Coeliac disease (CD) is an autoimmune enteropathy caused by permanent intolerance to cereal gluten proteins, in particular gliadins(Reference Schuppan, Junker and Barisani1). The main CD-related lesions occur in the intestinal epithelium, consisting of villous flattening, crypt cell hyperplasia and infiltration of the lamina propria by lymphocytes and macrophages. CD patients display many symptoms and signs, but is classically characterised by the defective absorption of nutrients and the failure to gain weight. Gliadin ingestion triggers inflammatory responses in the intestinal mucosa with the production of cytokines and related mediators such as IL-6(Reference Romaldini, Barbieri and Okay2, Reference Dema, Martínez and Fernández-Arquero3), TNF-α and NF-κB(Reference Jelínková, Tuckova and Cinová4). TNF-α and IL-6 play important roles in up-regulating T-cell functions and mediating tissue toxicity(Reference Bemelmans, Abramowicz and Gouma5, Reference Naka, Nishimoto and Kishimoto6). Currently, the mainstay of treatment is a strict lifelong adherence to a gluten-free diet; however, compliance with this dietary treatment is difficult because wheat flour is present in a great percentage of processed foods. Thus, in this context, the development of alternative or adjuvant nutritional strategies to improve the health status of coeliac sufferers acquires special relevance.
Recent epidemiological data indicate that environmental factors, other than gluten, can contribute to the pathogenesis and/or presentation of CD(Reference Medina, De Palma and Ribes-Koninckx7, Reference Sánchez, De Palma and Capilla8). In particular, the disease has been associated with reduced numbers of bifidobacteria and the increased prevalence of Enterobacteriaceae in the gut microbiota(Reference Nadal, Donat and Ribes-Koninckx9, Reference Collado, Donat and Ribes-Koninckx10). Data from in vitro and in vivo animal studies have revealed that some bifidobacterial strains can counteract the toxicity and inflammatory potential of gliadin on enterocytes(Reference Laparra and Sanz11), which are the cells responsible for Fe absorption in the upper intestine, and contribute to the gut immune function. A previous animal study has shown marked differences in the effects of gliadins in the jejunum of weanling animals, sensitised or not with interferon (IFN)-γ(Reference Laparra, Olivares and Gallina12). Moreover, oral administration of Bifidobacterium longum CECT 7347 to a gliadin-induced enteropathy model ameliorated the production of inflammatory cytokines in jejunal samples and decreased the number of both CD4+ and CD4+Foxp3+ T-regulatory cells. The positive effects of B. longum CECT 7347 feeding on alleviating inflammation were parallel to increased expression of proteins involved in energy and lipid metabolism, cell structure, protein folding and immune responses(Reference Olivares, Laparra and Sanz13).
In CD patients, ferropenic anaemia is one of the most common extra-intestinal manifestations(Reference Hernandez and Green14, Reference Ludvigsson, Brandt and Montgomery15), probably secondary to this is inadequate intestinal absorption due to gliadin-mediated alterations in the architecture of the intestinal epithelium. Fe is an essential micronutrient for nearly all living organisms, which is poorly absorbed from the diet, whose status is tightly controlled by signals sensing dietary composition, body Fe stores and metabolic requirements(Reference Ganong16). Inflammatory cytokines such as TNF-α(Reference Johnson, Bayele and Johnston17) and IL-6(Reference Kobune, Kohgo and Kato18) have been suggested to influence dietary Fe assimilation and systemic Fe homeostasis(Reference Hirayama, Kohgo and Kondo19). These cytokines mediate a decrease in serum Fe concentrations accompanied by increased Fe storage in both enterocytes and hepatocytes. In addition, systemic Fe homeostasis is regulated by hepcidin (Hamp), a β-defensin-like peptide mainly synthesised by hepatocytes, which acts as a key regulator of Fe metabolism reducing its intestinal absorption(Reference Ganz20, Reference De Domenico, Zhang and Koening21). Ferropenic anaemia in CD patients develops as a refractory to dietary Fe supplementation(Reference Rodrigo22). This particular characteristic implies the need to develop additional nutritional strategies to prevent or improve the health status of CD patients.
Traditionally, rats have been the animal model of choice when performing nutritional studies. There are a number of differences concerning Fe absorption in rats compared with humans, limiting their predictive value in relation to human responses(Reference Greger23, Reference Corpet and Pierre24). However, rats can develop Fe deficiency when fed deficient diets(Reference Alférez, Díaz-Castro and López-Aliaga25) and exhibit comparable responses of regulatory factors of systemic Fe homeostasis that involve liver transferrin receptor-2 (TfR2)(Reference Jenkitkasemwong, Broderius and Nam26) and Hamp (Reference Jenkitkasemwong, Broderius and Nam26, Reference Pigeon, Ilyin and Courselaud27). A recent study in rats has associated Fe depletion and metabolic activity with imbalances in the microbiota, showing higher abundances of lactobacilli and Enterobacteriaceae and a significant decrease in the Roseburia spp./Eubacterium rectale group(Reference Dostal, Chassard and Hilty28). Many members of the Enterobacteriaceae have developed mechanisms, including siderophores, to acquire Fe in competition with other bacteria and the host(Reference Andrews, Robinson and Rodriguez-Quinones29), and favour pro-inflammatory milieu(Reference Cinova, De Palma and Stepankova30).
The objective of the present study was to evaluate the effects of the oral administration of B. longum CECT 7347 on Fe homeostasis in weanling animals, sensitised or not with IFN-γ to induce an experimentally gliadin-sensitive enteropathy.
Materials and methods
Gliadin preparation
A commercially available extract of gliadin (G3375; Sigma-Aldrich) was subjected to in vitro digestion in order to obtain partially digested gliadin-derived peptides(Reference Laparra and Sanz11). The dialysate (15 kDa, molecular weight cut-off) was diluted in a commercially available hypoallergenic milk-based formula and administered orally by means of a micropipette.
Bacterial strain and culture conditions
B. longum CECT 7347 was isolated from the faeces of healthy infants as described elsewhere(Reference Medina, De Palma and Ribes-Koninckx7). Bacterial cultures were grown in Man–Rogosa–Sharpe agar and broth (Scharlau) supplemented with 0·05 % (w/v) cysteine (MRS-C; Sigma), and kept at 37°C in anaerobic conditions (AnaeroGen; Oxoid) for 24 h. For animal studies, a pure culture of the strain was grown overnight and used to inoculate fresh MRS-C broth for 22 h. Cells were harvested by centrifugation (6000 g for 15 min) at a stationary growth phase, washed twice in PBS (130 mm-NaCl and 10 mm-sodium phosphate, pH 7·4) and resuspended in 10 % (w/v) hypoallergenic milk-based formula (Nutramigen©; Mead Johnson B.V.). Aliquots of these suspensions (108 colony-forming units/ml) were frozen in liquid N2 and stored at − 80°C until used. More than 90 % of cells were alive upon thawing and no significant differences were found related to storage time (1 month). For every new experiment, one fresh aliquot was thawed to avoid variability in bacterial cell viability between the experiments.
Animals
All animal experiments were performed according to the University of Valencia Ethics Committee Guidelines for Animal Experiments (Central Support Service for Experimental Research). The experimental animals were female weanling Wistar rats. Adult females were date-mated, and fed ad libitum with a normal diet (Harlan Bioproducts). Shortly after spontaneous birth, offspring were randomly distributed in different groups (n 6)(Reference Laparra, Olivares and Gallina12): (1) artificially reared (AR); (2) AR+B. longum CECT 7347; (3) AR+gliadin-derived peptides (0·5 mg/ml hypoallergenic milk-based formula); (4) AR+gliadin-derived peptides+B. longum CECT 7347 (6·0 × 107 and 8·2 × 108 colony-forming units/ml); (5) AR+gliadin-derived peptides+IFN-γ (sensitised with 1000 U (1·6 × 103pg) IFN-γ intraperitoneally immediately after birth); (6) AR+gliadin-derived peptides+IFN-γ+B. longum CECT 7347 suspension.
Artificially fed animals
Newborn animals were hand-fed (100 μl) using a micropipette. They were hand-fed by using a micropipette with a hypoallergenic milk-based formula for newborns, every 4 h (four to five times per d) until 10 d old, with composition per 100 ml (13·6 %, w/v) being as follows: 285 kJ (68 kcal); 1·9 g protein; 3·4 g fat; 7·5 g carbohydrates; 260 mOsm/l. Changes in body weight were monitored every 2 d. Weanling rats were fed (0·1 ml) with a gliadin (0·5 mg/ml) solution in a single dose per d for 10 d, and received a provocative dose of gliadin (1 mg/ml) approximately 2 h before killing.
Liver tissue and blood collection
Rats were anaesthetised (Isofluran) and killed by exsanguination by intracardiac puncture. Whole-blood samples were preserved in EDTA-treated tubes to prevent coagulation. For total RNA isolation, one lobe from each liver section was quickly dissected and stored in RNAlater (Qiagen). Another liver lobe was used to quantify tissue Fe content. All tissue samples were stored at − 80°C until further analysis.
Hb measurement
Hb concentrations were measured photometrically using the cyanmethaemoglobin standard solution according to the manufacturer's instructions (Sigma-Aldrich). This method is based on the oxidation of Hb and its derivatives (except sulphhaemoglobin) to methaemoglobin in the presence of potassium ferricyanide to form cyanmethaemoglobin. Absorbance, measured at 540 nm, was proportional to the total Hb concentration.
Total iron quantification
Liver samples were washed twice with saline solution (140 mm-NaCl and 5 mm-KCl) and total Fe content was determined by atomic absorption spectrometry (Perkin-Elmer) in a solution of the ashes.
RT and real-time PCR analyses
Total RNA was extracted from liver tissue samples using an RNeasy mini kit (Qiagen) following the protocol provided by the manufacturer. Briefly, 1 μg of total RNA was converted to double-stranded complementary DNA using avian myeloblastosis virus RT (Promega). PCR was performed with primers designed for the following Rattus norvegicus genes: Hamp (forward: 5′-AGC GGT GCC TAT CTC CGG CA-3′; reverse: 5′-CGG AGG GGA GGC AGT GTG TTG-3′); TfR2 (forward: 5′-GGC AGA GTG GTC GCT GGG TG-3′; reverse: 5′-GGC CAG AGC TCG GCA GTG TG-3′); TNF-α (forward: 5′-AGG GTA CCA CAG AAA GAT GC-3′; reverse: 5′-GGA GAT GAG ACC CTT AGG TT-3′); IL-6 (forward: 5′-TCT CGA GCC CAC CAG GAA C-3′; reverse: 5′-AGG GAA GGC AGT GGC TGT CA-3′); NF-κB (forward: 5′-CTT CTC GGA GTC CCT CAC TG-3′; reverse: 5′-CCA ATA GCA GCT GGA AAA GC-3′); β-actin (forward: 5′-CTC TTC CAG CCT TCC TTC CT-3′; reverse: 5′-TAG AGC CAC CAA TCC ACA CA-3′), the latter used as a housekeeping gene. The PCR mix (20 μl reaction volume) consisted of 7·5 μl SYBR Green I Master Mix, 1·3 μmol/l primers and 2·5 μl complementary DNA. PCR were performed in triplicate in a LightCycler® 480 system (Roche) with the following conditions: one cycle at 95°C for 5 min, thirty-five cycles at 60°C for 20 s and 72°C for 45 s. The relative mRNA expression of the tested gene compared with β-actin expression was calculated using the 2−ΔΔC p method(Reference Livak and Schmittgen31). Samples of each animal tissue were measured in duplicate and gene expression was expressed as fold change.
Quantification of hepcidin in plasma (liquid chromatography–MS/MS)
All sample preparation steps were performed at room temperature as described previously(Reference Murphy, Witcher and Luan32) with slight modifications. Aliquots (50 μl) of plasma were pipetted into 200 μl cone tubes, and a 100 μl aliquot of acetonitrile (Burdick and Jackson) was added to each tube and mixed by pipetting. The samples were then centrifuged at 3000 g for 10 min at 4°C (Jouan). After centrifugation, the protein precipitation supernatant (100 μl) was mixed with 0·02 % (v/v) aqueous acetic acid and injected onto an Agilent HPLC system connected on line to an Esquire-liquid chromatography electrospray system equipped with a quadrupole ion trap mass spectrometer (Bruker Daltonics). The HPLC system was equipped with a quaternary pump, an in-line degasser, an automatic injector and a variable wavelength absorbance detector set at 214 nm (1100 Series; Agilent Technologies). The column used in these analyses was a BioBasic C18 5 μm 4·6 × 250 mm (Thermo). The elution phases consisted of mobile phase A (0·125:1:500, trifluoroacetic acid–isopropanol–water) and mobile phase B (0·125:1:50:350:100, trifluoroacetic acid–isopropanol–water–methanol–acetonitrile). Aliquots (50 μl) of the precipitation supernatants were injected in each cycle and the analysis was performed with the following gradient (min, %B): 0, 5; 30, 90; 33, 100; 35, 0; 40, 90; 45, 5.
N2 was used as the nebulising and drying gas, and He collision gas pressure was approximately 5 × 10− 3 bar. The capillary was held at 4 kV. Mass spectra were recorded over the mass/charge (m/z) range of 100–3500. About fifteen spectra were averaged in the MS analyses and about five spectra in the MS/MS analyses. The signal threshold to perform auto-MS/MS analyses was 5000, and precursor ions were isolated within a range of 4·0 m/z and fragmented with a voltage ramp from 0·39 to 2·6 V. The m/z spectral data were processed and transformed to spectra representing mass values using the program Data Analysis version 3·0 (Bruker Daltonics). BioTools version 2·1 (Bruker Daltonics) software was used to process the MS/MS spectra and to perform peptide sequencing. From each animal, two independent samples were analysed.
Statistical analysis
Data are expressed as means and standard deviations. Statistical analyses were performed using SPSS version 15 software (SPSS, Inc.). One-way ANOVA and Tukey's post hoc test were applied. In animal studies, comparison procedures were performed using a pairwise comparison to detect statistically significant differences. Simple linear regression analysis was used to determine the relationships between hepcidin expression, liver Fe contents and Hb concentrations. Statistical significance was established at P< 0·05 for all comparisons.
Results
Effect of gliadins on Hb and liver iron deposition
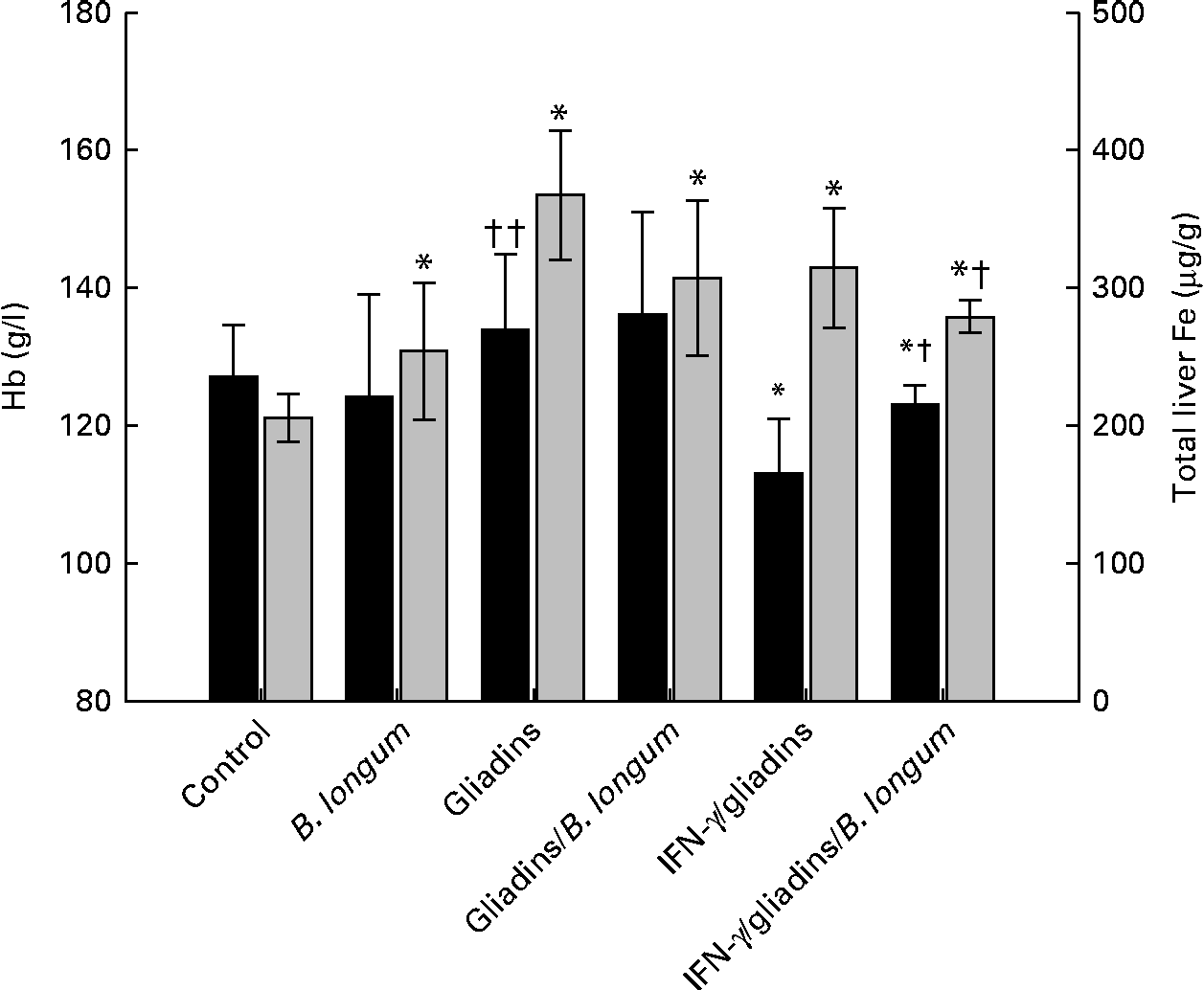
The animals fed with gliadins, alone (P= 0·275) or together with B. longum CECT 7347 (P= 0·168), did not show significant alterations in Hb concentrations compared with the controls (Fig. 1). This trend was also observed in the animals administered the bifidobacterial strain alone (P= 0·529). The animals sensitised with IFN-γ, but not fed gliadins, did not exhibit significant changes in Hb concentrations (data not shown). However, the IFN-γ-sensitised gliadin-fed animals showed significant lower Hb concentrations than the controls (P= 0·001) and non-sensitised gliadin-fed animals (P= 0·004). The administration of B. longum CECT 7347 to the IFN-γ-sensitised gliadin-fed animals partially restored (P= 0·043) Hb concentrations, although these animals still exhibited lower (P= 0·005) concentrations than the controls.

Fig. 1 Hb (■) and liver iron contents (![]() ) in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins with or without Bifidobacterium longum CECT 7347. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control animals (P< 0·05). Mean value was significantly different from that of the IFN-γ/gliadins-treated animals: † P= 0·04, †† P< 0·01.
) in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins with or without Bifidobacterium longum CECT 7347. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control animals (P< 0·05). Mean value was significantly different from that of the IFN-γ/gliadins-treated animals: † P= 0·04, †† P< 0·01.
The administration of B. longum CECT 7347 alone to weanling animals slightly, but significantly (P= 0·040), increased liver Fe contents compared with the controls. The non-sensitised animals fed gliadins alone (P< 0·001) or together with B. longum CECT 7347 (P= 0·002) exhibited significantly (P< 0·05) increased liver Fe contents compared with the controls. In the IFN-γ-sensitised gliadin-fed animals, liver Fe contents were also increased (P< 0·001), but were similar to those quantified in the non-sensitised gliadin-fed animals (P= 0·198). However, a significantly lower (P= 0·038) liver Fe content was quantified in the sensitised animals fed gliadins and the bifidobacterial strain compared with those sensitised animals fed gliadins alone, although also markedly higher (P< 0·001) than that in the controls. There were no significant (P>0·05) changes in liver Fe contents in the IFN-γ-sensitised animals not fed gliadins compared with the controls at the end of the treatment (data not shown).
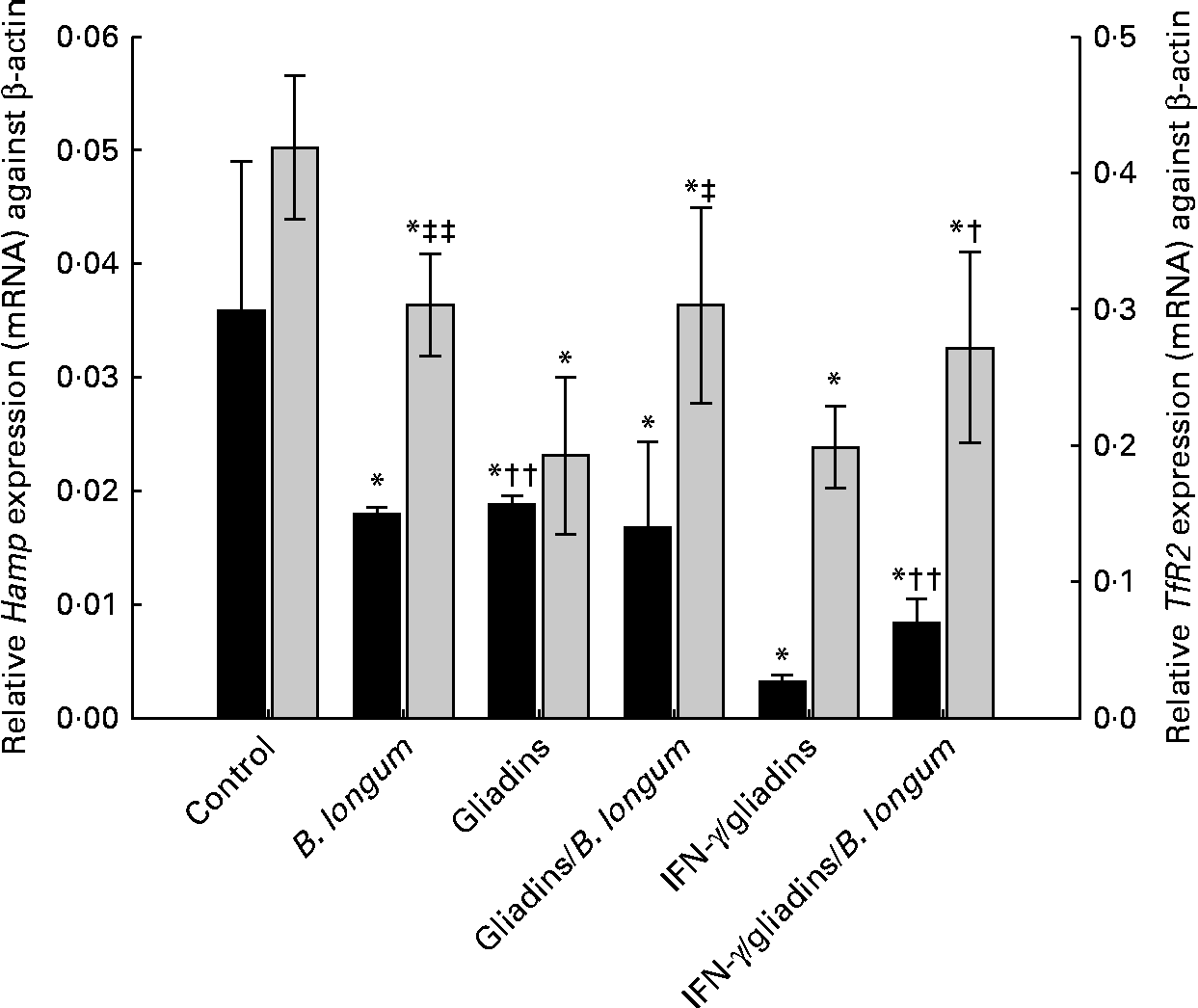
Changes in TfR2 expression levels in animals, sensitised or not with IFN-γ, fed gliadins and the bifidobacterial strain are shown in Fig. 2. Feeding B. longum CECT 7347 alone to weanling animals significantly (P= 0·003) decreased TfR2 expression levels compared with the controls. However, TfR2 expression levels were more markedly down-regulated (P= 0·006) in the animals fed gliadins alone. The co-administration of B. longum CECT 7347 slightly restored (P= 0·019) TfR2 expression levels, although it was still lower (P= 0·013) than those in the controls. In the sensitised animals, gliadin feeding down-regulated TfR2 expression levels, which were partially restored (P= 0·041) by the concurrent administration of the bifidobacterial strain. Feeding B. longum CECT 7347 alone also down-regulated (P= 0·003) TfR2 expression levels compared with the controls, but similar levels found in the IFN-γ-sensitised gliadin-fed animals.

Fig. 2 Hepatic hepcidin (Hamp;■) and transferrin receptor-2 (TfR2; ![]() ) expression (mRNA) levels in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins with or without Bifidobacterium longum CECT 7347. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control animals (P< 0·05). Mean value was significantly different from that of the IFN-γ/gliadins-treated animals: † P< 0·02, †† P< 0·01. Mean value was significantly different from that of the gliadins-treated animals: ‡ P< 0·02, ‡‡ P< 0·01.
) expression (mRNA) levels in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins with or without Bifidobacterium longum CECT 7347. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control animals (P< 0·05). Mean value was significantly different from that of the IFN-γ/gliadins-treated animals: † P< 0·02, †† P< 0·01. Mean value was significantly different from that of the gliadins-treated animals: ‡ P< 0·02, ‡‡ P< 0·01.
Effect of gliadins on plasma active hepcidin peptide concentrations and hepatic hepcidin expression (mRNA)
Liquid chromatography–MS/MS methods are able to quantify human urinary(Reference Kroot, Kemna and Bansal33) or human and animal plasma(Reference Murphy, Witcher and Luan32, Reference Kroot, Kemna and Bansal33) hepcidin (Hamp) from 0·25 to 500 ng/ml. However, protocol standardisation is still needed for the results to be comparable between laboratories. In the present study, to avoid misinterpretation of the reported results, we followed a previously optimised method for analysis in mouse plasma and serum samples(Reference Murphy, Witcher and Luan32). The active Hamp peptide was identified by sequencing its amino acid backbone (Table 1). To better understand the role of Hamp in the alterations described in Fe homeostasis, circulating concentrations of the active Hamp peptide were quantified in animals from the different treatment groups (Table 2). The median plasma Hamp concentration in the control animals was 24·5 ng/ml. This value is of a similar order to those previously reported in mice(Reference Murphy, Witcher and Luan32).
Table 1 Amino acid sequences used to identify hepcidin (Hamp) in animal plasma
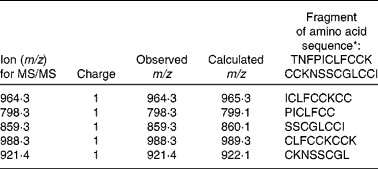
* Q99MH3, HEP_RAT (SwissProt).
Table 2 Plasma hepcidin (Hamp) peptide concentrations in animals administered with Bifidobacterium longum CECT 7347 or gliadins alone, gliadins together with the bifidobacterium strain, animals sensitised with interferon (IFN)-γ and fed gliadins and those co-administered with the bifidobacterial strain (Medians and ranges; six animals per group)
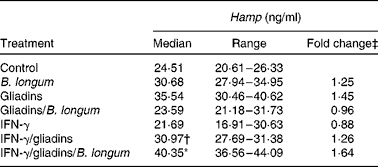
* Median value was significantly different from that of the control animals (P= 0·007; Student's t test).
† Median value was significantly different from that of the IFN-γ/gliadins-treated animals (P= 0·033; Student's t test).
‡ Fold change v. median plasma Hamp concentration quantified in the control animals.
The animals administered B. longum CECT 7347 alone exhibited slightly increased median values (1·25-fold), but not significantly different (P= 0·116) from the controls. Feeding gliadins produced Hamp concentrations up to 1·45-fold that of the controls, bordering statistical significance (P= 0·072). However, when the bifidobacterial strain was administered with gliadins, differences in plasma Hamp concentrations quantified were reduced to values close to those of the control animals (0·96-fold). Sensitisation of the animals with IFN-γ gave median plasma Hamp concentrations 0·88-fold (P= 0·965) of the controls, but after gliadin feeding, the values were quantified at 1·26-fold of the control values, albeit not statistically significant (P= 0·113). The only significant increase in plasma Hamp concentrations was found in the IFN-γ-sensitised gliadin-fed animals administered B. longum CECT 7347.
Initially, Hamp is synthesised as a pre-pro-peptide (84 amino acids; aa), which is finally processed to a 24 aa bioactive peptide(Reference Park, Valore and Waring34). Previous studies have reported differences in hepatic Hamp expression (mRNA) of anaemic rats(Reference Jenkitkasemwong, Broderius and Nam26), prompting us to investigate potential changes in the different animal groups in the present study. Animals from all the treatment groups showed a decreased expression (mRNA) of hepcidin (Hamp) compared with the controls (Fig. 2). The administration of gliadins significantly (P= 0·021) down-regulated Hamp expression, as well as when administered together with B. longum CECT 7347 (P= 0·021); however, statistical (P= 0·535) differences were not detected between either treatment. The administration of bifidobacteria alone also caused the down-regulation of the hepatic expression of Hamp. The animals sensitised with IFN-γ exhibited a higher down-regulated expression of Hamp after being fed with gliadins (P< 0·001), which was partially restored (P= 0·001) by the bifidobacterial strain. In the present study, a significant reciprocal correlation was observed between Hamp expression (mRNA) and total hepatic Fe contents (μg/g) (R Reference Romaldini, Barbieri and Okay2 0·504; P< 0·005). In addition, Hamp expression was significantly (R 2 0·453; P< 0·012) correlated with Hb concentrations through a square root model (Fig. 3). The correlation calculated for Hamp expression was able to explain 25·43 % (liver Fe = 1/0·0032+0·032 × Hamp expression) and 20·52 % (Hb = 11·49+10·13 × (Hamp expression)1/2) of the variations in total hepatic Fe content and Hb concentrations, respectively.
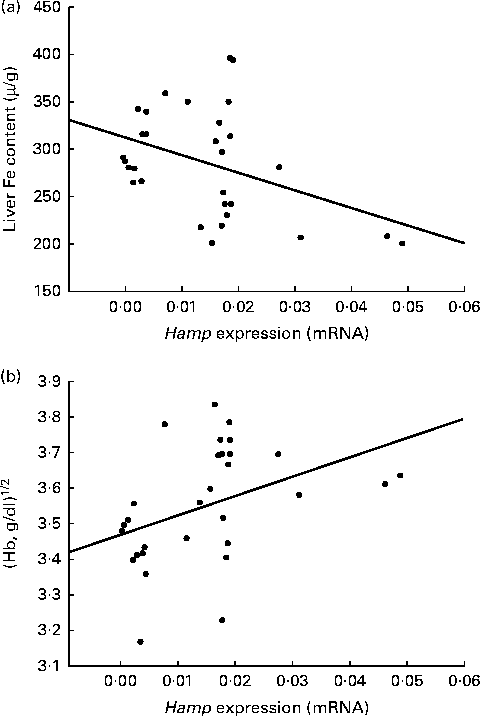
Fig. 3 Correlation of hepatic expression of hepcidin (Hamp) with liver iron contents (a) and Hb concentrations (b) in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins and/or Bifidobacterium longum CECT 7347. Hamp expression was significantly related to liver iron contents (P< 0·005, liver Fe = 1/0·0032+0·032 × Hamp expression) and Hb concentrations (P< 0·012, Hb = 11·49+10·13 × (Hamp expression)1/2).
Effect of gliadin on the hepatic expression of cytokines
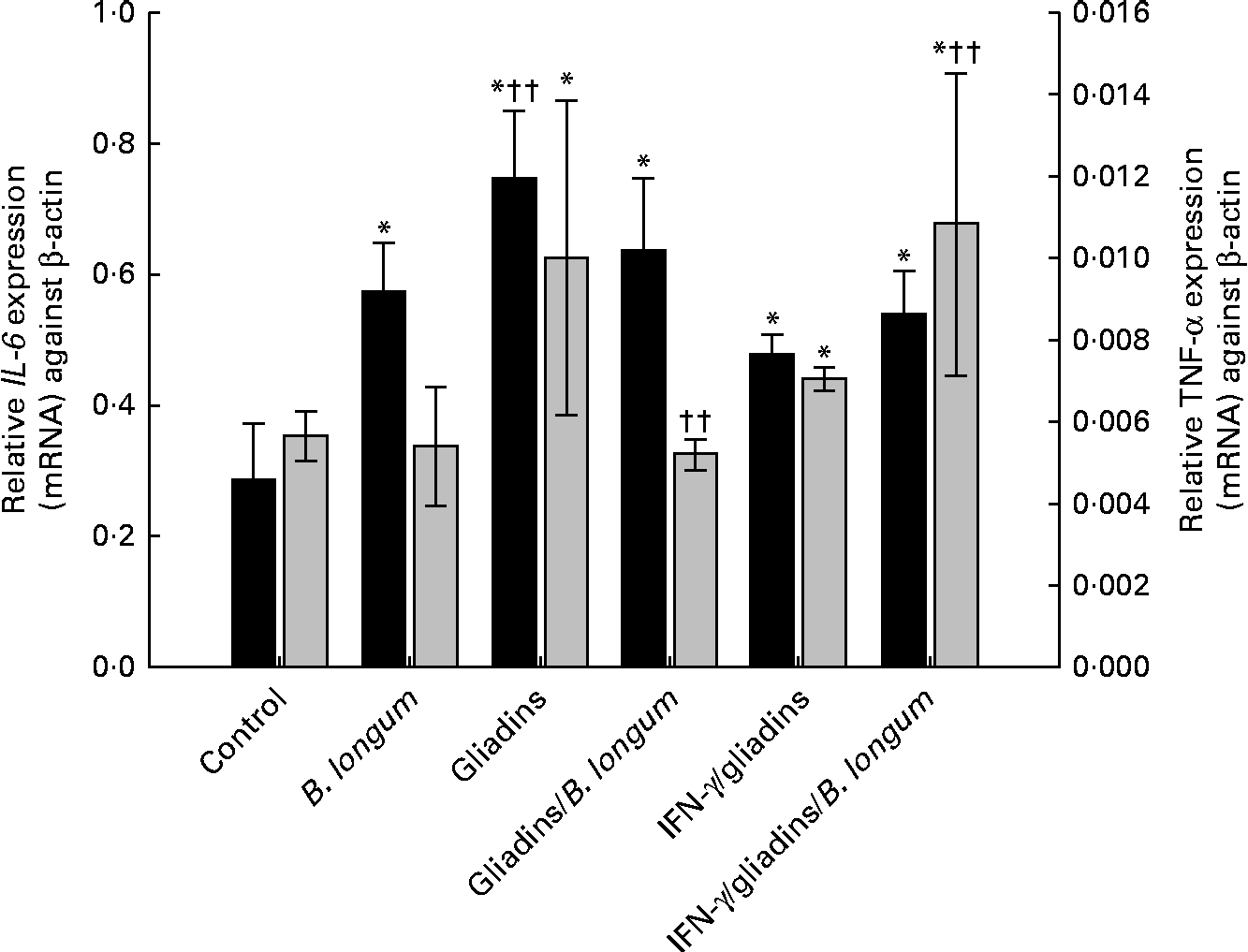
Changes in hepatic IL-6 and TNF-α expression levels in the different treatment groups are shown in Fig. 4. All animals from the different treatment groups showed an increased expression (mRNA) of IL-6 compared with the controls. There were no significant differences in IL-6 mRNA levels in animals fed gliadins with or without the bifidobacterial strain. A significant (P= 0·001) increase in IL-6 expression levels was also observed after feeding B. longum CECT 7347 alone. Feeding gliadins to the sensitised animals with IFN-γ increased (P= 0·002) hepatic IL-6 expression levels, although to a lesser extent (P< 0·001) compared with the non-sensitised animals. The administration of gliadins with B. longum CECT 7347 to the IFN-γ sensitised animals did not differ from the effect observed in the non-sensitised animals.

Fig. 4 Hepatic IL-6 (■) and TNF-α (![]() ) expression (mRNA) levels in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins with or without Bifidobacterium longum CECT 7347. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control animals (P< 0·05). †† Mean value was significantly different from that of the IFN-γ/gliadins-treated animals P< 0·01.
) expression (mRNA) levels in weanling animals, sensitised or not with interferon (IFN)-γ, fed with gliadins with or without Bifidobacterium longum CECT 7347. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control animals (P< 0·05). †† Mean value was significantly different from that of the IFN-γ/gliadins-treated animals P< 0·01.
The administration of B. longum CECT 7347 alone to weanling animals did not cause significant (P= 0·381) alterations in the basal expression of this inflammatory marker. Gliadin feeding significantly (P= 0·008) increased TNF-α mRNA expression, while the simultaneous administration of B. longum CECT 7347 decreased (P= 0·004) its levels to the values similar (P= 0·162) to those of the controls (Fig. 4). In the IFN-γ-sensitised gliadin-fed animals, TNF-α expression increased (P= 0·002) compared with the expression level found in the controls, but did not show a significant (P= 0·071) variation compared with the non-sensitised gliadin-fed animals. However, the IFN-γ-sensitised animals fed both gliadins and the bifidobacterial strain exhibited higher TNF-α expression levels than those animals fed gliadins without the bacterium.
Discussion
Gliadins cause different symptoms in CD patients, and nutritional Fe deficiency-related manifestations are common(Reference Ludvigsson, Brandt and Montgomery15, Reference Hin, Bird and Fisher35). Epidemiological studies have reported a higher prevalence of ferropenic anaemia in individuals with undiagnosed CD (50 %)(Reference Hin, Bird and Fisher35) and CD patients with normal mucosa (32 %)(Reference Ludvigsson, Brandt and Montgomery15) in comparison with healthy controls. According to the present data, deficient Fe status seems to be caused by the impairment of micronutrient homeostasis processes and liver physiology. The present study shows that gliadin feeding causes alterations in liver Fe deposition and mobilisation, and induces the expression of hepatic inflammatory mediators, even in weanling animals lacking perceptible mucosal damage(Reference Laparra, Olivares and Gallina12). In the IFN-γ-sensitised animals, changes in Fe homeostasis were greater, resulting in a more severe decrease in circulating Hb concentration. This effect on weanling animals with high physiological requirements of Fe during early growth stages can have a profound negative impact on the development of adequate physiological and immune responses. B. longum CECT 7347 administration to gliadin-fed animals, sensitised or not with IFN-γ, partially reduced the severity of the gliadin-mediated effects observed in these animals, and could theoretically contribute to ameliorating the severity of anaemia in CD patients.
Fe homeostasis is mediated through the regulation of duodenal Fe absorption and Fe release from macrophages, and other cell types, according to signals from different compartments reflecting dietary sources or availability, status of the body Fe stores and metabolic requirements. In this context, the liver plays a central role in the regulation of Fe metabolism, producing key regulators and mediators such as Hamp (Reference Ganz20, Reference Young, Glahn and Ariza-Nieto36). Hamp is predominantly expressed in the liver, although various tissues, such as the heart, brain and monocytes, can express Hamp at low levels in response to adhesion and pro-inflammatory cytokines(Reference Zhang and Rovin37), regulating not only Fe metabolism but also intestinal Fe absorption.
Previous research has demonstrated the inverse relationship between serum Hamp concentration and Fe absorption from food and supplemental sources(Reference Young, Glahn and Ariza-Nieto36). Besides, Hamp transcription is down-regulated when body Fe demand is high(Reference Jenkitkasemwong, Broderius and Nam26, Reference Pigeon, Ilyin and Courselaud27). In the present study, gliadin feeding increased hepatic Fe deposition, impairing Fe supply to peripheral tissues. This was reflected by the significant inverse relationship between Hamp transcription levels and liver Fe concentrations in animals fed with gliadins, administered either with or without B. longum CECT 7347. In accordance with impaired Fe status, expression levels of TfR2 were down-regulated in gliadin-fed animals. TfR2 is thought to serve as a body Fe sensor, relying on information from plasma Fe (holo-transferrin)(Reference Fleming38), which is necessary for hepatic Hamp expression(Reference Jenkitkasemwong, Broderius and Nam26). In human studies, serum protein concentration of Hamp has previously been associated with ferritin concentration, but not with Hb(Reference Dallalio, Fleury and Means39), as found in this study. B. longum CECT 7347 administration to the gliadin-fed animals partially restored TfR2 expression to levels similar to those of the controls, although it was insufficient to reduce the marked gliadin-induced hepatic Fe deposition. Sensitisation with IFN-γ reduced serum Hb concentrations below the normal range in these animals (120 g/l). It is noteworthy that feeding B. longum CECT 7347 significantly restored the Hb concentration and reduced the gliadin-mediated hepatic deposition of Fe, parallel with the small increase in Hamp expression levels. This trend is concordant with previous reports indicating that Hamp expression levels are lower in anaemic than in non anaemic individuals(Reference Kemna, Tjalsma and Podust40).
Inflammation and bacterial infection, in addition to body Fe status, are the main stimuli inducing Hamp synthesis(Reference Ganz20, Reference De Domenico, Zhang and Koening21). In CD patients, gliadins induce the production of pro-inflammatory cytokines, among others IL-6, which has been suggested as a marker of both CD activity and response to treatment(Reference Romaldini, Barbieri and Okay2). In the initial phase of the inflammatory process, IL-6 is a key inducer of hepatic acute-phase protein synthesis, but also plays diverse biological activities in immune regulation, inflammation and haematopoiesis(Reference Naka, Nishimoto and Kishimoto6, Reference Nemeth, Rivera and Gabayan41). The administration of gliadins to weanling animals, with or without B. longum CECT 7347, increased hepatic IL-6 expression levels, which are hypothesised to be mediated by the increased hepatic deposition of Fe. IL-6 exerts a wide range of biological activities in immune regulation, favouring T-cell growth and the differentiation of cytotoxic T cells(Reference Lotz, Jirik and Kabouridis42) and macrophages(Reference Nicola, Metcalf and Matsumoto43), and may be important in Th-17 commitment(Reference Dema, Martínez and Fernández-Arquero3). In this respect, the administration of B. longum CECT 7347 to weanling animals has proven effective to reduce both peripheral CD4+ and CD4+Foxp3+ T cells in gliadin-fed animals, revealing the positive immunomodulatory characteristics of this bacterial strain(Reference Laparra, Olivares and Gallina12). It should be noted that two types of IL-6-responsive elements have been identified, in genes encoding acute-phase proteins, acting through increased gene expression or its post-translational modification(Reference Naka, Nishimoto and Kishimoto6). However, from the limited data available at present, one cannot speculate which of these specific mechanisms underlies the bifidobacterial-induced activation of IL-6.
In vitro studies using the intestinal epithelial Caco-2 cell line have shown that TNF-α production reduces Fe intestinal absorption(Reference Johnson, Bayele and Johnston17) and favours its hepatic deposition(Reference Hirayama, Kohgo and Kondo19). In animals exhibiting significantly increased TNF-α expression, compared with the controls, a similar relationship could be found between hepatic TNF-α expression and Fe deposition levels. Moreover, increased TNF-α expression levels in the non-sensitised gliadin-fed animals and the sensitised animals fed gliadins with or without the bifidobacterial strain could be associated with increased median values for plasma active hepcidin quantified in the different treatment groups. However, this association has not been reported for in vitro studies with human-derived hepatocytes(Reference Ganz20), suggesting that Hamp response activation may require the interaction of liver and immune cells(Reference Jacolot, Férec and Mura44). Gliadin-induced production of pro-inflammatory cytokines such as IL-1β and TNF-α favours lymphocyte infiltration and, thereby, further adaptive responses to counteract gliadin toxicity(Reference Hoffman45). Hepcidin production, which greatly induces IL-6 and TNF-α production by macrophages(Reference De Domenico, Zhang and Koening21), would suggest its potential contribution to antigen processing as well as to direct effects causing Fe sequestration.
Conclusions
An alteration in Fe homeostasis, leading to ferropenic anaemia, is one of the most common extra-intestinal manifestations of CD. The data reported herein, on the administration of B. longum CECT 7347 to weanling animals, reveal novel findings on how administration of this strain affects Fe homeostasis in a gliadin-induced enteropathy model. The sensitised gliadin-fed animals displayed low Hb concentrations, below the normal range in these animals, demonstrating enteropathy-associated anaemia; however, administration of B. longum CECT 7347 normalised the expression levels of TfR2, thus contributing to restoring normal Hb concentrations. Although both TfR2 and IL-6 are involved in Hamp expression regulation, their action seems to be dependent on Fe serum levels. To the best of our knowledge, this is the first study to provide evidence for the entero-hepatic link with the bifidobacterial-mediated regulation of essential micronutrient homeostasis, such as that of Fe. Further studies and human trials are needed to gain a better understanding of the clinical relevance of B. longum CECT 7347 in controlling CD-related ferropenic anaemia.
Acknowledgements
The present study was supported by grants AGL2011-25169 and Consolider Fun-C-Food CSD2007-00063 from the Spanish Ministry of Science and Innovation (MICINN, Spain). J. M. L. has a postdoctoral contract under the programme JAE-Doc (CSIC, Spain) and M. O. has a JAE-preDoc contract (CSIC, Spain).
M. O., J. M. L. and Y. S. designed the experiments. J. M. L. and M. O. performed the experiments. J. M. L. and Y. S. drafted the manuscript and all authors revised the final version of the manuscript.
The authors declare no conflicts of interest.