Introduction
The Qinghai-Tibetan Plateau (QTP) of western China covers an area of about 2.5 million km2 with a mean altitude of more than 4,000 m (Schaller Reference Schaller1998). The rangelands of the QTP support c.5 million pastoral herders by providing forage for an estimated 12 million yak and 30 million sheep and goats (Sheehy et al. Reference Sheehy, Miller and Johnson2006), whilst its varied landscapes and alpine ecosystems support endangered and endemic species (Foggin and Smith Reference Foggin, Smith, Peter, Wang and Xie1996). Large areas of rangeland on the QTP are severely degraded (Zhou et al. Reference Zhou, Zhao, Tang, Gu and Zhou2005, Wen et al. Reference Wen, Dong, Li, Li, Shi, Wang, Liu and Ma2013), though there is some ambiguity in relation to the extent, magnitude and form of this degradation (Harris Reference Harris2010). Causes of rangeland degradation are attributed to overgrazing by livestock (Wang et al. Reference Wang, Wang and Wang2006), burrowing and grazing activity of small mammals (Fan et al. Reference Fan, Zhou, Wei, Wang, Jiang, Singleton, Hinds, Leirs and Zhang1999; Zhang et al. Reference Zhang, Zhong, Fan, Singleton, Hinds, Krebs and Spratt2003), socio-economic development (Ding et al. Reference Ding, Zhang, Shen, Liu, Zhang, Wang, Bai and Zheng2006) and global climate change (Cui and Graf Reference Cui and Graf2009). However, rangeland degradation is not readily attributable to a single cause, but instead may reflect a range of mechanisms operating at different spatial and temporal scales across the QTP (Li et al. Reference Li, Perry, Brierley, Gao, Zhang and Yang2013).
Grazing and burrowing by plateau pika Ochotona curzoniae is considered by some to be a major causal factor in grassland degradation, or possibly an exacerbating factor, whereas others regard most of the effects of pika activity as primarily a consequence of rangeland degradation (Smith and Foggin Reference Smith and Foggin1999, Smith et al. Reference Smith, Zahler, Hinds, McNeely, McCarthy, Smith, Olsvig-Whittaker and Wikramanayake2006, Wangdwei et al. Reference Wangdwei, Steele and Harris2013). Management strategies to address grassland degradation include the control of plateau pikas by poisoning (Pech et al. Reference Pech, Jiebu, Arthur, Zhang and Lin2007). This poisoning, which has been carried out for at least 50 years (Smith et al. Reference Smith, Formozov, Hoffmann, Zheng, Erbajeva, Chapman and Flux1990, Fan et al. Reference Fan, Zhou, Wei, Wang, Jiang, Singleton, Hinds, Leirs and Zhang1999), has fuelled fears that the policy could have negative consequences for biodiversity (Smith and Foggin Reference Smith and Foggin1999, Lai and Smith Reference Lai and Smith2003, Arthur et al. Reference Arthur, Pech, Davey, Jiebu, Zhang and Lin2008, Delibes-Mateos et al. Reference Delibes-Mateos, Smith, Slobodchikoff and Swenson2011), as the plateau pika, is the primary or secondary prey resource for a wide range of predatory species such as brown bear Ursus arctos, Pallas’s cat Otocolobus manul, Tibetan fox Vulpes ferrilata, Upland Buzzard Buteo hemilasius and Saker Falcon Falco cherrug (Schaller Reference Schaller1998, Ross et al. Reference Ross, Munkhtsog and Harris2010, Harris et al. Reference Harris, Zhou, Ji, Zhang, Yang and Yu2014, Chen et al. Reference Chen, Jiang, You and Chen2015).
Information on the type, timing, distribution, scale and outcome of Plateau Pika poisoning campaigns are scarce. Wilson and Smith (Reference Wilson and Smith2015), citing a press article, noted that in the Sanjiangyuan region extermination work had been carried out on 78,000 km2 of land by 2013, and that more than 31,000 km2 were earmarked for pika poisoning in 2014. However, the same press report notes that funding also supports alternative methods of controlling pikas and managing grasslands. One such method involves the erection of artificial nests and perching sites for birds of prey that feed on plateau pikas. A number of county rodent control stations on the Qinghai-Tibetan Plateau have been erecting such structures for several years; for example, by 2012, 6,125 nests and perches had been in erected in Madoi County, Qinghai at a cost of 4 million yuan (data from Rodent Control Department, Agriculture and Forestry Authority, Madoi County).
Measures to limit overgrazing by livestock include settlement of herders, privatisation and enclosure of pastures and control of livestock density (Harris Reference Harris2010, Foggin Reference Foggin2012). Infrastructure development associated with the resettlement policy includes the construction of highways, settlements and power supply networks, and can also have impacts on wildlife distributions (Xia et al. Reference Xia, Yang, Li, Wua and Feng2007, Ogutu et al. Reference Ogutu, Owen-Smith, Piepho, Kuloba and Edebe2012, Dixon et al. Reference Dixon, Maming, Gunga, Purev-Ochir and Batbayar2013a). Additionally, in recent years there has been a dramatic increase in tourism to the QTP (Cui and Graf Reference Cui and Graf2009), creating possibilities for local herders to diversify their income and acting as a major driver for further infrastructure development and the expansion of settlements. This increasing human footprint (Sanderson et al. Reference Sanderson, Jaiteh, Levy, Redford, Wannebo and Woolmer2002) requires sensitive and informed management if its impact on the environment and biodiversity of the QTP is to be ameliorated.
In conjunction with the unprecedented economic growth and social changes witnessed in recent decades (Bawa et al. Reference Bawa, Koh, Lee, Liu, Ramakrishnan, Yu, Zhang and Raven2010), China has established numerous nature reserves within its development strategy for Western China (Foggin Reference Foggin2012), where the Chang Tang and Sanjiangyuan National Nature Reserves on the QTP cover areas of 298,000 km2 and 152,000 km2 respectively, and are ranked the largest and second largest in China. The Saker Falcon, a globally ‘Endangered’ predator (Appendix S1 in the online supplementary material), occurs as a breeding bird and as winter migrant on the QTP, particularly in alpine grassland habitats (Dixon et al. Reference Dixon, Ma and Batbayar2015). As a predator of the plateau pika, and its susceptibility to anthropogenic developments (e.g. electrocution; Dixon et al. Reference Dixon, Maming, Gunga, Purev-Ochir and Batbayar2013a), the Saker Falcon is potentially an indicator of the condition of vast areas of grassland including extensive protected areas on the QTP. In this study we have examined the landscape-scale ranging behaviour of the species using satellite telemetry and remote imagery to overcome difficulties of studying a wide ranging predator across an extensive, remote and largely inaccessible environment. We discuss our results in context of infrastructure development and rangeland management on the QTP.
Methods
We analysed data from eight platform transmitter terminals (PTTs; ‘satellite tags’) that had been deployed from 1998 to 2009 on female Saker Falcons in Russia and Mongolia, comprising six adults and two juveniles (Appendix S2; see also Eastham et al. Reference Eastham, Quinn, Fox, Chancellor and Meyburg2000, Potapov et al. Reference Potapov, Fox, Sumya and Gombobaatar2002). All PTTs were attached using a Teflon-ribbon harness (Kenward et al. Reference Kenward, Pfeffer, Al-Bowardi, Fox, Riddle, Bragin, Levin, Walls and Hodder2001) but over this period three different models of PTT were used to obtain location data via the Argos system only (n = 4 PTTs) and a fourth model also provided GPS location data (n = 4 PTTs; Appendix S2). Birds were tracked when wintering on the QTP between January 1998 and February 2011.
The Argos system uses the Doppler effect to provide location data, the accuracy of which varies according to the geometrical conditions of the satellite pass, the stability of the transmitter oscillator, the number of messages collected and their distribution in the pass (CLS 2014). Consequently the Argos system provides an estimate of accuracy and assigns a location class (LC) for each location, which have reported accuracies of: LC3 accurate to within 250 m, LC2 < 500 m, LC1 < 1,500 m, LC0 > 1,500 m. LC A and B have no associated accuracy estimates. However, there can be significant discrepancies in these error estimates because oscillator instability or a fast moving platform may lead to underestimation of the errors (CLS 2014). To assess accuracy of our Argos location data we compared location points for four birds that were deployed with transmitters that provided both GPS and Argos location data. We considered all GPS locations to be accurate to within ± 25 m (CLS 2014), thus used GPS locations as reference points to compare with Argos locations of different location classes. As GPS and Argos locations were obtained at different times we could not directly compare GPS and Argos locations for the same moment in time, thus we restricted our comparison to locations that were received within ± 4 hours of a GPS location.
The Argos locations that corresponded most closely to GPS locations were location classes 3, 2, 1 and A, thus we used these four location classes to define the home range areas of Saker Falcons that were deployed with Argos only PTTs (Appendix S3). We found that 70% of Argos location points with a location class of 3, 2, 1 and A were located ≤ 1.2 km of a GPS location obtained within ± 4 hrs, which compared favourably with sequential GPS locations obtained within ± 4 hrs of each other, where 79% were located ≤ 1.2 km. Consequently, we used a 70% MCP to define the home range of birds deployed with Argos only PTTs (n = 4) and a 95% MCP of the GPS locations to define the home range of birds deployed with Argos/GPS PTTs (n = 4).
We quantified the home range area by using the Minimum Convex Polygon (MCP), which is more robust when using unfiltered data (Nilsen et al. Reference Nilsen, Pedersen and Linnell2008) and used 70% and 95% of fixes to define the boundaries of Saker Falcon movements for Argos and GPS data respectively (Börger et al. Reference Börger, Dalziel and Fryxell2008). For birds with GPS data we also estimated the core area of the home range through fixed-kernel methods (Worton Reference Worton1989) and calculated 50% fixed kernels using the least-squares cross-validation procedure to determine the optimal value of the smoothing parameter for a given kernel and sample size (Seaman and Powell Reference Seaman and Powell1996, Seaman et al. Reference Seaman, Millspaugh, Kernohan, Brundige, Raedeke and Gitzen1999, Gitzen and Millspaugh Reference Gitzen and Millspaugh2003).
Subsequently, we used a machine learning algorithm, Random Forest, to detect the association between Saker Falcon occurrences and environmental variables (i.e. altitude, land cover, and human footprint index). Random Forest is an ensemble classifier that consists of many decision trees, implementing Breiman’s random forest algorithm for classification and regression (Breiman Reference Breiman2001). It compares environmental variables at the presence sites (Saker Falcon occurrences) and absence sites (evenly distributed points representing environmental gradient, i.e. pseudo-absence). The values of the environmental variables at the presence and absence sites were derived from layers of elevation (DAAC 2004), land cover (1:1 million raster data of vegetation and land cover; Chinese Academy of Surveying and Mapping), and human footprint index (Sanderson et al. Reference Sanderson, Jaiteh, Levy, Redford, Wannebo and Woolmer2002). The resolution of the three layers was 1 km2. Occurrences within the same grid cell have the same value and replicated records were not removed, so that the frequently used cells were weighted by the number of records inside.
We used a chi-square test to examine whether the proportion of land with > 50% grassland cover differed within Saker Falcon wintering ranges of different sizes. We used Z-tests to compare land cover types within the overall area occupied by wintering Saker Falcons with that of the Qinghai-Tibetan Plateau as a whole.
In order to obtain data on the abundance of plateau pikas across a large area of the eastern Qinghai-Tibetan Plateau, we undertook spot surveys along a road transect route between 23 July and 04 August 2015 (Figure S1). At c.10 km intervals we classified by eye the density of plateau pika holes in c.1 ha of habitat adjacent to the observation point, and then allocated them to two categories: 0–25 and > 25 holes per ha. At the same time we quantified the number of animals seen and allocated them to two categories: none/few (1–4 individuals) and moderate/many (> 5 individuals). Within the same 1 ha area we recorded, by eye, the percentage cover of grass, classifying each survey spot as either above or below 50% grassland cover. We used a chi-square test to compare hole density and two categories of observed animal numbers in relation to the proportion of the 1 ha plots with > 50% grassland cover.
Results
In total, we obtained location data from satellite transmitters deployed on eight falcons wintering on the QTP for periods of 33–164 days (median duration = 130 days; Table 1). They primarily occupied southern Qinghai, with location data for all the birds coming from within an area of 143,292 km2 (i.e. 100% MCP; Fig. 1). We were able to directly compare the 70% MCP derived from Argos locations with the 95% MCP derived from GPS locations for two individuals, which showed close correspondence in both area and shape (Table 1; Figure S2). The number of locations was too few to calculate 70% MCPs of Argos locations for three birds, though for two of these birds we were able to calculate 95% MCPs using GPS location data (Table 1). With the exception of one individual, all birds occupied spatially discrete winter home ranges that ranged in size from 5 to 515 km2 (Mean = 166 km2, Median = 86 km2; Table 1, Fig. 2).
Table 1. Winter home range sizes and duration of tracking period of eight Saker Falcons on the Qinghai-Tibetan plateau. 95% and 70% MCPs calculated from GPS and Argos location data respectively. N values in parentheses are the number of location points used to derive minimum convex polygons. 50% kernels derived from GPS data only.
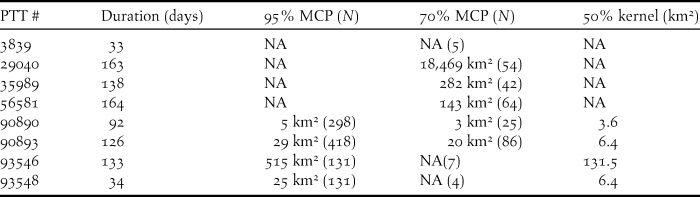

Figure 1. Overall wintering range (100% MCP) of eight Saker Falcons on the Qinghai-Tibetan plateau.
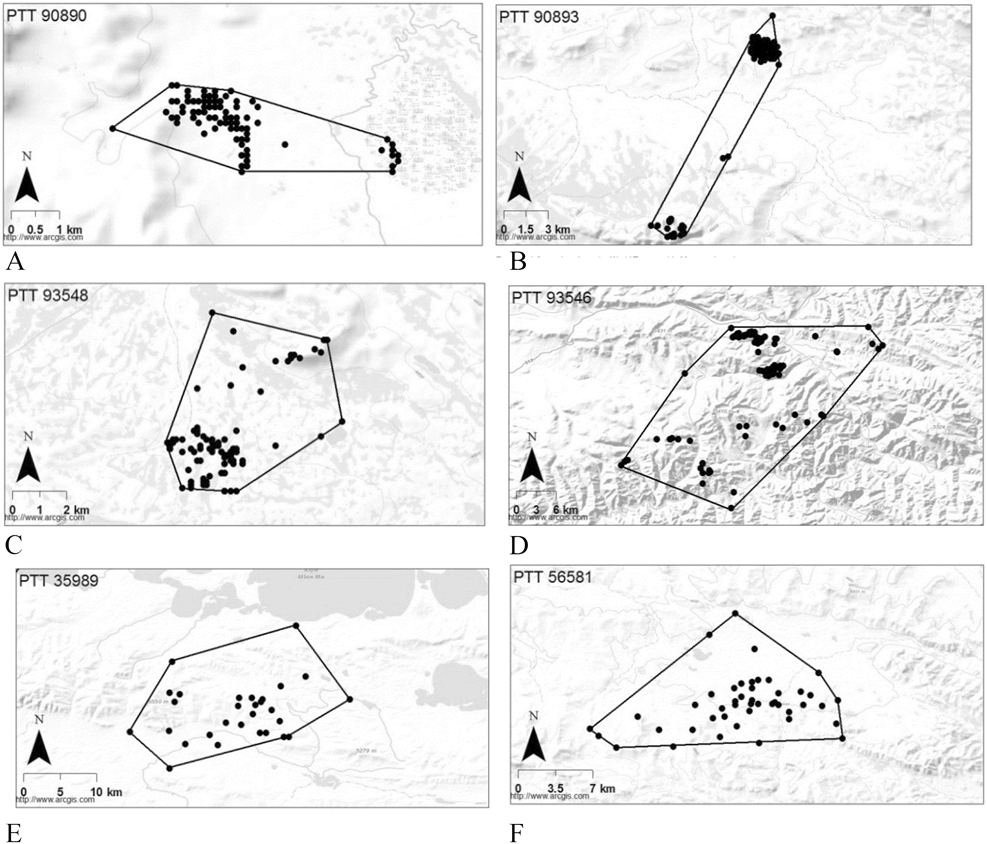
Figure 2. Winter home ranges of six Saker Falcons on the Qinghai-Tibetan plateau. 95% MCP of GPS location data for PTTs (a) 90890, (b) 90893, (c) 93548 and (d) 93546, and 70% MCPs of Argos location data for PTTs (e) 35989 and (f) 56581.
Of the birds tracked in detail via GPS, the adult with the smallest range of 5 km2 alternated between two restricted areas c.3 km apart at the edge of a wide plain whilst making occasional excursions of 10–20 km to mountainous landscape (Fig. 2a), and the adult with a range of 29 km2 exhibited a similar pattern of behaviour, alternating between two restricted areas of mountainous landscape c.14 km apart either side of a wide valley (Fig. 2b). A juvenile remained in its wintering range of 25 km2 for a period of 34 days (Fig. 2c), occupying a plain and adjacent hills, before embarking on a 268 km circuitous excursion sometime between 18 and 22 November, returning briefly to its origin on 26 November, before again departing sometime between 26 and 28 November on a northward excursion where transmission ceased 275 km away on 04 December, when the bird probably died. The adult with the largest GPS range of 515 km2 occupied a mountainous area with the maximum distance between locations being 47 km (Fig. 2d).
Of the birds tracked using Argos location data, one adult with a home range of 282 km2 occupied a hill range between two large lakes (Fig. 2e), and a juvenile with a home range of 143 km2 occupied a hilly landscape and an adjacent plain over a period of 164 days (Fig. 2f). From 25 September to 3 March, one bird utilised a large area of 66,090 km2 (100% MCP) sequentially occupying a series of four sub-ranges for at least 26, 27, 26 and 53 days that were spaced 350, 118 and 92 km apart respectively. These discrete sub-ranges with 70% MCP areas of 15.9, 639.9, 120.5 and 308.5 km2 and were all located in hilly areas (Figure 3).
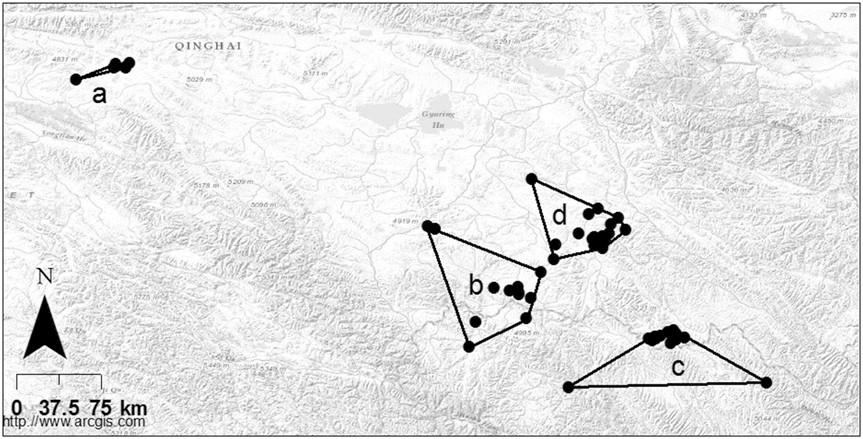
Figure 3. Four spatially and temporally discrete home ranges occupied by a single Saker Falcon in one winter period (PTT #29040). These home ranges were occupied from 25 September-21 October 2000 (a), 26 October-01 December 2000 (b), 02 December-28 December 2000 (c) and 30 December 2000-03 March 2001 (d).
There was a significantly greater proportion of grassland with > 50% cover within the home ranges of the three birds with relatively small winter ranges of 3–29 km2 compared with the three that occupied larger winter ranges of 143–515 km2 i.e. 95% cf. 66% (χ 2 = 702.7, df = 1, P < 0.0001).
Of the 18 different land cover types only six occurred at a frequency of ≥ 5%, either on the QTP as whole or within the distribution range of wintering Saker Falcons (Appendix S4; Figure 4). Land cover type 31 (‘grassland with > 50% cover’) was the predominant land cover type on the QTP as whole (41%), yet it occurred in a significantly greater proportion of grid cells within the overall winter distribution range of all eight Saker Falcons (64%; z = 25.09, P < 0.01). The proportion of ‘grassland > 50% cover’ within occupied cells of individual winter home ranges was 72% ± SE 9% (median = 76%; range 40–99%). Compared with the QTP as a whole, there was also a significantly greater proportion of land cover type 67 (‘other non-vegetated ground’) within the overall distribution range of Saker Falcons (z = 2.7315, P < 0.01), though it only comprised 5% of the land cover. Conversely, there was significantly less of land cover types 21 (‘forest > 30% cover’; z = -11.5932, P < 0.01), 33 (‘grassland 5–20% cover’; z = -16.4627, P < 0.01) and 66 (‘bare rock’; z = -9.9348, P < 0.01) within the overall winter distribution range of Saker Falcons than across the QTP as a whole.
Figure 4. Percentage of six main land cover types within the Qinghai-Tibetan plateau (black), overall winter distribution (grey) and winter ranges (white) of Saker Falcons. 21 = forest canopy >30%; 31 = grassland cover > 50%, 32 = grassland cover 20-50%; 33 = grassland cover 5-20%; 66 = bare rock; 67 = other non-vegetated; other = total of all other land cover types identified (see Appendix S4).
We used a machine learning algorithm, random forest, to detect the association between Saker Falcon occurrences and environmental variables (i.e. altitude, land cover, and human footprint index; Figure 5). The random forest model based on these three variables explained 47.2% of the variance in the presence/absence data of Saker Falcons on the QTP as a whole. Our results indicated a nonlinear association between the Saker Falcon occurrences and altitude, as well as human footprint index (HFI). Saker Falcons preferred to establish winter ranges within the altitude range 4,200–5,000 m, where there was a HFI within the range 0–25, and land cover type 31 (grassland > 50% cover) predominated.
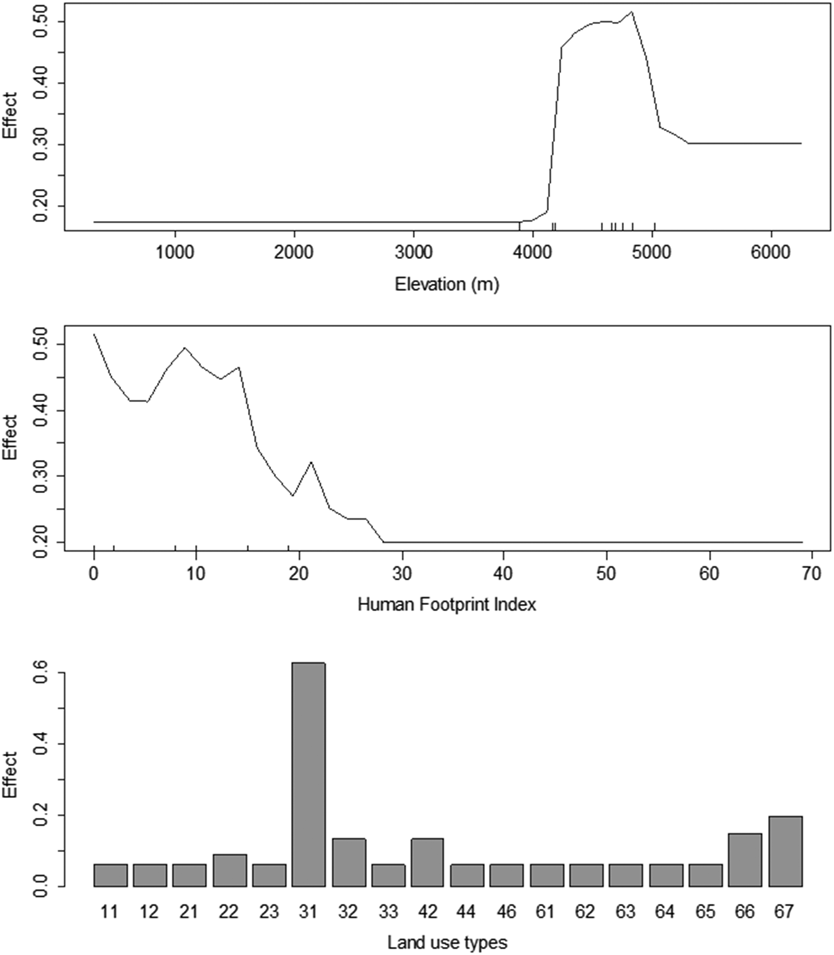
Figure 5. Result of random forest model showing Saker Falcon preference for high altitude areas with low anthropogenic influence, where the land cover is predominantly grassland with more than 50% cover. The codes for land cover types are defined in Appendix S3.
We recorded plateau pika and hole densities at 185 survey plots along a 1,910 km road transect on the eastern Qinghai-Tibetan Plateau at altitudes > 3000 m (Figure S1). There was a strong relationship between density of holes and plateau pikas with land cover type, with significantly more holes (χ2 = 95.2, df = 1, P < 0.001) and animals (χ2 = 15.8, df = 1, P < 0.001) observed in 1 ha survey plots with grassland cover > 50%.
Discussion
Saker Falcons occur across a large part of the QTP, with the majority of records coming from eastern Qinghai (Vaurie Reference Vaurie1972), although the paucity of records from the west and the south of the plateau may equally reflect the relative inaccessibility of these regions. Nevertheless, the distribution of Saker Falcons tracked by satellites in this study suggests that the main wintering area of migrants is south and east Qinghai. However, it must be noted that we have only studied female Saker Falcons and the ranging behaviour or distribution of males wintering on the QTP may differ. The distribution of the plateau pika in Qinghai is skewed towards alpine grasslands in the south and east (Smith and Xie Reference Smith and Xie2008). Eastern Qinghai is characterised by high altitude alpine grasslands, where the winter ranges of Saker Falcons comprised mostly grasslands with > 50% cover. Grazing by herbivores removes virtually all available useable plant biomass by the end of winter, and higher plant biomass in autumn may increase winter survival of plateau pikas (Pech et al. Reference Pech, Jiebu, Arthur, Zhang and Lin2007). There is evidence that an increase in precipitation and temperature has contributed to a more favourable growing season in the alpine grassland of eastern Qinghai in recent years (Zhang et al. Reference Zhang, Guo, Ji, Lei, Wang, Yan, Li and Li2013), which may benefit wintering predators such as the Saker Falcon by increasing the area of grassland cover > 50% and the over-winter survival of plateau pikas.
We found that among Saker Falcons winter home range size was strongly associated with grassland cover, with significantly more grassland of > 50% cover being found in smaller home ranges than larger ones. Furthermore, we found that plateau pika abundance was higher in survey plots with > 50% grassland cover. Resource availability can influence home range size (McLoughlin and Ferguson Reference McLoughlin and Ferguson2000), and the distribution and abundance of prey species is likely to be an important determinant in the movements of a wide ranging predator (Macdonald and Carr Reference Macdonald, Carr, Standen and Folley1989). Saker Falcon home ranges are predicted to be smaller where prey is abundant. Consequently, our data indicates that grassland cover > 50% is likely to be a good proxy indicator of plateau pika availability on the QTP.
The plateau pika is the main prey for most predatory animals on the QTP (Smith and Foggin Reference Smith and Foggin1999), thus factors that influence its abundance and distribution are likely to have an effect on a wide range of predatory species (Delibes-Mateos et al. Reference Delibes-Mateos, Smith, Slobodchikoff and Swenson2011). Extensive poisoning programmes have been employed on the QTP since the late 1950s, which now primarily utilise anticoagulants such as botulin toxin C and bromadiolone that are distributed on wheat bait in spring (Fan et al. Reference Fan, Jing, Wang and Zhou1986, Pech et al. Reference Pech, Jiebu, Arthur, Zhang and Lin2007, Arthur et al. Reference Arthur, Pech, Davey, Jiebu, Zhang and Lin2008). However, there is little information published on the implementation of these programmes or the effects of poisoning on plateau pika populations. Despite more than half a century of control measures the plateau pika generally remains an abundant and widespread species on the QTP, suggesting that the poisoning programme has had limited lasting impact on their population. The indirect impact of this management practice on other (e.g. predatory) species on the QTP, however, is unknown but the use of anticoagulants to control small mammal populations has been implicated in unintentional poisoning of predatory and scavenging species elsewhere (Olea et al. Reference Olea, Sanchez-Barbudo, Vinuela, Barja, Mateo-Tomas, Pineiro, Mateo and Purroy2009, Winters et al. Reference Winters, Rumbeiha, Winterstein, Fine, Munkhtsog and Hickling2010, Coeurdassier et al. Reference Coeurdassier, Riols, Decors, Mionnet, David, Quintaine, Truchetet, Scheifler and Giraudoux2014) and therefore remains concerning. The Buddhist religion and culture of herders on the QTP means that, in contrast to some other regions of the world (e.g., Margalida Reference Margalida2012), deliberate poisoning of scavenging species does not apparently occur to any great extent.
Recent decades have witnessed an increasing human influence on the ecosystem of the QTP. The human footprint index (HFI) uses measures of population density, land transformation, accessibility and electrical power infrastructure as proxies for human influence (Sanderson et al. Reference Sanderson, Jaiteh, Levy, Redford, Wannebo and Woolmer2002). Saker Falcons wintered in ranges with a low HFI, suggesting that the species may be adversely affected by the rapid infrastructure developments witnessed on the QTP in recent years including railway and highway construction, creation and expansion of settlements, and the proliferation of electricity transmission and distribution lines. Conversely, infrastructure development may provide new nesting places for Saker Falcons in areas of the QTP that are otherwise nest site limited (Ellis et al. Reference Ellis, Ellis and Tsengeg1997, Dixon et al. Reference Dixon, Purev-Ochir, Galtbalt and Batbayar2013b, Reference Dixon, Ma and Batbayar2015). It should be noted that HFI only provides a coarse ‘snapshot’ of human influence on the ecosystem of the QTP, while the rapidity of infrastructure development and social change means that the current situation may not be accurately represented in existing datasets. For example, large scale tourism can result in road developments together with associated expansion of settlements; such mass tourism can already be seen at Qinghai Lake. However, different forms of community and environmentally focused tourism may not have such an impact on the ecosystem and provide greater benefits to local communities (e.g. Sopheap et al. Reference Sopheap, Qiong and Tatar2015). Further study is required to identify the specific features of human socio-economic development in the region that have the greatest impact on wide-ranging predators such as the Saker Falcon.
It has been postulated that climate warming may increase grassland productivity (Du et al. Reference Du, Kawashima, Yonemura, Zhang and Chen2004), which could potentially benefit plateau pikas and their predators, whereas Klein et al. (Reference Klein, Harte and Zhao2007) suggested warming temperatures can decrease rangeland productivity, which may have a negative effect on plateau pikas and their predators. Any influence exerted by climate change will undoubtedly involve complex interacting factors, thus its potential effect, alongside anthropogenic influences, on the grassland ecosystem of the region remains largely unknown (Harris Reference Harris2010). Wide ranging predatory species generally require large areas and productive ecosystems, thus they can be useful indicators of biodiversity and/or ecological integrity in protected areas (Sergio et al. Reference Sergio, Newton and Marchesi2005, Reference Sergio, Newton and Marchesi2008). Furthermore, predators can act as ‘sentinels’ for anthropogenic changes that may impact many different taxa (Sergio et al. Reference Sergio, Newton and Marchesi2008). Remote tracking of wide-ranging predators like the Saker Falcon, when used in conjunction with remote sensing data, can potentially be used to monitor the condition of vast, inaccessible protected areas like the Chang Tang and Sanjiangyuan National Nature Reserves on the QTP. The dispersion of wintering or breeding birds and the characteristics of their home range areas can provide insights into prey availability and habitat quality. However, further work is still required to understand the relationship between ranging behaviour of Saker Falcons and the availability of Plateau Pikas, and how this is influenced by or reflects differences in land cover types.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000319
Acknowledgements
This study was funded by the Environment Agency-Abu Dhabi (EAD). We thank HE M. Al Bowardi for his support in undertaking this work. XZ was supported by the National Key Research and Development Program of China (2016YFC0503200) and Recruitment Program of Global Youth Experts of China, and AD by the CAS President’s International Fellowship Initiative. The World Bank (Netherlands-Mongolia Trust Fund for Environmental Reform) and BirdLife International (the Rio Tinto-BirdLife Programme) funded the purchase and deployment of two of the PTTs included in this study. PTTs used in this study were deployed on Saker Falcons in Russia by C. Eastham and in Mongolia by E. Potapov, S. Gombobaatar, G. Purev-Ochir, D. Ragyov and AD.








