- UIC
-
urinary iodine concentration
Background to the problem
Iodine deficiency is currently estimated to affect 241 million school-aged children worldwide, despite great improvements in global iodine nutrition since the turn of the century( Reference Andersson, Karumbunathan and Zimmermann 1 ). This improvement is largely due to successful iodine fortification of populations through salt-iodisation programmes that have resulted in greater access to iodised salt( Reference Andersson, Karumbunathan and Zimmermann 1 ). As the World Health Organisation (WHO) considers iodine deficiency to be ‘the single most important preventable cause of brain damage’ worldwide( 2 ) this issue is of considerable public-health interest. Many industrialised countries often mistakenly consider that iodine deficiency is an historical, rather than current, problem( Reference Zimmermann 3 ). This appears to be the situation in the UK where iodine deficiency, once endemic in certain parts of the country, is believed to have been eradicated in the 1960s( Reference Phillips 4 ) and subsequently the country has been assumed to be iodine sufficient. Very little attention has since been paid to iodine status in the UK, either in terms of public-health policy or in terms of research, a situation that we have tried to address through a series of studies.
This review describes historical iodine deficiency in the UK, gives current information on dietary sources of iodine and summarises recent evidence of iodine deficiency in the UK and our own findings on its association with child neurodevelopment.
Iodine deficiency disorders: effect on the developing brain
The clinical consequences of inadequate iodine intake are grouped under the umbrella of ‘iodine deficiency disorders’( Reference Zimmermann 5 ). Iodine deficiency is problematic owing to the role of iodine as a vital component of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3), which are required for brain and neurological development( Reference Zimmermann 5 ). Thyroid hormones are required throughout brain development, both prior to the fetus producing its own thyroid hormones (when they must be supplied by the mother) and post-natally (when they are dependent on neonatal and infant iodine supply)( Reference Williams 6 ). Thyroid hormones are required for the proliferation and migration of neurones, development of the brain cytoarchitecture and glial differentiation and migration( Reference Williams 6 ). A lack of thyroid hormone, particularly maternal T4, which the fetus converts to the active form, T3, has deleterious effects on brain development( Reference Zimmermann 5 , Reference Williams 6 ).
Severe iodine deficiency is well known to cause cretinism, a condition associated with severe learning disabilities, deafness and impaired motor development( Reference Zimmermann 5 ). It is suggested that countries with moderate-to-severe iodine deficiency have population IQ scores that are twelve–thirteen IQ points lower than those of iodine-sufficient regions( Reference Qian, Wang and Watkins 7 , Reference Bleichrodt, Born and Stanbury 8 ). The effects of mild-to-moderate iodine deficiency on cognition are less well known than those of severe iodine deficiency( Reference Zimmermann 9 ) but it is believed that there is a continuum of disability relating to iodine deficiency in pregnancy that ranges from severe impairment in the offspring (associated with severe deficiency) to subtle impairments of IQ and motor ability (associated with a less severe deficiency)( Reference Dunn and Delange 10 ). Trial evidence is lacking from regions of mild-to-moderate iodine deficiency as child cognitive outcomes were not measured in the six randomised, controlled trials of iodine supplementation of pregnant women in such areas( Reference Glinoer, De Nayer and Delange 11 – Reference Pedersen, Laurberg and Iversen 16 ); the two studies that did include neurodevelopmental outcomes were neither randomised nor placebo-controlled( Reference Berbel, Mestre and Santamaria 17 , Reference Velasco, Carreira and Santiago 18 ). There is, however, evidence from randomised controlled trials of a beneficial effect on cognitive and motor scores of iodine supplementation in schoolchildren from mildly-to-moderately iodine-deficient countries( Reference Zimmermann, Connolly and Bozo 19 , Reference Gordon, Rose and Skeaff 20 ). Furthermore, several observational studies on pregnant women have found altered neurological outcomes (motor and/or mental scores and behavioural scores) in mild maternal thyroid dysfunction, either subclinical hypothyroidism or hypothyroxinaemia( Reference Haddow, Palomaki and Allan 21 – Reference Ghassabian, Bongers-Schokking and Henrichs 28 ). However, as many of these studies did not measure iodine status in the pregnant women, the role of mild-to-moderate iodine deficiency during pregnancy on neurological outcomes is not well documented.
Iodine requirements and population status monitoring
Iodine requirements
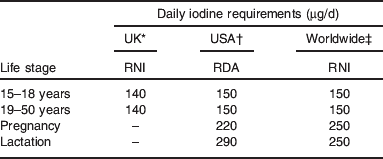
UK iodine requirements were set in 1991( 29 ); the adult requirement was set on the basis that 70 μg/d was required to prevent goitre (this was set as the lower reference nutrient intake) and the reference nutrient intake was set at twice that value, i.e. at 140 μg/d. Table 1 compares iodine requirements in the UK( 29 ) to those set by other international bodies( 2 , 30 ). The comparison highlights the fact that there is no increment in the iodine requirement for pregnancy in the UK, which is in marked contrast to the near-doubling of intake recommended by the WHO( 2 ) and the US Institute of Medicine( 30 ). The UK requirements for pregnancy are clearly outdated as pregnant women require additional iodine for three reasons: (i) to facilitate the 50% increase in thyroid hormone production required in pregnancy to cover the needs of the mother and the fetus, (ii) to cover the increase in renal clearance of iodine and (iii) to provide the fetal requirement for iodine after the onset of fetal thyroid function around mid-gestation( Reference Zimmermann 5 , Reference Delange 31 ).
Table 1. Iodine requirements by life stage according to different international bodies
Although it is possible for the higher iodine requirement of pregnancy to be met in part by drawing on thyroidal iodine stores( Reference Glinoer 32 ) (and possibly placental stores, although it is not known whether these can be utilised by the fetus( Reference Burns, O'Herlihy and Smyth 33 )), this is only feasible if these stores are replete prior to conception, which would not be the case in a situation of iodine deficiency. It is thus important that pregnant women and those planning a pregnancy are provided with information on dietary sources of iodine to ensure that requirements are met. There is very little information about iodine in dietary or nutritional advice given to the UK public; in particular, there is a complete lack of such information in advice for pregnant women( Reference Gordon 34 , 35 ). The absence of advice on iodine intake in pregnancy may reflect the fact that UK iodine requirements for pregnancy are outdated and out of step with those of other international bodies.
Excessive intake of iodine
Although this review is concerned with iodine deficiency, it is worth noting that iodine excess may be as problematic as iodine deficiency, having implications for altered thyroid function, including hypothyroidism and hyperthyroidism( Reference Crawford, Cowell and Emder 36 – Reference Orito, Oku and Kubota 39 ). The UK report on Dietary Reference Values suggests a safe upper limit of 17 μg/kg body weight per d, or 1000 μg/d, for adults( 29 ). The Tolerable Upper Intake Level of iodine for pregnant women has been set at 600 μg/d by the European Commission and 1100 μg/d by the Institute of Medicine( 30 ). The upper limit for iodine intake is a somewhat arbitrary cut-off as some individuals, particularly those with long-standing iodine deficiency, may respond adversely to intakes below the suggested safe level( Reference Burgi 37 ).
Measurement of iodine status
Iodine is classified as a micronutrient for which status should be assessed by biomarkers rather than by dietary intake( Reference Ovesen and Boeing 40 ). This is because the use of food-table iodine values to estimate exposure from dietary intake is confounded by the fact that the iodine content of foods varies considerably, both between similar species (e.g. of fish) and even within the same species reared under different conditions (e.g. because of farming methods), thus resulting in inaccurate estimates of iodine intake from dietary analysis( Reference Rasmussen, Ovesen and Bulow 41 ). Iodine is renally excreted and, as >90% of ingested iodine is eventually excreted by this route, urinary iodine concentration (UIC) is considered an excellent biomarker of iodine intake( 2 , Reference Zimmermann 5 ). The WHO recommends that population iodine status assessment is based on the median UIC in spot-urine samples and has published cut-offs to indicate iodine sufficiency in populations (Table 2)( 2 ). A spot-urine sample is not suitable for iodine assessment of individuals due to the intra-individual variation in daily urine volume and iodine intake( Reference Zimmermann 42 ) but the variation in urine volume can be reduced by measuring urinary creatinine concentration, particularly if adjusted for the age and sex of the individual( Reference Knudsen, Christiansen and Brandt-Christensen 43 ).
Table 2. Classification of iodine status of a population according to WHO criteria( 2 )

Population monitoring of UK iodine status has been sparse and poor since the 1940s. Indeed, the 2007 WHO report on iodine status worldwide listed the UK as one of the countries with no population iodine data( Reference de Benoist, McLean and Andersson 44 ). It was not until 2011 when Vanderpump and co-workers published the results of a study funded by the Clinical Endocrinology Trust( Reference Vanderpump, Lazarus and Smyth 45 ) that the UK had any country-wide data on the iodine status of the population.
Historical perspective of iodine deficiency in the UK
Iodine deficiency used to be widespread in the UK, as evidenced by endemic goitre in some regions( Reference Berry 46 – Reference Murray, Ryle and Simpson 49 ). The first estimates of a regional distribution of goitre in England were made in the early part of the twentieth century( Reference Berry 46 ), and in 1924, a survey of schoolchildren (total of 3 75 000) revealed levels of thyroid enlargement that were of concern; the findings prompted a recommendation by the author of that survey for ‘prophylactic administration of iodine to girls in some endemic areas of England and Wales’( Reference Stocks 47 ), but this was not implemented on a wide-scale basis. A ‘goitre belt’ existed in England and extended from the West Country (Somerset, Dorset, Devon and Cornwall) through Gloucestershire and Derbyshire and into parts of Wales( Reference Phillips 4 , Reference Stocks 47 ); goitre was also evident in Northern Ireland and parts of Scotland( Reference Kelly and Snedden 50 ). The phrase ‘Derbyshire neck’ was frequently used and refers to the fact that Derbyshire was an area with endemic goitre until about the 1930s( Reference Saikat, Carter and Mehra 51 ). Following the Second World War, the Medical Research Council recommended the iodisation of salt to prevent iodine deficiency and goitre( Reference Murray, Ryle and Simpson 49 , 52 ) but no action was taken( Reference Phillips 4 , Reference Kelly and Snedden 50 ). These recommendations were based on commissioned surveys in the UK, through the ‘Goitre Sub-committee’ of the Medical Research Council which found goitre rates of up to 50% in adult women in Oxfordshire, enlarged thyroids in 43% of girls in Dorset and an incidence of goitre up to 23% in Scotland( Reference Murray, Ryle and Simpson 49 , 52 ). There were reports of thyroid enlargement in antenatal clinics in the West Country( Reference Kelly and Snedden 50 ), highlighting the fact that women of childbearing age and pregnant women were exposed to iodine deficiency during these years. As a follow-up to these Medical Research Council surveys, several authors in the 1950s and 1960s documented thyroid enlargement in certain areas of the UK( Reference Hughes, Rodgers and Wilson 48 , Reference Trotter, Colchrane and Benjamin 53 , Reference Kilpatrick, Milne and Rushbrooke 54 ). Thus, it is clear that iodine deficiency was still persistent in the UK until the 1960s.
Eradication of goitre in the UK
Despite historical reports of thyroid enlargement and calls to action by the Medical Research Council, the UK never introduced a formal iodisation programme to ensure optimal iodine status. Instead the country experienced iodisation by default and the eradication of goitre in the UK happened for two main reasons, neither of which was Government-driven( Reference Phillips 4 ). Firstly, milk iodine concentration increased in the UK as a result of iodine-fortified concentrates being given to cattle and the increased use of iodophor (iodine-based) disinfectants in the dairy sector( Reference Phillips 4 ). Secondly, at about the same time, the Government promoted the consumption of milk for general health, for example through campaigns in the 1950s to ‘Drinka Pinta Milka Day’( 55 ); the establishment of the Milk Marketing Board and school-milk schemes also caused an increase in milk consumption( Reference Phillips 4 ). The combined effect of increased milk-iodine content and increased consumption of milk led to a three-fold increase in iodine intake between the 1950s and the 1980s( Reference Phillips 4 , Reference Wenlock, Buss and Moxon 56 ), which was sufficient to cause a decline in the incidence of goitre; the population was subsequently assumed to be iodine-sufficient. Indeed, in the late 1980s there were concerns that the iodine content of milk had reached levels that were too high and might require regulation, particularly in the winter months when cattle are in barns and receive more mineral-supplemented concentrates( Reference Phillips, Nelson and Barker 57 ); the iodine concentration in milk has been regularly monitored by Government bodies for this reason( 58 – 61 ).
Correction of iodine deficiency in a population – the UK perspective
The method recommended by the WHO to correct iodine deficiency in a population is Universal Salt Iodisation( 2 ), which is the iodisation of all salt for human and animal consumption; salt is usually iodised with potassium iodide or iodate( Reference Sullivan 62 ). Salt has been the vehicle of choice for iodine fortification programmes as it is relatively inexpensive, easy to monitor and regulate and consumed at constant levels by most people in a population( Reference Zimmermann 5 ). However, there are obvious conflicts in industrialised countries such as the UK that have strong salt-reduction campaigns. Nevertheless, the WHO considers that the two policies can work in harmony; the concentration of iodine in salt can be raised to a level that takes into account the salt intake recommendations for the country (e.g. <6 g/d in the UK)( 63 ).
The UK does not currently have a programme for the elimination of iodine deficiency through the use of iodised salt. A study in Cardiff found that only four of thirty-six samples of salt purchased had detectable iodine concentrations, and of these four, only two had levels suitable for the prevention of iodine deficiency( Reference Lazarus and Smyth 64 ). We surveyed the availability of iodised table salt for household consumption in seventy-seven supermarkets (from the five major retail chains) across the south of the UK in 2009; all branches of a sixth supermarket chain (Lidl) sold exclusively iodised salt. We found that only thirty-two (41·5%) of the supermarkets surveyed stocked iodised salt. More importantly, when the market share( 65 ) of all six supermarket chains was taken into account, the weighted availability was just 21·5%, as the two major supermarket chains, Tesco and Asda, in the UK did not stock iodised salt in any of their branches, while supermarkets with a lower market share more often stocked the product (Fig. 1)( Reference Bath, Button and Rayman 66 ).
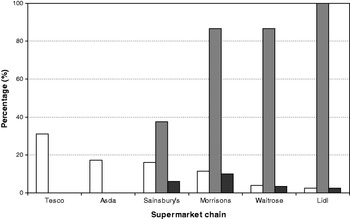
Fig. 1. Availability of iodised salt according to supermarket chain in the UK. Iodised salt availability (![]() ) according to supermarket chain. Weighted iodised salt availability (
) according to supermarket chain. Weighted iodised salt availability (![]() ) is shown for each chain that takes into account the market share (□) of the supermarket chain(
65
). Availability was determined by a shelf-survey in summer 2009 and information from the buying department of Lidl (reproduced from(
Reference Bath, Button and Rayman
66
)).
) is shown for each chain that takes into account the market share (□) of the supermarket chain(
65
). Availability was determined by a shelf-survey in summer 2009 and information from the buying department of Lidl (reproduced from(
Reference Bath, Button and Rayman
66
)).
Without an iodised salt programme, UK iodine intake is dependent entirely on food choices and in view of the low popularity of rich-iodine sources (e.g. fish and milk) in some population groups, pregnant women are vulnerable to iodine deficiency( Reference Smyth 67 ).
Alternative vehicles for iodine fortification in the UK
As an alternative to salt, other foods can be used as a vehicle for iodine fortification. For example, in Australia and New Zealand, there has been a mandatory requirement that all bread should be fortified with iodine (through the use of iodised salt) since 2009( 68 ). Iodine intake can also be improved in a population by indirect or unintentional iodisation through, for instance, iodine-enriched milk and dairy produce as a result of farming practice( Reference Phillips 4 ). The latter approach has improved iodine supply to the UK population and milk and dairy produce are currently the principal dietary sources of iodine intake in the UK as evidenced by the fact that milk contributes up to 40% of iodine intake in adults( Reference Henderson, Irving and Gregory 69 ). Milk and dairy products are clearly a vital UK iodine source and this is underlined by correlations between the iodine concentration of milk and UIC measured in seven towns across the UK( Reference Nelson, Phillips and Morris 70 ). In addition, UIC in both the UK( Reference Vanderpump, Lazarus and Smyth 45 , Reference Nelson, Phillips and Morris 70 , Reference Broadhead, Pearson and Wilson 71 ) and Ireland( Reference Nawoor, Burns and Smith 72 ) shows seasonal variation, reflecting changes in milk iodine concentration between summer and winter, again highlighting the dependence of iodine status on milk and dairy produce consumption. However, UK milk is an unregulated and haphazard source of iodine( Reference Phillips 4 ) and, as such, is unlikely to be wholly successful for the long-term eradication of iodine deficiency.
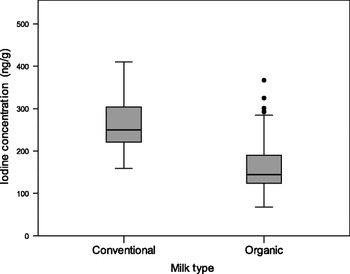
Indeed, we have shown that milk can be an unreliable source of iodine on the basis of our findings of a lower iodine concentration in organic milk than in conventional milk( Reference Bath, Button and Rayman 73 ). We measured iodine concentration in a total of ninety-two organic milk samples and eighty conventional milk samples from retail outlets across the South of the UK in the summer of 2009. The median iodine concentration value for organic samples (144·5 ng/g) was 42·1% lower than that of conventional milk samples (249·5 ng/g; Fig. 2 ( Reference Bath, Button and Rayman 74 ))( Reference Bath, Button and Rayman 73 ). As it was iodine from milk that rescued the UK from iodine deficiency in the past, any change in milk consumption or in milk iodine concentration will have an impact on the iodine status of the country. In the UK, as in other countries in the Western world, consumer trends show an increase in the purchasing of organic milk( 75 ). The fact that the iodine concentration of organic milk is over 40% lower than that of conventional milk is likely to compromise iodine intake in those that regularly use organic milk and dairy products. This is of particular concern when pregnant women who have higher iodine requirements( 2 ) switch to organic milk, perceiving it to be a healthier choice. However, there are as yet no data on the effect of organic-milk consumption by UK pregnant women on their iodine status.

Fig. 2. Iodine concentration of organic (n 92) and conventional milk samples (n 80) purchased from retail outlets in summer 2009. Organic milk had an iodine concentration that was 42·1% lower than conventional milk (reproduced from( Reference Bath, Button and Rayman 74 )).
Current iodine status in the UK
Iodine intake data
The National Diet and Nutrition Survey is one of the few sources of information on iodine intake in the UK, but as it comes from dietary rather than urinary analysis the data may not be accurate( Reference Rasmussen, Ovesen and Bulow 76 ). Nevertheless, the National Diet and Nutrition Survey data give a means of monitoring trends in iodine intake in the UK. The mean iodine intake for adult (19–64 years) males and females in the 2000/01 survey was above the reference nutrient intake for adults( Reference Henderson, Irving and Gregory 69 ). However, the mean iodine intake for women in the lowest age group (19–24 years) fell short of the adult requirement and 12% had an intake of iodine below the UK lower reference nutrient intake, which is 70 μg/d( Reference Henderson, Irving and Gregory 69 ). It is noteworthy that significantly lower intakes of iodine were found in the 2000/01 adult survey than in the 1986/87 survey, suggesting a possible fall in iodine intake( Reference Henderson, Irving and Gregory 69 ). This fall in intake is further evidenced from the results of years 1 and 2 (2008–2010) of the National Diet and Nutrition Survey rolling programme where the mean iodine intake has dropped since 2000/01 (e.g. 143 v. 161 μg/d for adult females) and there has been an increase in the percentage of adult females having intakes below the lower reference nutrient intake (i.e. 8 v. 6%)( Reference Bates, Lennox and Bates 77 ).
Iodine status of pregnant women and women of childbearing age – the results from small-scale, localised studies
Although there were no national data on UK iodine status until 2011( Reference Vanderpump, Lazarus and Smyth 45 ), there were a number of small-scale, localised studies that evaluated iodine status through urinary iodine assessment in women of childbearing age and pregnant women.
We collected urine samples from a total of fifty-seven UK women of childbearing age in 2007( Reference Bath, Walter and Taylor 78 ) and 2008( Reference Rayman, Sleeth and Walter 79 ) in Surrey; the median UIC (66 and 55 μg/l, respectively) revealed mild iodine deficiency in the cohort when compared with the WHO criteria for adults (Table 2)( 2 ). This is of concern as women of child-bearing age should meet the iodine requirement prior to conception to maximise thyroidal stores( Reference Glinoer 80 ). The benefit of pre-pregnancy thyroidal stores of iodine has been demonstrated in Italian studies where women who used iodised salt in the two years before conception had a reduced risk of thyroid failure than those who began to use iodised salt during pregnancy( Reference Moleti, Lo Presti and Campolo 81 ), or who commenced iodine supplements during pregnancy( Reference Moleti, Di Bella and Giorgianni 82 ).
Iodine deficiency has been demonstrated in a study of pregnant women from the North East of England( Reference Kibirige, Hutchison and Owen 83 ) of whom 40% had a UIC between 50 and 100 μg/l, indicating inadequate iodine status. That study( Reference Kibirige, Hutchison and Owen 83 ) was published prior to recommendations by the WHO that the median should be between 150 and 249 μg/l( 2 ) and so the prevalence of deficiency in the cohort in the North East may have been higher than that reported. Iodine deficiency was also studied in pregnant women in Scotland (though the study was only published as an abstract) and showed that 40% of pregnant women had an iodine status of less than half the recommended amount for pregnancy( Reference Barnett, Visser and Williams 84 ). Finally, iodine status has been reported in pregnant women recruited to a trial in Cardiff that aimed to evaluate the impact of screening and treatment (with thyroxine) of hypothyroidism and hypothyroxinaemia in pregnancy on child cognitive outcomes( Reference Lazarus, Bestwick and Channon 85 ); the median UIC of both the euthyroid and hypothyroid women was indicative of iodine deficiency (98 and 117 μg/l, respectively)( Reference Pearce, Lazarus and Smyth 86 ) when compared with WHO criteria for pregnancy (Table 2)( 2 ).
As there were no data on iodine status of pregnant women in the South of the UK, we studied 100 pregnant women in their first trimester of pregnancy, recruited from the Royal Surrey County Hospital in Guildford( Reference Bath, Wright and Taylor 87 ). The median UIC (85 μg/l) and iodine: creatinine ratio (122·9 μg/g) classified the women as mildly-to-moderately iodine deficient( 2 , Reference Zimmermann 9 ). The 42% of women who took an iodine-containing supplement had a significantly higher iodine status than women relying on diet alone (median 111 v. 61 μg/l, P<0·001; Fig. 3). It is important to note that these supplements were multivitamin and mineral preparations that contained iodine, not kelp supplements which should be avoided owing to the risk of excessive iodine intake from this highly variable source( Reference Zimmermann and Delange 88 ).

Fig. 3. Iodine concentration in pregnant women of the Royal Surrey County Hospital around 12 weeks’ gestation. Results are shown for the whole group (n 100), those on iodine-containing supplement (n 42) and not taking such a supplement (n 58; reproduced from( Reference Bath, Wright and Taylor 87 )).
These small-scale surveys raise concern; they not only suggest that UK pregnant women are not meeting their iodine requirement, but that women may be entering pregnancy with low thyroidal iodine stores, as evidenced by iodine deficiency in women of child-bearing age.
National survey of iodine status in 2011
Concern that iodine status in the UK is suboptimal was heightened in 2011 when the first national survey of iodine status of the country for more than sixty years revealed mild iodine deficiency in UK schoolgirls( Reference Vanderpump, Lazarus and Smyth 45 ). UIC was measured in 737 adolescent schoolgirls (14–15 years) across nine regions in the UK( Reference Vanderpump, Lazarus and Smyth 45 ). The median UIC (80·1 μg/l) indicated mild iodine deficiency in the cohort and suggested that the underlying assumption that UK iodine intake is adequate is no longer tenable; authors of that study urged a comprehensive review of iodine status in the UK( Reference Vanderpump, Lazarus and Smyth 45 ).
Using the median UIC in schoolgirls( Reference Vanderpump, Lazarus and Smyth 45 ) and the number of school-aged children with an insufficient iodine intake, the UK is now in the top ten iodine-deficient countries worldwide, positioned between Angola and Mozambique( Reference Andersson, Karumbunathan and Zimmermann 1 ). The editorial that accompanied that study of UK schoolgirls( Reference Vanderpump, Lazarus and Smyth 45 ) stated that it was ‘unconscionable that a country with the resources of the UK should be iodine-deficient in 2011’( Reference Pearce 89 ). Researchers from the International Council for the Control of Iodine Deficiency Disorders have urged the UK to adopt a salt iodisation policy, based on success in other countries worldwide( Reference van der Haar, Gerasimov and Haxton 90 ).
The fact that iodine deficiency has been revealed in the UK in the present time despite the assumption that the problem was confined to history, mirrors the experience of other industrialised countries, for example the US( Reference Hollowell and Haddow 91 – Reference Perrine, Herrick and Serdula 94 ), Australia and New Zealand( Reference Li, Waite and Ma 95 , Reference Skeaff, Thomson and Gibson 96 ). All three countries have witnessed a decrease in iodine intake despite having being considered as iodine-replete and having successfully eradicated iodine deficiency in the past( Reference Zimmermann 3 ). Complacency has been named as the ‘greatest enemy in the war against iodine deficiency’( Reference Dunn 97 ) and it appears as though the UK has fallen into this trap.
Was iodine deficiency ever truly eradicated in the UK?
Lower milk consumption in recent years( 98 ) has been cited as the likely reason for the decline in iodine intake in the UK( Reference Vanderpump, Lazarus and Smyth 45 ), as noted by the recent studies. However, this explanation may be too simplistic, especially as consumption of other dairy products has increased in recent years (e.g. yoghurt)( Reference Dairy 99 ). In fact, it is questionable whether iodine deficiency was ever truly eradicated in the UK; iodine intake may simply have increased sufficiently to eradicate visible goitre but remained at a suboptimal or mild iodine-deficiency level.
This notion can be explored by delving into the data presented in the few UK studies that report UIC that were carried out in the time of assumed iodine sufficiency( Reference Nelson, Phillips and Morris 70 , Reference Nelson, Quayle and Phillips 100 , Reference Chow, Phillips and Lazarus 101 ). The UIC values are suggestive of mild iodine deficiency, especially in the summer months when milk-iodine concentration is lower( Reference Nelson, Phillips and Morris 70 , Reference Nelson, Quayle and Phillips 100 , Reference Chow, Phillips and Lazarus 101 ). For example, in a study that evaluated the impact of iodine supplements on thyroid function, baseline UIC was 49–62 μg/l indicating mild iodine deficiency, but at the time, the relatively low value was suggested to be a result of a mild winter, thus a lower reliance on mineral-supplemented feed, the previous year( Reference Chow, Phillips and Lazarus 101 ).
These figures suggest that iodine status has not increased to an adequate level in the UK since the days of goitre and that recent studies that show certain groups to be iodine deficient may have revealed an underlying problem, rather than suggesting that iodine deficiency has re-emerged.
Impact of current level of iodine deficiency in the UK on child cognition
Although iodine deficiency in the UK population is suggested by a few studies( Reference Vanderpump, Lazarus and Smyth 45 , Reference Kibirige, Hutchison and Owen 83 , Reference Barnett, Visser and Williams 84 , Reference Pearce, Lazarus and Smyth 86 ), including our own( Reference Bath, Walter and Taylor 78 , Reference Rayman, Sleeth and Walter 79 , Reference Bath, Wright and Taylor 87 ), there is no information on whether the level of deficiency has a negative impact on the development of the child in the womb. Such information is crucial to the instigation of public-health action to eradicate UK iodine deficiency.
We therefore aimed to relate maternal iodine deficiency in pregnancy to cognitive outcomes in the offspring up to the age of 11 years( Reference Bath, Steer and Golding 102 ). This was achieved through collaboration with Avon Longitudinal Study of Parents and Children (ALSPAC)( Reference Bath, Steer, Golding, Emmett and Rayman 103 ), a study that recruited pregnant women in the early 1990s from the South West of the UK, collected data during pregnancy and followed the health and development of their children( Reference Golding, Pembrey and Jones 104 ). We therefore measured iodine (and creatinine to correct for urine volume) concentration in stored urine samples collected during pregnancy as a measure of iodine status and assessed cognition using the results from IQ tests at age 8 years, reading ability at age 9 years and school performance at Key Stage 2( Reference Bath, Steer and Golding 102 ). The group of pregnant women was classified as being iodine deficient by WHO criteria (median UIC 91·8 μg/l); this level of deficiency is similar to the value for our recent study in pregnant women in Surrey( Reference Bath, Wright and Taylor 87 ), meaning that although the results are 20 years old, they are relevant to the situation today.
The women were broadly grouped as iodine deficient (<150 μg/g) or iodine sufficient (>150 μg/g) based on WHO criteria( 2 ) and the relationship with child cognition was explored( Reference Bath, Steer and Golding 102 ). We found that children born to mothers classified as being iodine deficient were more likely to have scores in the bottom quartile for total IQ (OR 1·58, 95% CI 1·09, 2·29), reading accuracy (OR 1·83, 95% CI 1·22, 2·74) and Key Stage 2 Mathematics (OR 1·60, 95% CI 1·07, 2·41), even after adjustment for twenty-one potential confounders, including breastfeeding, parental education, maternal age and estimated n-3 fatty-acid intake from seafood( Reference Bath, Steer and Golding 102 ).
Although these results cannot prove causality, they suggest that the mild-to-moderate iodine deficiency found in UK pregnant women is having a negative impact on child cognitive outcomes up to the age of 11 years. In addition, there are negative implications at the population level as even a marginally lower mean IQ affects economic success and productivity( Reference Dickerson 105 ).
Conclusions and recommendations
Despite a worldwide focus on the elimination of iodine deficiency, iodine is a largely overlooked nutrient in the UK and there is a dearth of information on iodine intake and status which we have endeavoured to address. Our findings, and those of others, suggest that a UK public-health policy is required to minimise the risk of iodine deficiency and its adverse effects, particularly in pregnancy.
The UK should implement the monitoring of population iodine status. This could be done very easily through the National Diet and Nutrition Survey where urine samples are already collected and stored allowing ready measurement of iodine concentration as is done in the US-equivalent National Health and Nutrition Examination Study. This would provide additional data on the risk of iodine deficiency in vulnerable subgroups of the UK population, such as children whose brains are still developing. Dietary information for pregnant women, those planning a pregnancy and women of childbearing age should include information on iodine so that the risk of iodine deficiency can be minimised during critical life stages. Trials need to be carried out to establish whether iodine supplementation of UK pregnant women (or indeed schoolchildren) beneficially affects cognitive scores and we are working towards that end.
Acknowledgements
The authors declare no conflict of interest. S. C. B. was funded by Wassen International and the Waterloo Foundation. S. C. B. conducted the studies described and wrote the manuscript. M. P. R. raised the funding, supervised the studies and helped in the preparation of the manuscript. The authors are grateful to all participants of the studies described and for the advice and help of the co-authors on their studies (listed in the reference list).