujita, Uchida and Maruyama23 ). SMP30 is also known as gluconolactonase, and it has a role in VC biosynthesis as a lactone hydrolysing enzyme. Ishigami et al. generated SMP30 knockout mice (SMP30-KO mice) that developed symptoms of scurvy when fed a VC-deficient diet( Reference Ishigami, Fujita and Handa24 ). Thus, SMP30-KO mice may be a useful animal model for investigating the physiological functions of VC( Reference Kondo, Inai and Sato25 ). Recently, the daily dietary VC requirement of SMP30-KO mice was estimated and it was also demonstrated that high VC intake (ten times of the requirement) suppressed the age-related thymic atrophy and decline in the T-cell count in the peripheral blood, thymus and spleen in these mice( Reference Uchio, Hirose and Murosaki26 ).
To investigate the effect of high VC intake on lymphoid organ weight, splenic immune function and the intracellular redox status of splenocytes after GC treatment, we measured spleen and thymus weights as well as lymphocyte count, T-cell proliferation, cytokine production and intracellular antioxidants/lipid peroxide in the splenocytes after administration of DEX to VC-deficient SMP30-KO mice fed a normal-VC diet or high-VC diet.
Materials and methods
Animals
Specific pathogen-free male C57BL/6 (wild type (WT)) mice were purchased from Charles River Japan and were acclimatised for 7 d before the experiments. Throughout the experiments, mice were housed in individual cages and were maintained under specific pathogen-free conditions in a controlled environment (room temperature: 22 to 24°C, relative humidity: 50 to 60 % and 12 h light–12 h dark cycle). SMP30-KO mice were generated from C57BL/6 mice by gene targeting, as described previously( Reference Ishigami, Fujita and Handa24 ). Experiments were performed with 7- to 9-week-old male SMP30-KO mice and 9- to 10-week-old male WT mice. Animal experiments were performed in accordance with the animal care and use protocol approved by the Institutional Animal Care and Use Committee of Tokyo Metropolitan Institute of Gerontology (TMIG) (permit number: 18038) and the TMIG Guidelines for the Care and Use of Laboratory Animals.
Experimental design
The daily VC requirement of SMP30-KO mice was previously reported to be 20 mg/kg( Reference Uchio, Hirose and Murosaki26 ). Animal and clinical studies have shown that supplementation with antioxidants such as melatonin and VC for 2 months reduces oxidative stress of peripheral blood lymphocytes and improves immune function such as T-cell proliferation( Reference Block, Jensen and Morrow27 – Reference Tian, Zhang and Dai29 ). Thus, SMP30-KO mice were randomised to two groups based on body weight and fed a purified diet containing 0·02 %VC (20 mg/kg per d, n 10) or 0·2 %VC (200 mg/kg per d, n 10) for 2 months. WT C57BL/6 mice (WT mice; n 10) were fed the purified diet without VC for 2 months as a control group. Mice were given access to each diet and drinking water ad libitum throughout the study. The purified diet was based on the American Institute of Nutrition (AIN) 93M diet( Reference Reeves, Nielsen and Fahey30 ) and its composition is shown in Table 1. α-Maize starch, casein, soyabean oil, cellulose powder, AIN93M mineral mixture and AIN93 vitamin mixture were purchased from Oriental Yeast Co. Maize starch and sucrose were obtained from Matsutani Chemical Industry and Mitsui Sugar Co., Ltd, respectively. Choline bitartrate, l-cystine, tert-butylhydroquinone and l-ascorbic acid were purchased from Wako Pure Chemicals. After 2 months, SMP30-KO mice receiving the 0·02 %VC or 0·2 %VC diet were randomised by body weight to a vehicle subgroup (n 5) or a DEX subgroup (n 5 each), and WT mice were also randomly allocated to two subgroups in a similar manner. The size of the subgroups was determined from the results of a previous investigation into the effect of betulinic acid, a natural plant triterpenoid, on the decrease of splenocytes after DEX administration( Reference Yi, Zhu and Wu10 ). A size of n 5 was estimated to be sufficient based on the following assumptions: a splenocyte count of 5·0 (sd 1·5) × 107 cells after DEX administration to SMP30-KO mice receiving the 0·02 %VC diet, 25 % restoration of the splenocyte count by 0·2 %VC, statistical power of 80 % and type I error of 5 %. Mice in the two DEX subgroups were administered DEX (Wako) intraperitoneally at a dose of 2·5 mg/kg once daily for 2 d, while mice in the vehicle groups were given the same volume of 0·9 % saline (Otsuka Chemical) containing 10 % v/v ethanol. Before the start of the present study, we performed the preliminary examination for determining the optimum DEX conditions (solvent, dosage and exposure period) that lead to a significant reduction in lymphoid organ weight as well as splenic lymphocyte count and immune function compared with vehicle by reference to previous reports( Reference Mumtaz, Hoyer and Panne31 – Reference Purton, Monk and Liddicoat33 ). At 12 h after DEX administration on day 2, the plasma ascorbic acid concentration, body weight, thymus weight and spleen weight were measured, and splenocytes were prepared for further analyses. After mice were anesthetised with isoflurane, blood samples were collected from the inferior vena cava using a 1-ml disposable syringe (Termo) containing heparin (Ajinomoto). Then the animals were killed by exsanguination, and the thymus and spleen were harvested and washed with saline to minimise contamination.
Table 1. Composition of the basal diet*
(Values are the amount of ingredients (g/kg diet) in each group)
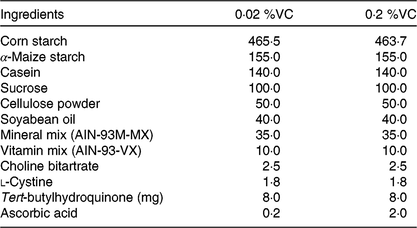
VC, vitamin C; AIN, American Institute of Nutrition.
* The basal diet was based on the AIN-93M diet, which was supplemented with 0·02 or 0·2 % ascorbic acid.
Measurement of the plasma ascorbic acid concentration
The plasma level of ascorbic acid was measured by HPLC with electrochemical detection, as described previously( Reference Uchio, Hirose and Murosaki26 ). Briefly, a plasma sample was mixed with an equal volume of 10 % w/v metaphosphoric acid containing EDTA-2Na and then was centrifuged at 8000 g for 10 min at 4°C. Before HPLC, the supernatant was mixed with 10 % w/v TCA and was centrifuged at 5200 g for 10 s at room temperature, after which the resulting supernatant was diluted with the mobile phase and injected into a semi-micro HPLC system (Nanospace SI-2, Shiseido). Separation was performed using a Capcell Pak C18 MG column (Shiseido) with an isocratic mobile phase (0·1 M potassium phosphate buffer, pH 2·0), and electrochemical detection (Model 3016, Shiseido) was done in the amperometric mode at an oxidation potential of +700 mV.
Preparation and counting of splenocytes
Spleen cells were prepared according to the reported method( Reference Uchio, Hirose and Murosaki26 ). In brief, the spleen was initially minced with scissors and then mashed with the plunger of a syringe to obtain a cell suspension that was centrifuged at 250 g for 5 min at 4°C. After removing the supernatant, erythrocytes were lysed by incubation in lysis solution (17 mm NH4Cl and 140 mm Tris-HCl, pH 8·0) for 5 min at 4°C. Then an excess of Hank’s solution (Sigma) containing 5 % fetal bovine serum (FBS; Hyclone) was added and the cell suspension was centrifuged at 250 g for 5 min at 4°C. After removing the supernatant, the pellet was resuspended in the PBS containing 2 % FBS to measure the total splenocyte count and the splenic T-cell population by flow cytometry or was resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco) supplemented with 0·2 % w/v NaHCO3, 10 % v/v heat-inactivated FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 5 ng/ml 2-mercapto-ethanol) to evaluate cytokine production and proliferative capacity. Single-cell suspensions of splenocytes were obtained by passage through a 70-µm nylon cell strainer (Becton-Dickinson). The total splenocyte count was obtained with an automated cell counter (CDA-500; Sysmex), after which the splenocyte suspension was stored at –80°C until measurement of the intracellular ascorbic acid content, SOD activity, GSH content and lipid peroxide content.
Flow cytometry
Splenocytes were pre-incubated with anti-cluster of differentiation (CD)16/CD32 antibody (10139, Clone: 93, Biolegend) to block the Fc receptor and then were stained with monoclonal antibodies according to each manufacturer’s instructions. Splenocytes were stained with fluorescein isothiocyanate-labelled anti-CD3 antibody (100306, Clone: 145-2C11; Biolegend), phycoerythrin-labelled anti-CD4 antibody (12-0042-82, Clone: RM4-5; eBioscience) and peridinin chlorophyll protein complex-labelled anti-CD8 antibody (100732, Clone: 53-6.7; Biolegend). CD3+CD4+ cells were defined as helper T cells and CD3+CD8+ cells were defined as cytotoxic T cells. Splenocyte subpopulations were analysed by flow cytometry (EPICS XL ADC using EXPO 32 ADC software; Beckman Coulter), and the absolute count of each subpopulation was calculated as the total splenocyte count multiplied by the percentage of each cell type.
Cell culture and measurement of cytokine production
First, ninety-six-well polystyrene microplates (Greiner) were precoated by incubation with 5·0 μg/ml anti-CD3ϵ antibody (MAB484; R&D Systems) in 50 μl of PBS overnight at 4°C. After aspiration of the antibody solution, splenocytes (5·0 × 105 per well) were seeded in the coated wells and were cultured in RPMI medium at 37°C under a 5 % CO2 atmosphere. After 24 h, supernatants were collected and the levels of IL-2, IL-4, IL-10, IL-12 and interferon (IFN)-γ in each supernatant were determined.
Determination of cytokine levels
Cytokine levels in culture supernatants were measured by sandwich ELISA, as reported previously( Reference Murosaki, Muroyama and Yamamoto34 ). The reagents used for ELISA are summarised in online Supplementary Table S1. To evaluate the balance between T helper type 1 (Th1) and T helper type 2 (Th2) cells, the Th1:Th2 ratio was calculated indirectly as the ratio of Th1 cytokine (IFN-γ) production/Th2 cytokine (IL-4) production.
Measurement of T-cell proliferation
Splenocytes (5·0 × 105 per well) were seeded into 96-well plates containing RPMI medium with or without concanavalin A (ConA; C2010; Sigma) at a final concentration of 2 μg/ml and were incubated for 48 h at 37°C under a 5 % CO2 atmosphere. At 4 h before the end of incubation, 4-(3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzol-disulfonate (WST-1) solution (10 mm WST-1 (Dojindo) and 0·4 mm 1-methoxy-5-methylphenazinium methyl sulphate (Dojindo in PBS) were added to the plate. On completion of incubation, the absorbance of each well was measured at 450 and 630 nm (for reference) with a microplate reader. Proliferative activity was assessed by calculating the stimulation index (absorbance of ConA-stimulated wells/absorbance of unstimulated wells).
Measurement of intracellular total protein
Splenocytes (1·0 × 107 cells) were lysed in 100 μl of radioimmunoprecipitation assay (RIPA) buffer (Cayman Chemical) supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and were homogenised twice with a Sonifire SLPe 40 (Branson) for 1 s at 20 % amplitude on ice. Then the homogenate was centrifuged at 10 000 g for 10 min at 4°C, after which the supernatant was diluted 5-fold with deionised water. Subsequently, the total protein content of the homogenate was determined by the bicinchoninic acid assay( Reference Smith, Krohn and Hermanson35 , Reference Burgess, Deutscher, Abelson and Simon36 ).
Measurement of intracellular ascorbic acid
The intracellular ascorbic acid content was measured by HPLC, as described previously( Reference Uchio, Hirose and Murosaki26 , Reference Mitsuzumi, Kusamiya and Kurimoto37 ). After splenocytes (5·0 × 106 cells) were homogenised twice with a Sonifire SLPe 40 in 50 μl of 5 % w/v metaphosphoric acid containing EDTA-2Na, the homogenate was centrifuged at 8000 g for 10 min at 4°C. Before HPLC, the supernatant was diluted 5-fold with 10 % w/v TCA and centrifuged at 5200 g for 10 s at room temperature, after which the resulting supernatant was diluted 5-fold with the mobile phase and injected into a semi-micro HPLC system (Shiseido). The intracellular ascorbic acid content of splenocytes was normalised per mg of protein.
Measurement of intracellular superoxide dismutase activity
Splenocytes (5·0 × 106 cells) were homogenised twice with a Sonifire SLPe 40 in 100 μl of sucrose buffer (0·25 m sucrose, 10 mm Tris (hydroxymethyl) aminomethane and 1 mm EDTA, pH 7·40). Then the homogenate was centrifuged (10 000 g for 15 min at 4°C), and the total SOD activity in the supernatant was measured with an SOD assay kit-WST (Dojindo), according to the manufacturer’s directions. One unit (U) of SOD activity was defined as causing 50 % inhibition of the assay reaction, and intracellular SOD activity was normalised per mg of protein.
Measurement of intracellular glutathione
Splenocytes (1·0 × 107 cells) were homogenised twice with a Sonifire SLPe 40 in 100 μl of 5 % w/v 5-sulphosalicylic acid solution, after which the homogenate was centrifuged at 8000 g for 10 min at 4°C and the supernatant thus obtained was diluted 5-fold with deionised water. Subsequently, total GSH and oxidised glutathione (GSSG) levels were determined in the supernatant with a GSSG/GSH quantification kit (Dojindo), according to the manufacturer’s instructions. The GSH content was calculated as the difference between total GSH and GSSG, and the GSH:GSSG ratio was also calculated. Intracellular GSH and GSSG contents of splenocytes were normalised per mg of protein.
Measurement of intracellular lipid peroxide
To measure the intracellular level of thiobarbituric acid-reactive substances (TBARS), splenocytes (1·0 × 107) were homogenised twice using a Sonifire SLPe 40 in 100 μl of RIPA buffer (Cayman) supplemented with protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged (1600 g for 10 min at 4°C) and the supernatant collected for measurement with a TBARS assay kit (Cayman Chemical), according to the manufacturer’s protocol( Reference Uchio, Higashi and Kohama38 ). The TBARS level was normalised per mg of protein.
Statistical analysis
Differences between the vehicle and DEX subgroups were assessed with Student’s unpaired t test. Within the vehicle or DEX subgroups, data were analysed by one-way ANOVA, followed by the Tukey–Kramer test, for comparisons between the 0·02, 0·2 %VC and WT groups. All analyses were performed with Statcel3 software (OMS Publishing). Results are shown as means and standard deviations. A probability (P) < 0·05 was considered to indicate statistical significance.
Results
Effects of high vitamin C intake on body weight, organ weights and plasma ascorbic acid in SMP30-KO mice
To examine the influence of high dietary VC intake on DEX-induced changes in body weight, immune organ weights and the ascorbic acid level in SMP30-KO mice, these parameters were compared after administration of DEX or the vehicle. No adverse events were observed in all experimental groups throughout the experiment. While vehicle treatment did not result in any significant differences in body weight, thymus weight and spleen weight among the three groups (0·02, 0·2 %VC and WT groups), the plasma ascorbic acid concentration was significantly higher in the 0·2 %VC group than in the other two groups (Table 2). After DEX administration, body weight showed no significant differences among the three groups (Table 2). However, thymus weight was significantly decreased by DEX in all three groups, with no significant differences among the groups (Table 2). Spleen weight was significantly decreased by DEX in all three groups, but it was significantly higher in the 0·2 %VC group compared with the other two groups (Table 2). In addition, the plasma ascorbic acid concentration showed a significant decrease in the 0·02 and 0·2 %VC groups after DEX administration, whereas it did not decrease significantly in the WT group (Table 2). Despite being decreased by DEX, the plasma ascorbic acid concentration was still significantly higher in the 0·2 %VC group compared with the other two groups (Table 2).
Table 2. Effect of high dietary vitamin C (VC) intake on body weight, thymus weight, spleen weight and plasma ascorbic acid after administration of dexamethasone (DEX)
(Mean values and standard deviations for n 5 (vehicle or DEX subgroups of the 0·02 %VC, 0·2 %VC and wild type (WT) groups))
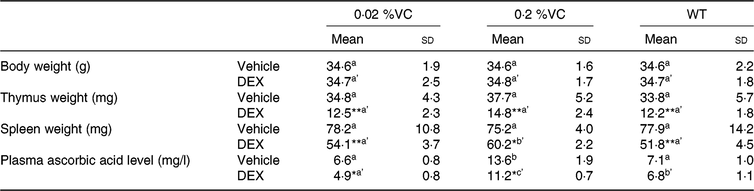
a,b (vehicle subgroups) or a’,b’,c’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test).
Mean value was significantly different from that of the vehicle subgroup: * P < 0·05, ** P < 0·01 (unpaired Student’s t test).
Effect of high vitamin C intake on the splenocyte count and splenic T-cell subpopulations
It is well known that GC administration decreases the lymphocyte count in mice( Reference Mumtaz, Hoyer and Panne31 , Reference Neishabouri, Hassan and Azizi32 ). To investigate the effect of high VC intake on DEX-induced changes in splenic T cells, we measured the total splenocyte count and splenic T-cell subpopulations after administration of the vehicle or DEX to SMP30-KO mice. After vehicle treatment, the splenocyte count and the number of splenic CD3+ T cells, CD3+CD4+ T cells and CD3+CD8+ cells did not differ significantly among the three groups. DEX administration caused a significant decrease in these cells in all three groups compared with vehicle administration (Fig. 1(A)–(D)). However, the splenocyte count and the number of splenic CD3+ T cells, CD3+CD4+ T cells and CD3+CD8+ T cells were significantly higher in the 0·2 %VC group compared with the other two groups after DEX treatment (Fig. 1(A)–(D)).
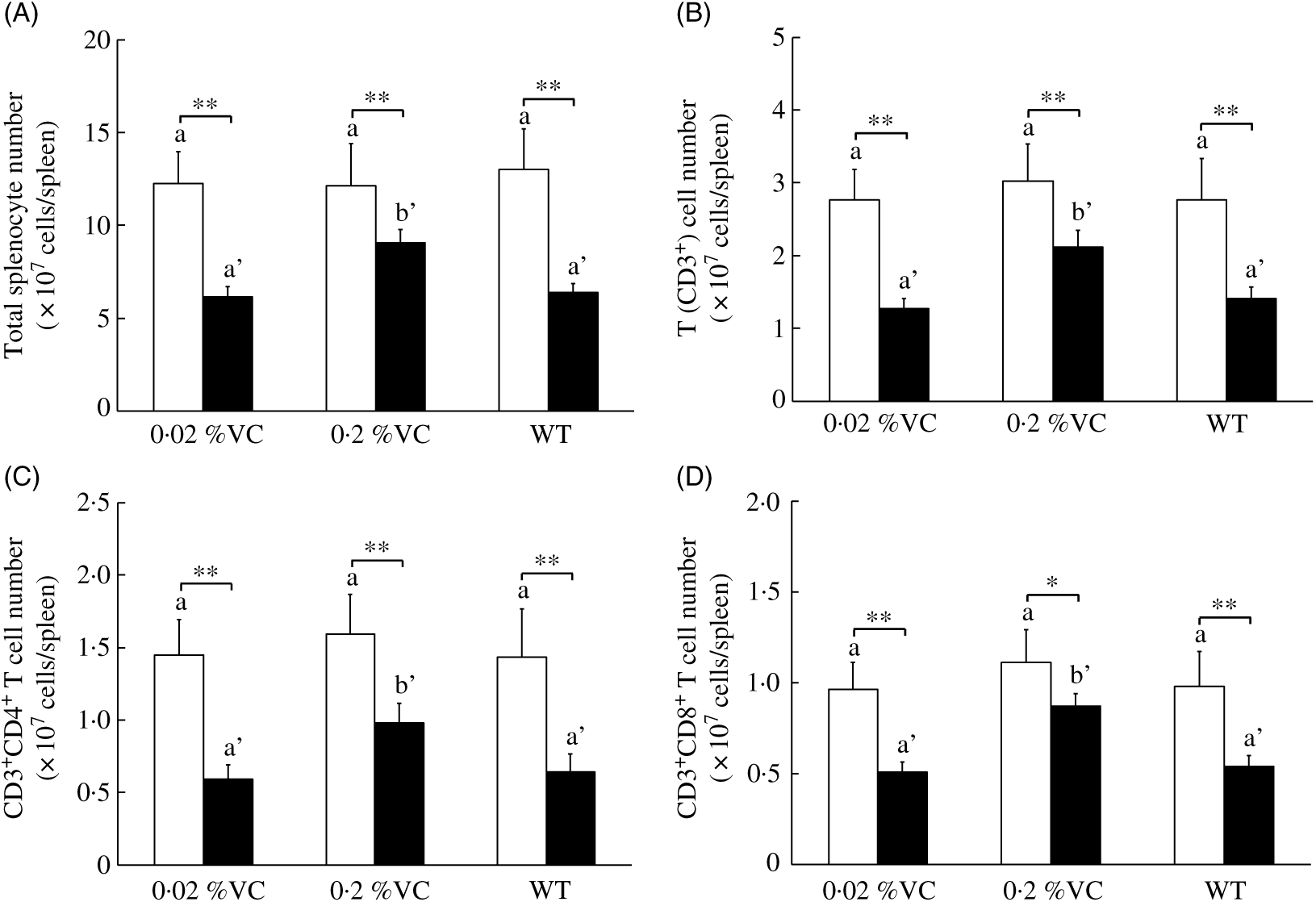
Fig. 1. Effect of high dietary vitamin C (VC) intake on splenocytes and T-cell subsets after administration of dexamethasone (DEX; ![]() ). Values are means and standard deviations for n 5 (vehicle (
). Values are means and standard deviations for n 5 (vehicle (![]() ) or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups). a,b (vehicle subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test). Mean value was significantly different from that of the vehicle subgroup: * P < 0·05, ** P < 0·01 (unpaired Student’s t test).
) or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups). a,b (vehicle subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test). Mean value was significantly different from that of the vehicle subgroup: * P < 0·05, ** P < 0·01 (unpaired Student’s t test).
Effect of high vitamin C intake on cytokine production by splenocytes stimulated with anti-CD3 antibody
GC treatment inhibits the production of IL-2 and IFN-γ by T helper type 1 (Th1) cells and also suppresses IL-12 production by APC, causing a decrease of the Th1:Th2 cytokine ratio( Reference Elenkov11 , Reference Yao, Wang and Zhang39 ). To examine the influence of high VC intake on DEX-induced changes in cytokine production, we administered DEX or the vehicle to SMP30-KO mice and then measured the concentrations of IL-2, IL-4, IL-10, IL-12p40 and IFN-γ in splenocytes cultured with anti-CD3 antibody. After vehicle treatment, the levels of all cytokines and the Th1:Th2 ratio did not differ significantly among the three groups (Fig. 2(A)–(F)). DEX administration significantly reduced the IL-2 level in all three groups, but it was significantly higher in the 0·2 %VC group compared with the other two groups (Fig. 2(A)). IL-4 was slightly, but significantly, decreased in the 0·02 %VC and 0·2 %VC groups after DEX administration, while it was slightly higher in the WT group than in the other two groups (Fig. 2(B)). IL-10 did not decrease in either of the VC groups after DEX administration, but it showed a significant decrease in the WT group. However, there was no significant difference in IL-10 among the three groups after DEX treatment (Fig. 2(C)). DEX administration caused a significant decrease in IL-12p40 in the 0·02 %VC group and the WT group, but the decrease in IL-12p40 was significantly smaller in the 0·2 %VC group and the IL-12p40 level was significantly higher in this group compared with the other two groups (Fig. 2(D)). IFN-γ was significantly decreased in all three groups after DEX administration, but it was significantly higher in the 0·2 %VC group than in the other two groups (Fig. 2(E)). Finally, the Th1:Th2 ratio was significantly decreased by DEX administration in all three groups, but it was significantly higher in the 0·2 %VC group compared with the other two groups (Fig. 2(F)).
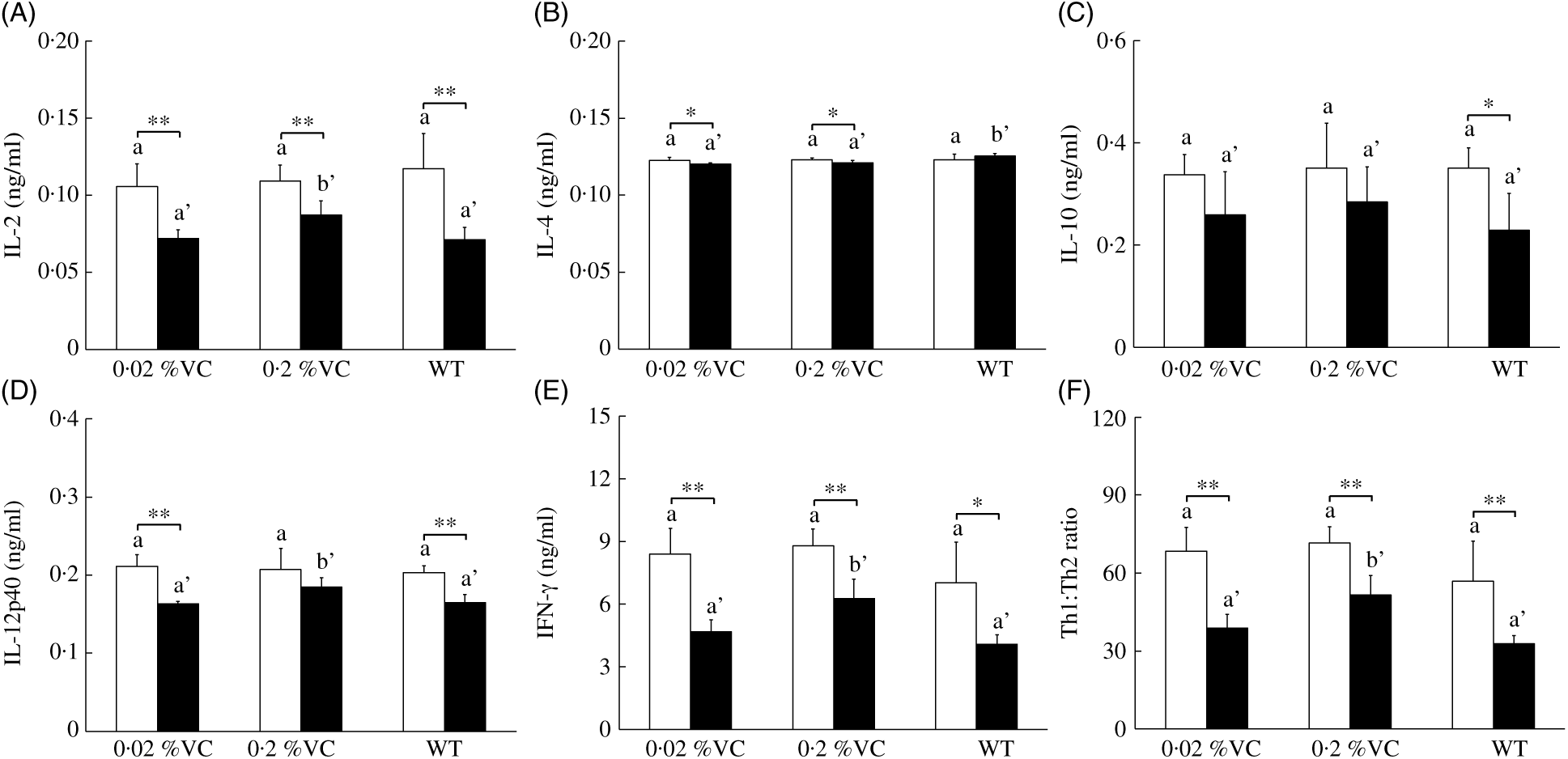
Fig. 2. Effect of high dietary vitamin C (VC) intake on cytokine production by splenocytes stimulated with anti-CD3 antibody after administration of dexamethasone (DEX; ![]() ). Values are means and standard deviations for n 5 (vehicle (
). Values are means and standard deviations for n 5 (vehicle (![]() ) or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups). a,b (vehicle subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test). Mean value was significantly different from that of the vehicle subgroup: *P < 0·05, ** P < 0·01 (unpaired Student’s t test).
) or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups). a,b (vehicle subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test). Mean value was significantly different from that of the vehicle subgroup: *P < 0·05, ** P < 0·01 (unpaired Student’s t test).
Effect of high vitamin C intake on proliferation of splenic T cells stimulated with concanavalin A
It was reported that GC treatment suppressed T-cell proliferation when splenocytes were stimulated with a T-cell mitogen( Reference Neishabouri, Hassan and Azizi32 , Reference Yao, Wang and Zhang39 ). To investigate the influence of high VC intake on T-cell proliferation after DEX administration to SMP30-KO mice, we measured the proliferative activity of T cells among splenocytes treated with ConA. There were no significant differences in T-cell proliferative activity among the three groups after vehicle treatment (Fig. 3). On the other hand, T-cell proliferative activity showed a significant decrease in the 0·02 %VC and WT groups after DEX administration, while it did not decrease in the 0·2 %VC group and was significantly higher in this group compared to the other two groups (Fig. 3).
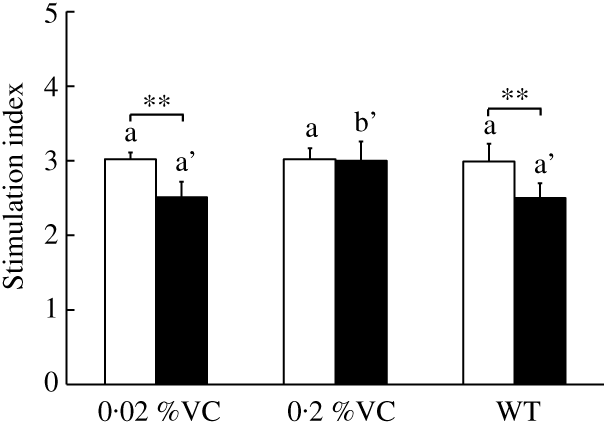
Fig. 3. Effect of high dietary vitamin C (VC) intake on proliferative activity of splenocytes stimulated with concanavalin A after administration of dexamethasone (DEX; ![]() ). Values are means and standard deviations for n 5 (vehicle or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups). a (vehicle (
). Values are means and standard deviations for n 5 (vehicle or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups). a (vehicle (![]() ) subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test). Mean value was significantly different from that of the vehicle subgroup: ** P < 0·01 (unpaired Student’s t test).
) subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test). Mean value was significantly different from that of the vehicle subgroup: ** P < 0·01 (unpaired Student’s t test).
Effect of high vitamin C intake on intracellular antioxidant activity and lipid peroxide content in splenocytes
GC were reported to decrease antioxidant activity and increase the lipid peroxide level in the spleen( Reference Yi, Zhu and Wu10 ). We investigated the influence of high VC intake on intracellular antioxidant and lipid peroxide levels in splenocytes from SMP30-KO mice treated with DEX by measuring the intracellular levels of ascorbic acid, GSH, SOD and TBARS. After vehicle treatment, the intracellular levels of ascorbic acid, GSH, SOD and TBARS showed no significant differences among the three groups (Table 3). DEX administration significantly decreased the intracellular ascorbic acid level, SOD activity and total GSH level in the 0·02 %VC group and WT group but not in the 0·2 %VC group. As a result, the intracellular ascorbic acid level, SOD activity and total GSH level were significantly higher in the 0·2 %VC group than in the other two groups after DEX administration (Table 3). The intracellular GSH level and GSH:GSSG ratio were significantly decreased by DEX in all three groups but were significantly higher in the 0·2 %VC group compared with the other two groups (Table 3). DEX significantly increased the intracellular TBARS level in the 0·02 %VC and WT groups, but not in the 0·2 %VC group, with the TBARS level being significantly lower in the 0·2 %VC group compared with the 0·02 %VC group (Table 3).
Table 3. Effect of high dietary vitamin C (VC) intake on intracellular antioxidant and lipid peroxide levels in splenocytes from mice treated with dexamethasone (DEX)
(Mean values and standard deviations for n 5 (vehicle or DEX subgroups of the 0·02, 0·2 %VC and wild type (WT) groups))
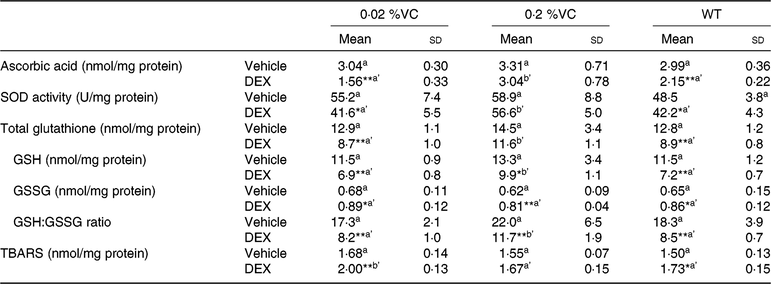
SOD, superoxide dismutase; GSH, reduced glutathione; GSSG, oxidised glutathione; TBARS, thiobarbituric acid-reactive substances.
a (vehicle subgroups) or a’,b’ (DEX subgroups): mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA with a post hoc Tukey–Kramer test).
Mean value was significantly different from that of the vehicle subgroup: * P < 0·05, ** P < 0·01 (unpaired Student’s t test).
Discussion
In the present study, high VC intake (ten times of the requirement) significantly inhibited the induction of splenic atrophy by DEX in SMP30-KO mice and also maintained the number of splenocytes and splenic CD4+and CD8+ T cells. In addition, high VC intake significantly ameliorated DEX-induced suppression of IL-2, IL-12 and IFN-γ production by splenocytes, alteration of the splenocyte Th1:Th2 ratio and inhibition of T-cell proliferation among splenocytes. Furthermore, high VC intake inhibited the reduction in intracellular ascorbic acid, SOD and GSH levels in splenocytes after administration of DEX and suppressed elevation of the intracellular TBARS level. These results suggest that high dietary intake of VC may decline the intracellular antioxidant activity and maintain the T-cell count, Th1 cytokine production, Th1/Th2 balance and T-cell proliferative capacity, thus preventing GC-induced T-cell dysfunction in response to stress.
SMP30 is a lactone-hydrolysing enzyme with an essential role in VC biosynthesis, and SMP30-KO mice fed a VC-deficient diet display scurvy symptoms( Reference Ishigami, Fujita and Handa24 , Reference Kondo, Inai and Sato25 ). Recently, the dietary VC requirement of SMP30-KO mice was proposed to be 20 mg/kg per d (0·02 %VC in the diet) equivalent to human RDA (100 mg/d) in the case of dose per body surface area, because the plasma ascorbic acid concentration and splenocyte count were similar in mice on a 0·02 %VC diet and WT mice( Reference Uchio, Hirose and Murosaki26 ). In agreement with that report, the plasma ascorbic acid level, the T-cell count among splenocytes, cytokine production by splenocytes, splenic T-cell proliferation and splenocyte intracellular antioxidant content after administration of the vehicle were similar in the 0·02 %VC and WT groups (Fig. 1–3, Tables 2 and 3). On the other hand, the plasma ascorbic acid concentration after DEX administration was significantly higher in the WT group compared with the 0·02 %VC group (Table 2). Normal mice with the SMP30 gene can synthesise VC in the liver for supply to the peripheral blood and tissues, and it is known that hepatic VC synthesis increases under conditions causing oxidative stress such as physical-restraint stress( Reference Nakano and Suzuki40 ), lipopolysaccharide injection( Reference Victor, Guayerbas and Puerto41 , Reference Kuo, Tan and Dragan42 ) and feeding a high-fat diet( Reference Tranberg, Hansen and Lykkesfeldt43 ). Thus, it seems that WT mice increased hepatic VC production to maintain the plasma ascorbic acid concentration after DEX administration, but the intracellular levels of ascorbic acid, SOD and GSH were still significantly decreased by DEX, as were the T-cell count, cytokine production and T-cell proliferation among splenocytes. In contrast, these effects of DEX were significantly suppressed in SMP30-KO mice on the high-VC diet (Fig. 1–3, Tables 2 and 3). These findings suggest that endogenous VC production in the liver may not have been sufficient to maintain the intracellular antioxidant level and immune parameters in WT mice after treatment with DEX.
Physical or psychological stress activates the hypothalamic–pituitary–adrenal axis and increases the production of adrenal cortical hormones such as GC that promote the atrophy of lymphoid organ, including spleen and thymus, with a decreased in their lymphocyte count and suppress immune function( Reference Purton, Monk and Liddicoat33 , Reference Glaser and Kiecolt-Glaser44 , Reference Herold, McPherson and Reichardt45 ). In agreement with previous reports, the decreased weight of these lymphoid organs were observed in DEX-treated mice, while high VC intake reduced spleen atrophy but had no effect on thymic involution, possibly due to the difference between organs’ sensitivity to DEX (Table 2). Furthermore, we demonstrated that DEX administration reduced the total number of splenocytes, along with a decrease in splenic CD4+ and CD8+ T cells, in SMP30-KO mice receiving the 0·02 %VC diet, while these changes were significantly ameliorated in the mice receiving the 0·2 %VC diet (Fig. 1). It was previously reported that DEX increases the production of ROS, including H2O2, and induces apoptosis of T cells by activation of caspase-3, while these changes are suppressed in T cells overexpressing catalase, a potent scavenger of H2O2 ( Reference Tome, Jaramillo and Briehl46 ). Administration of antioxidants such as betulinic acid and melatonin maintains antioxidant activity, suppresses ROS production/caspase activation, and increases expression of B cell lymphoma-2 (Bcl-2), which prevents apoptosis by inhibiting the caspase pathway, thus preventing GC-induced lymphocyte apoptosis and splenic atrophy( Reference Vishwas, Mukherjee and Haldar9 , Reference Yi, Zhu and Wu10 ). Similar to these findings, intracellular levels of antioxidants (ascorbic acid, SOD and GSH) were maintained in the splenocytes of SMP30-KO mice receiving the high-VC diet, and elevation of lipid peroxide after DEX administration was also significantly inhibited in these mice (Table 3). Treatment of thymocytes with VC was reported to inhibit monosodium glutamate-induced apoptosis by up-regulation of Bcl-2 protein expression( Reference Pavlovic, Pavlovic and Kocic21 ). Our findings suggested that high VC intake may maintain antioxidant activity and reduce oxidative damage, which may lead to cell death inhibition and preservation of the total number of the splenic lymphocytes.
GC cause T-cell apoptosis, inactivate T cells and APC and inhibit cytokine production to suppress the adaptive immune system that normally eliminates cancer cells and pathogens such as viruses( Reference Cain and Cidlowski7 , Reference Glaser and Kiecolt-Glaser44 ). Oxidative damage to immune cells caused by excessive ROS production and/or reduced antioxidant activity has also been reported to decrease the production of various cytokines. For example, preincubation of T cells with H2O2 or buthionine sulfoximine (a potent inhibitor of GSH biosynthesis) induces oxidative stress and suppresses activation of these cells as well as suppressing the production of IL-2 and IFN-γ ( Reference Malmberg, Arulampalam and Ichihara47 , Reference Hadzic, Li and Cheng48 ), which induces IL-12 mRNA expression by macrophages( Reference Schroder, Hertzog and Ravasi49 , Reference Yoshida, Koide and Uchijima50 ). In addition, elevation of endogenous ROS levels by H2O2 or by GSH synthesis inhibitors was reported to suppress IL-12 expression in activated APC( Reference Khan, Rahim and Boddupalli20 , Reference Dobashi, Aihara and Araki51 ). In the present study, administration of DEX to the 0·02 %VC group decreased the secretion of IL-2, IL-12p40 and IFN-γ by splenocytes treated with anti-CD3 antibodies which were used as potent T-cell activator, while inhibition of cytokine production by DEX was prevented in the 0·2 %VC group (Fig. 2). These results suggested that maintenance of antioxidant activity by high VC intake may have prevented DEX from suppressing IL-2 and IFN-γ production by T cells as well as IL-12 production by APC.
Activated helper CD4+ T cells differentiate into at least two classes of effector T cells, which are IFN-γ-producing Th1 cells that promote cellular immunity and IL-4 producing-Th2 cells that promote humoral immunity, and Th1 and Th2 cells can antagonise each other. In addition, IFN-γ which is produced by not only Th1 cells but also activated CD8+ cells, and NKT cells activate macrophages associated with secretion of IL-12 that promotes Th1 differentiation by phosphorylating the signal transducer and activator of transcription 4( Reference Schroder, Hertzog and Ravasi49 , Reference Pennock, White and Cross52 ). A Th1-dominant immune response increases the risk of autoimmune diseases, while a Th2-dominant response increases the risk of asthma and allergy. Therefore, an appropriate Th1/Th2 balance is essential for acquisition and maintenance of immune homeostasis( Reference Kidd53 ). Since Th1 cells are more susceptible to GC-induced apoptosis than Th2 cells, GC selectively suppress the Th1 immune response and cause a shift toward Th2-dominant immunity( Reference Elenkov11 ). In the present study, reduction in Th1:Th2 ratio by DEX administration was prevented in SMP30-KO mice receiving the high-VC diet (Fig. 2), suggesting that high VC intake may sustain IFN-γ and IL-12 production and prevent Th1:Th2 ratio reduction by GC to maintain an appropriate Th1/Th2 balance.
Proliferation of helper CD4+ T cells and cytotoxic CD8+ T cells has a key role in the development of adaptive immunity. GC suppress proliferation of these T cells by reducing the production of IL-2, a T-cell growth factor( Reference Glaser and Kiecolt-Glaser44 , Reference Boyman and Sprent54 ), as well as by inhibiting the production of IL-12 that promotes the survival and proliferation of activated T cells( Reference Bright and Sriram55 , Reference Yoo, Cho and Lee56 ). It was reported that reducing the intracellular SOD level and GSH:GSSG ratio decreased T-cell proliferation when splenocytes were treated with T-cell mitogens such as ConA( Reference Massilamany, Gangaplara and Kim18 , Reference Lee, Son and Park57 ). In addition, accumulation of membrane lipid peroxides in mice with T-cell-specific deficiency of GSH peroxidase 4, a scavenger of phospholipid hydroperoxides, prevented T-cell expansion and abrogated protection against infection by acute lymphocytic choriomeningitis virus or Leishmania major, while these immune effects were suppressed by intake of high-dose vitamin E (a potent antioxidant)( Reference Matsushita, Freigang and Schneider16 ). Taken together, high VC intake may have contributed to maintenance of T-cell proliferation by preventing a decrease in IL-2 and IL-12 production and by blocking reduction in antioxidants and an increase in lipid peroxide. It may be important to investigate the mechanism of high VC intake on T-cell proliferation in the future.
In conclusion, high dietary VC intake inhibited various immunosuppression effects of DEX, ameliorating the decrease in intracellular antioxidant activity, the increase in lipid peroxide and the decrease in T-cell numbers, Th1 cytokine production, Th1:Th2 ratio and T-cell proliferation in splenocytes, which reflect systemic immunity. Our results suggest that high dietary VC intake may have the potential to maintain systemic immune homeostasis in response to stress.
Acknowledgements
The authors appreciate Hiroko Nakai (Kyushu University, Fukuoka, Japan) for technical assistance with the experiment.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The author’s contributions are as follows: R. U., Y. H., S. M. and A. I. designed the study; R. U. and conducted the study; R. U. analysed data; R. U. and Y. H. participated in interpretation of the results; R. U., Y. H., S. M., H. I. and A. I. wrote the manuscript; S. M. and A. I. had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001922









