Introduction
Throughout the world, many populations of long-distance migratory shorebirds are in decline (e.g. International Wader Study Group 2003, Thomas et al. Reference Thomas, Lanctot and Szekely2006). Most of them rely on a few coastal staging wetlands along flyways to replenish energy and nutrient reserves (Skagen Reference Skagen2006). These wetlands are scarce and they are often threatened by factors such as coastal development, agriculture, disturbance (hunting, fishing, recreation), competition with fisheries for food, and sea level rise (see Weber et al. Reference Weber, Houston and Ens1999), so the availability of these wetlands may pose a serious constraint on the conservation of migratory shorebirds (e.g. Piersma et al. Reference Piersma, Van Gils, Wiersma, del Hoyo, Elliott and Sargatal1996, Piersma and Baker Reference Piersma, Baker, Gosling and Sutherland2000). All these factors that reduce the availability or profitability of natural staging wetlands for migratory shorebirds may increase the future importance of anthropogenic wetlands as buffer habitats (Masero Reference Masero2003).
Today, rice fields cover 11% of the world’s arable land area, but biodiversity conservation in landscapes dominated by rice fields remains a relatively new and unexplored topic globally (Amano Reference Amano2009). During the last two decades, recognition that rice fields provide important habitats for waterbirds, including several species of high conservation concern, has increased (see review by Elphick et al. Reference Elphick, Taft and Lourenço2010). These functional wetlands support large numbers of migrating shorebirds around the world (e.g. Elphick Reference Elphick2000, Blanco et al. Reference Blanco, López-Lanús, Dias, Azpiroz and Rilla2006, Sánchez-Guzmán et al. Reference Sánchez-Guzmán, Morán, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007, Amano Reference Amano2009) and may constitute the primary habitat in a region. In Japan, for example, the loss of flooded rice fields is partly responsible for the decline in shorebird populations (Amano Reference Amano2009). Despite the potential importance of rice fields as buffer areas, fundamental aspects of the stopover ecology of shorebirds and other migratory waterbirds in rice fields remain unknown. The length of stay largely determines the migration strategy of a bird, and it is among the important parameters in models of optimal migration (e.g. Alerstam and Lindström Reference Alerstam, Lindström and Gwinner1990, Alerstam and Hedenström Reference Alerstam and Hedenström1998, Weber et al. Reference Weber, Ens and Houston1998). However, to the best of our knowledge, it is presently unknown whether migrating shorebirds have long lengths of stay (usually > 1.5–2 weeks; see Skagen and Knopf Reference Skagen and Knopf1994) in rice fields and depart suddenly at a threshold date or, in contrast, they have shorter lengths of stay (a few days; see Skagen and Knopf Reference Skagen and Knopf1994) and exhibit little site fidelity. The shorebird strategies of refuelling and patterns of stopover site use obtained in natural wetlands cannot be directly extrapolated to rice fields since, for example, in natural wetlands most shorebirds rely on macroinvertebrates as an early food source (see review by Skagen and Oman Reference Skagen and Oman1996), but in rice fields the diet of several shorebirds may be based on rice seeds left on the ground after harvest (e.g. Tréca Reference Tréca1994, Lourenço and Piersma Reference Lourenço and Piersma2008a, Santiago-Quesada et al. Reference Santiago-Quesada, Masero, Albano, Villegas and Sánchez-Guzmán2009). Accordingly, further research on staging duration and other aspects of the ecology of shorebirds migrating through rice fields is required to provide a basis for informed decision making when considering management options for shorebirds and other waterbirds.
The Black-tailed Godwit Limosa limosa is a long-distance migratory shorebird whose global population is estimated to be declining at such a rate that the species was recently listed as ‘Near Threatened’ in the IUCN Red List of Threatened Species (IUCN 2008). More specifically, the Western European population of Black-tailed Godwit L. l. limosa has declined dramatically, by more than 50% over the last 20 years (Stroud et al. Reference Stroud, Davidson, West, Scott, Haanstra, Thorup, Ganter and Delany2004, Gill et al. Reference Gill, Langston, Alves, Atkinson, Bocher, Vieira, Crockford, Gelinaud, Groen, Gunnarsson, Hayhow, Hooijmeijer, Kentie, Kleijn, Lourenço, Masero, Meunier, Potes, Roodbergen, Schekkerman, Wymenga and Piersma2007). Recent updates indicate a total population of 60,000 breeding pairs (Gill et al. Reference Gill, Langston, Alves, Atkinson, Bocher, Vieira, Crockford, Gelinaud, Groen, Gunnarsson, Hayhow, Hooijmeijer, Kentie, Kleijn, Lourenço, Masero, Meunier, Potes, Roodbergen, Schekkerman, Wymenga and Piersma2007), which mainly winter in West Africa south of the Sahara, as well as in Iberia (Spain and Portugal) (Stroud et al. Reference Stroud, Davidson, West, Scott, Haanstra, Thorup, Ganter and Delany2004, Jensen et al. Reference Jensen, Béchet and Wymenga2008). A key research priority for the declining Western European population of Black-tailed Godwit is to improve understanding of the location, timing, and duration of the use of staging sites in southern Europe and Africa (Gill et al. Reference Gill, Langston, Alves, Atkinson, Bocher, Vieira, Crockford, Gelinaud, Groen, Gunnarsson, Hayhow, Hooijmeijer, Kentie, Kleijn, Lourenço, Masero, Meunier, Potes, Roodbergen, Schekkerman, Wymenga and Piersma2007, Jensen and Perennou Reference Jensen and Perennou2007, Jensen et al. Reference Jensen, Béchet and Wymenga2008).
Black-tailed Godwits as well as many other shorebird species have traditionally been largely restricted to estuaries and large inland natural wetlands, but in recent decades rice fields have become increasingly important (e.g. Tréca Reference Tréca1994, Blanco et al. Reference Blanco, López-Lanús, Dias, Azpiroz and Rilla2006, Lourenço and Piersma Reference Lourenço and Piersma2008a). Spain’s rice production has doubled since the 1980s (FAO 2006) and as a result there are new potential staging sites for godwits and other shorebirds crossing Spain during spring migration. In Extremadura (south-west Spain) large expanses of rice fields have been created since the 1970s. Extremadura has rarely been monitored during this time even though several thousand Black-tailed Godwits have been recorded foraging in its rice fields (Prieta Reference Prieta2003, Sánchez-Guzmán et al. Reference Sánchez-Guzmán, Morán, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007). The goals of this study were twofold. Firstly, we estimated for the first time the length of stay of a shorebird species migrating through rice fields, using the Black-tailed Godwit as a model. Secondly, we investigated the international importance of Extremadura’s rice fields, an unknown and unprotected staging site, for the Black-tailed Godwit, and addressed the question of whether, as has been recently suggested (Stroud et al. Reference Stroud, Davidson, West, Scott, Haanstra, Thorup, Ganter and Delany2004, Sánchez-Guzmán et al. Reference Sánchez-Guzmán, Morán, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007, Lourenço and Piersma Reference Lourenço and Piersma2008b), the increasing numbers of Black-tailed Godwits in southern Europe reflect a population shift towards the northern part of the winter range.
Methods
Study area
Extremadura’s rice fields are located in the south-west of the Iberian Peninsula (39°01′N 5°58′W; Figure 1). In the 1960s and 1970s, large expanses of extensive drylands in the basin of the River Guadiana were gradually transformed into rice fields (Sánchez et al. Reference Sánchez, Sánchez, Fernández and Muñoz del Viejo1993), presently covering 30,000 ha. The climate is typically Mediterranean, and to regulate the hydrological system many dams have been constructed, preventing flooding and providing water for irrigation. Each year, the rice production cycle begins with preparation of the fields from late February-March until mid-April, followed by flooding and sowing from the second half of April until the end of May. Then germination, growth, flowering and ripening continue until September. Harvesting is in October-early November. From then until the end of December, farmers roll the rice straw left after harvest into the mud under flooded conditions, leaving large expanses of shallow water, which usually remain throughout the winter until this annual cycle begins again. A few pans remain accidentally flooded throughout the winter due to the proximity of some water reservoirs.

Figure 1. Map of the Iberian Peninsula showing study area (Extremadura rice fields) and the main stopover sites for Black-tailed Godwits in south-west Iberia.
Length of stay
Radio-tagging. Radio-tags (model TW-3, Biotrack, England) were glued to the back-feathers of adult godwits mist-netted during spring migration 2005, following the procedure of Warnock and Takekawa (Reference Warnock and Takekawa2003). Each transmitter (8.9 g) represented a small percentage of the tagged godwit’s body mass (< 4%), and it had a lifespan of more than 60 days. Concerning the tag effect on behaviour, during a parallel study of godwit foraging behaviour, we observed that radio-tagged godwits had similar behaviour to unmarked birds (pers. obs). In addition, three godwits still equipped with tags were observed a few months later attending nests in Western Europe, and we verified that tags were lost after the study period since eight birds were seen without their radio-tags in the following years in the study area.
To determine a minimum length of stay of radio-tagged godwits, we conducted daily ground surveys of the roosting sites using hand-held Yagi antennas to locate each radio-tagged bird. Every year, all Black-tailed Godwits staging in the study area roost at night in a few flooded pans (Masero et al. Reference Masero, Santiago-Quesada and Sánchez-Guzmán2008). This godwit roosting behaviour made easier the monitoring of radio-tagged birds at sunset, and they were detected daily throughout their staging period. If a signal was not detected at sunset, we considered that the bird migrated in the late afternoon. On the days subsequent to bird departure, we surveyed all potential Godwit habitat in the study area and surroundings to ensure that they had all departed from Extremadura.
Additionally, we estimated the length of stay for each batch of tagged birds assuming a probable arrival date (Table 1). In the study area, and probably in most godwit staging sites of south-west Iberia, there are two main periods of migration waves during spring (see Hortas Reference Hortas1997). The first one occurs in mid-late January, while the second wave occurs a few weeks later (early-mid February). Regular surveys (several times per week from early January onwards) of the roosting area (see above) allowed us to detect the migration waves. The first and second wave of migration probably arrived on 17 January and 7 February 2005, respectively (Table 1). We tagged godwits on 25 January (n = 10) and 7 February (n = 7) (Table 1) and assumed they arrived during the first and second wave of migration, respectively. In the latter case, to try to tag godwits that arrived during the second wave of migration, the selection criterion for radio-tagging was the extent of breeding plumage. Most birds arriving in mid-January still showed winter plumage feathers and they only acquired a few breeding plumage feathers (< 30%) during the next three weeks (pers. obs. during foraging behaviour studies). However, most birds arriving on 7 February showed a high percentage of breeding plumage feathers, so we only tagged godwits with more than 60% of breeding plumage. Finally, we also tagged godwits on 23 February (n = 7) (Table 1) that were assumed to belong to the first wave. All these birds showed signs of strong wear in their bills, which was probably caused by foraging for rice seeds for a long time (> 4 weeks) in relatively dry feeding grounds (pers. obs. from birds recaptured during the same winter). Also, godwit numbers in late February were similar to those in mid-January (see Figure 2), meaning that these godwits probably arrived in Extremadura during the first wave of migration (note that all godwits of the second wave of migration left the study area three weeks after being radio-tagged; see results).
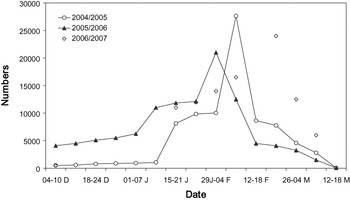
Figure 2. Black-tailed Godwit numbers in Extremadura rice fields. Data are weekly counts of the entire study area, except in winter 2006/2007 (counts in the roosting sites at sunset).
Table 1. Stay length of radio-tagged Black-tailed Godwits in Extremadura’s rice fields. C = date of capture and tagging. The assumed arrival date for each batch of tagged birds (see text for details), as well as Godwit numbers around this date, are showed in the body, and at the bottom of the table respectively.

* Godwits around 17 January. 15–16 January: 1,000–1,200 birds; 17 January: 8,125 birds; 18–19 January: 8,000–8,500 birds
** Godwits around 07 February. 5–6 February: 10,000–14,000 birds; 7 February: 27,643; 8–9 February: 25,000–27,000 birds
Capture-recapture models. Black-tailed Godwits were captured with mist-nets from mid-January to early March 2007 and individually marked with a combination of colour rings and lime flag (see codes in www.cr-birding.be). We searched for colour-ringed godwits in the study area (12 surveys; mean length of the intervals: 3.2 ± 2.9 days), and capture-resighting data were analysed with Cormarck-Jolly-Seber capture-recapture models (Schaub et al. Reference Schaub, Pradel, Jenni and Lebreton2001). We used the “recaptures only” model in MARK (White and Burnham Reference White and Burnham1999), and specified the unequal interval lengths in days. We examined 4 basic models of survival and resighting rates: (1) constant survival and resighting rates, (2) time-specific survival and constant resighting rates, (3) constant survival and time-specific resighting rates, and (4) time-specific survival and resighting rates. Goodness-of-fit test of the most general model was evaluated using the RELEASE-GOF utility implemented in program MARK and median ĉ. Akaike’s Information Criterion modified to account for small sample size (AICc) was used to select the most parsimonious model (Burnham and Anderson Reference Burnham and Anderson2002). For estimation of staging duration we used the mean life expectation equation derived by Brownie et al. (Reference Brownie, Anderson, Burnham and Robson1985): MLS = –1 ÷ ln (daily survival probability) using the survival rate of the model with the lowest AICc (e.g. Rice et al. Reference Rice, Collazo, Alldredge, Harrington and Lewis2007). The determination of staging duration with capture-recapture models consists of modelling the staging duration before capture by means of a recruitment analysis, and modelling of staging duration after capture, with ordinary survival analysis (Schaub et al. Reference Schaub, Pradel, Jenni and Lebreton2001). As we were releasing newly banded birds into the study area while collecting resightings, we could not calculate the recruitment probability, and therefore our estimate of length of stay represents only a minimum value corresponding to the staging time since banding.
Occurrence of Black-tailed Godwits in Extremadura
Weekly counts of godwits using Extremadura’s rice fields were carried out in December–March 2004–2006. The study area was divided into five sectors ranging from 4,000 to 7,000 ha, which were counted simultaneously in the morning. Due to some logistic constraints, it was impossible to carry out diurnal counts of the entire study area in 2007, so godwits were only counted in the roosting sites (see above) at sunset. We considered that Extremadura’s rice fields should be considered internationally important for Black-tailed Godwits if they regularly supported the 1% threshold proposed by Delany and Scott (Reference Delany and Scott2006).
Godwit numbers in coastal sites
We reviewed all the published reports and studies with estimations of godwit numbers carried out in coastal staging sites of south-west Spain and Portugal during spring migration. We selected the most important staging sites in south-west Spain, namely Doñana (including National and Natural Park, as well as surrounding rice fields; www.ebd.csic.es), Cadiz Bay Natural Park (GEAM 2002), and Odiel Marshes Natural Reserve (Sanchez et al. 2006) (Figure 1). In Portugal we selected the Tagus and Sado estuaries (Ramsar sites), Mondego estuary (Ramsar site), Ria Formosa Natural Park, Santo Andre Natural Reserve, Ria de Aveiro lagoon and Castro Marim (Figure 1). Most Portuguese counts were carried out by the Instituto da Conservação da Natureza e da Biodiversidade (ICNB) (Rufino Reference Rufino1988, Reference Rufino1989, Reference Rufino1990, Reference Rufino1991, Reference Rufino1992; Rufino and Costa Reference Rufino and Costa1993, Costa and Rufino Reference Costa and Rufino1994, Reference Costa and Rufino1996a, Reference Costa and Rufino1996b, Reference Costa and Rufino1997, ICNB unpubl. data).
Additionally, Doñana aerial counts were used to determine population trends (years 1998–2007). Doñana supports consistently large numbers of Black-tailed Godwits (Rendón et al. Reference Rendón, Green, Aguilera and Almaraz2008), and all aerial counts were carried out by the same experienced observer and following a similar method (see www.ebd.csic.es).
Statistical analysis
Length of stay was compared with Mann-Whitney U-test. Population trends were analysed using a generalised linear model with Poisson error distribution and log-link function. Data are presented as the mean ± SD.
Results
Length of stay
Radio-tagged birds. The minimum lengths of stay of Black-tailed Godwits tagged in late January, early February, and late February were 34.7 ± 1.7 d (n = 10), 14.4 ± 2.0 d (n = 7), and 8.3 ± 1.2 d (n = 7), respectively. According to the assumptions about arrival dates, the length of stay of godwits arriving in mid-January and early February would be 40.4 ± 4.5 d (n = 17) and 17.1 ± 4.6 d (n = 7), respectively, the latter being significantly shorter (Z = 3.7, P < 0.001).
Capture-recapture models. The goodness-of-fit test of the most general model fitted to the data set was not significant (![]() , P > 0.1), and no evidence of over-dispersion was found (median ĉ = 0.9). Survival rates measured over 11 intervals were best described by a model with constant survival and resighting probabilities (lowest AICc, Table 2). Daily survival probability based on the selected model was 0.955 ± 0.01, and capture probability was 0.11 ± 0.02. The extrapolated mean staging length was 21.7 days.
, P > 0.1), and no evidence of over-dispersion was found (median ĉ = 0.9). Survival rates measured over 11 intervals were best described by a model with constant survival and resighting probabilities (lowest AICc, Table 2). Daily survival probability based on the selected model was 0.955 ± 0.01, and capture probability was 0.11 ± 0.02. The extrapolated mean staging length was 21.7 days.
Table 2. Results of the survival analysis used to estimate stopover duration of Black-tailed Godwit during spring migration. Parameters: Φ = survival rate; p = resighting rate. Conditions: c = constant parameter; t = time-dependent parameter.

Godwit numbers
Large numbers of Black-tailed Godwits consistently used the Extremadura rice fields, peaking in February with an average number of 24,214 ± 3,327 individuals (Figure 2). Godwit numbers counted by ICNB in the main Portuguese sites during January are shown in Table 3.
Table 3. National counts of Black-tailed Godwits in Portugal during January. For the Tagus and Sado estuaries the percentage of coverage is shown in brackets. Counts were performed by ICNB (Instituto da Conservação da Natureza e da Biodiversidade). nc: no counts.

Populations trends in Doñana (Figure 3) showed a significant increase in godwits staying in southern Europe (December: χ 2 = 71,246, P < 0.01; January: χ 2 = 304, P < 0.01; February: χ 2 = 9,265, P < 0.01). In Cadiz Bay, the number of godwits in January was 1,706 birds, while in Odiel Marshes the number of godwits ranged between 0 and 6,684 birds over the course of the annual cycle (Sánchez et al. Reference Sánchez, Green and Castellanos2006) with a number of overwintering godwits close to 1,000 birds (M. Sánchez pers. comm.).
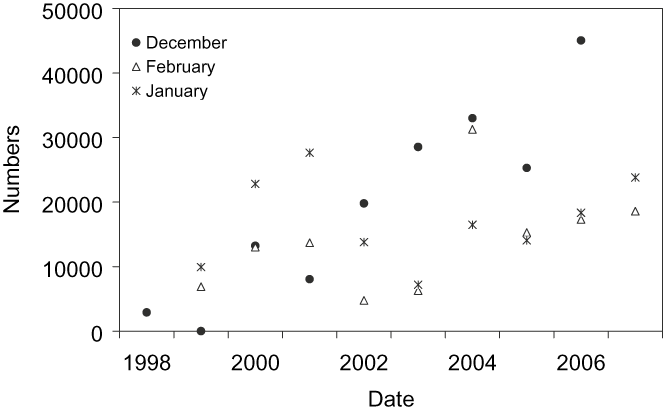
Figure 3. Population trends of Black-tailed Godwit in Doñana (south-west Iberia) during winter (December–February 1998–2007).
Discussion
We showed for the first time that large numbers of a migrating shorebird species such as Black-tailed Godwit had long lengths of stay in rice fields. Previous studies (Beintema and Drost Reference Beintema and Drost1986) suggested that godwits wintering in Africa perform a fast spring migration through southern Europe. However, this study showed that presently the situation is quite different, with thousands of Black-tailed Godwits staying longer than expected in rice fields, with Extremadura probably being a crucial staging site for most of them. In long-distance migratory birds, migration is a fundamental aspect of their life history and decisions made during migration from wintering to breeding grounds can have major effects on the fitness of individuals (e.g. Bêty et al. Reference Bêty, Giroux and Gauthier2004, Newton Reference Newton2006). It has been shown in Black-tailed Godwit L. l. islandica that timing of spring migration is likely to be a key parameter determining the subsequent breeding success (Gunnarsson et al. Reference Gunnarsson, Gill, Atkinson, Gelinaud, Potts, Croger, Ruth, Gudmundsson, Appleton and Sutherland2006). Thus, the longer length of stay in rice fields is probably critical in determining the subsequent breeding success for a considerable fraction of the global population of the nominate race of Black-tailed Godwit.
We documented the regular use of Extremadura’s rice fields by large numbers of migrating Black-tailed Godwits. Most (> 90%) Black-tailed Godwits staging in Extremadura during spring migration belong to the subspecies limosa (Masero et al. Reference Masero, Santiago-Quesada, Sánchez-Guzmán, Abad-Gómez and Albano2009), and Extremadura’s rice fields supported a minimum of 14% of the declining Western European population of Black-tailed Godwit. The increasing number of Black-tailed Godwits in Doñana and the appearance of a new staging site (Extremadura) probably reflects a shift of the Western European population towards the northern part of their wintering range, as has been recently suggested (Stroud et al. Reference Stroud, Davidson, West, Scott, Haanstra, Thorup, Ganter and Delany2004, Gill et al. Reference Gill, Langston, Alves, Atkinson, Bocher, Vieira, Crockford, Gelinaud, Groen, Gunnarsson, Hayhow, Hooijmeijer, Kentie, Kleijn, Lourenço, Masero, Meunier, Potes, Roodbergen, Schekkerman, Wymenga and Piersma2007, Sánchez-Guzmán et al. Reference Sánchez-Guzmán, Morán, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007, Lourenço and Piersma Reference Lourenço and Piersma2008b). Changes in the timing of arrival and departure may reflect adaptive responses of migratory birds to global climate change (e.g. Cotton Reference Cotton2003, Both et al. Reference Both, Bouwhuis, Lessells and Visser2006, Reif et al. Reference Reif, Vorísek, St’astný, Koschová and Bejcek2008), and the population shift towards the northern part of the winter range could be associated with these effects.
Conservation implications
Black-tailed Godwits staging in rice fields feed almost exclusively on rice seeds left on the ground after harvest (e.g. Kuijper et al. Reference Kuijper, Wymenga, van der Kamp and Tanger2006, Lourenço and Piersma Reference Lourenço and Piersma2008a, pers. obs.), so this habitat may be certainly important as an accessible and predictable food resource. Godwits feed efficiently on rice seeds (Santiago-Quesada et al. Reference Santiago-Quesada, Masero, Albano, Villegas and Sánchez-Guzmán2009), and their intake rates (1.3 kJ·min-1 in mid-February; Masero et al. Reference Masero, Santiago-Quesada, Sánchez-Guzmán, Albano, Abad-Gómez, Villegas, Corbacho and Costillo2007) feeding on these seeds are as high as those obtained in natural wetlands (1.2 kJ·min-1 on intertidal mudflats; Moreira Reference Moreira1994). The long lengths of stay in the rice fields, together with these aspects of the Black-tailed Godwit’s feeding ecology, suggest that rice fields are suitable staging habitats for migrating godwits, and therefore, they could play an important role as buffer habitats against the loss or degradation of natural wetlands. For example, the degradation of coastal wetlands such as Merja Zerga and the Loukos Delta in Morocco has caused a severe decline in Godwit numbers in north-west Africa during the last 30 years (Jensen et al. Reference Jensen, Béchet and Wymenga2008). The increasing area of rice fields in Spain and Portugal (200–500 km away to the north) during that same time period may have contributed to buffering the degradation of these spring staging sites in north-west Africa. Other migratory waterbirds using rice fields such as geese, ducks or cranes also feed on rice seeds (e.g. Gammonley and Fredrickson Reference Gammonley and Fredrickson1995, Sánchez-Guzmán et al. Reference Sánchez-Guzmán, Morán, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007, Amano Reference Amano2009), and rice fields could provide suitable staging sites for these seed-eating species.
The most recent conservation recommendations for the Western European population of Black-tailed Godwit (Gill et al. Reference Gill, Langston, Alves, Atkinson, Bocher, Vieira, Crockford, Gelinaud, Groen, Gunnarsson, Hayhow, Hooijmeijer, Kentie, Kleijn, Lourenço, Masero, Meunier, Potes, Roodbergen, Schekkerman, Wymenga and Piersma2007, Jensen and Perennou Reference Jensen and Perennou2007, Jensen et al. Reference Jensen, Béchet and Wymenga2008) include the improved protection of key wetland habitats in Iberia and Africa, either through maintenance of rice fields or restoration of wetlands, and the designation of more sites under relevant legislation and conventions (for example, EU Birds Directive and Habitats Directive or Ramsar Convention). Accordingly, we strongly suggest the inclusion of Extremadura’s rice fields as a special protection area for birds (SPA) under the European Union Directive on the conservation of wild birds (79/409/CEE). Considering the large number of solar energy plants and wind farms being proposed in Extremadura (Cardalliaguet Reference Cardalliaguet2008), the inclusion of these rice fields as an SPA would avoid, or at least reduce greatly, the possibility that these infrastructures are built in rice fields.
The rice fields surrounding the Tagus and Sado estuaries are of prime importance for non-breeding Black-tailed Godwits, and they have currently no special protection status (Kuijper et al. Reference Kuijper, Wymenga, van der Kamp and Tanger2006, Lourenço and Piersma Reference Lourenço and Piersma2008a). The ICNB counts for godwits in January 2006 for the Tagus and Sado estuaries contrast with the high number of godwits estimated during simultaneous counts in late February 2006 in the two estuaries (44,700 birds; see Kuijper et al. Reference Kuijper, Wymenga, van der Kamp and Tanger2006). Probably this great difference is because the national counts of waterbirds by ICNB underestimated Black-tailed Godwits using rice fields (pers. obs.). The new Lisbon airport will be built on the south side of the Tagus river, close to the rice fields and between both estuaries (LNEC 2008), which may lead to disturbance and loss of habitat (Jensen et al. Reference Jensen, Béchet and Wymenga2008). The degradation of this key staging-wintering site will probably increase the importance of the Extremadura rice fields (< 300 km away; see Figure 1) for godwits, which reinforces the need to protect the foraging and resting areas of Black-tailed Godwits in Extremadura rice fields.
Lastly, rice fields worldwide may be managed with the use of many herbicides, insecticides, and other agrochemicals, and when these products are applied to these agro-ecosystems might result in a serious threat to waterbirds (Blanco et al. Reference Blanco, López-Lanús, Dias, Azpiroz and Rilla2006). The current disproportionately heavy reliance of Black-tailed Godwits on rice fields for their food supply along the flyway may pose a serious threat if godwits are significantly exposed to these agrochemicals.
Acknowledgements
We are very grateful to all GIC members for their useful help during ringing activities and godwit counts in Extremadura, especially to F. Sanz and N. Albano. M. Mañez, coordinator of the team ‘Seguimiento de Procesos Naturales’ of Doñana biological station, provided useful comments about the counts in Doñana. The project PRI 2PR03A010 provided financial support for this study. F. Santiago was given a grant by the Spanish Ministry of Education and Science.








