The metabolic syndrome (MetS) is a clinical entity of substantial heterogeneity, represented by the combination of obesity (especially central obesity), insulin resistance and impaired glucose tolerance, atherogenic dyslipidaemia (high levels of TAG and low levels of HDL-cholesterol (HDL-C)) and hypertension(Reference Eckel, Grundy and Zimmet1). This cluster of factors co-occurs to a greater degree than expected by chance alone, affecting approximately 10–25 % of adults worldwide. The International Diabetes Federation states that this syndrome is driving the twin global epidemics of type 2 diabetes and CVD(2). People with the MetS have three times more risk of suffering from a heart attack or stroke – and twice the risk of dying from such an event – compared with people without the syndrome(2).
The dietary treatment of the MetS should address the different cornerstones presented in this syndrome(Reference Zulet, Bondia-Pons and Abete3). Therefore, since most individuals with the MetS are overweight, dietary treatment should be primarily focused on weight reduction. Moreover, the Mediterranean diet(Reference Kesse-Guyot, Ahluwalia and Lassale4), n-3 fatty acids (FA)(Reference Lopez-Huertas5, Reference Navas-Carretero, Perez-Granados and Schoppen6), total antioxidant capacity(Reference Bahadoran, Golzarand and Mirmiran7, Reference de la Iglesia, Lopez-Legarrea and Celada8) and meal frequency increment(9) are dietary patterns that have been reported to show positive effects on the MetS. Furthermore, the type and percentage of carbohydrates (CHO), glycaemic index or glycaemic load (GL), and dietary fibre content are some of the most relevant aspects related to insulin resistance and impaired glucose tolerance(Reference Salmeron, Manson and Stampfer10–Reference Hermsdorff, Barbosa and Volp12), which are important co-morbidities of the MetS.
Many subjects can follow a prescribed diet for a few months, but most people have difficulty in maintaining the acquired habits over the long term(Reference Ebbeling, Swain and Feldman13). In this context, although many studies have separately examined the impact of different dietary factors, mainly during nutritional interventions, none of them has apparently considered all of them integrated within a unique dietetic plan to ameliorate the co-morbidities of the MetS during an autonomous period after a nutritional-learning period. Therefore, the RESMENA-S (MEtabolic Syndrome REduction in NAvarra-Spain) study (www.clinicaltrials.gov; NCT01087086)(Reference Zulet, Bondia-Pons and Abete3) aimed to evaluate the effect of a novel dietary strategy involving together all these dietary elements and to compare it with the American Heart Association (AHA) guidelines, which are considered a reference for dietary strategies, in order to improve the features of the MetS and maintain them over the long term(Reference Grundy, Cleeman and Daniels14).
Methods
Subjects
A total of ninety-three Caucasian adults (fifty-two men and forty-one women) with a BMI of 36·11 (se 0·5) kg/m2 aged 49 (se 1) years diagnosed with the MetS according to the International Diabetes Federation criteria(Reference Alberti, Zimmet and Shaw15) were recruited for the intervention trial. Exclusion criteria were the presence of psychiatric or psychological disorders, difficulty in changing dietary habits, eating disorders, body-weight changes during the last 3 months, chronic diseases related to energy or nutrient metabolism, pursuit of special diets, or food allergies or intolerances, as has been described elsewhere(Reference Zulet, Bondia-Pons and Abete3). During the 6-month study, twenty-six volunteers dropped out: nine during the first intervention period and seventeen during the autonomous period. Therefore, sixty-seven individuals completed the study and were included in the final statistical analysis (Fig. 1).
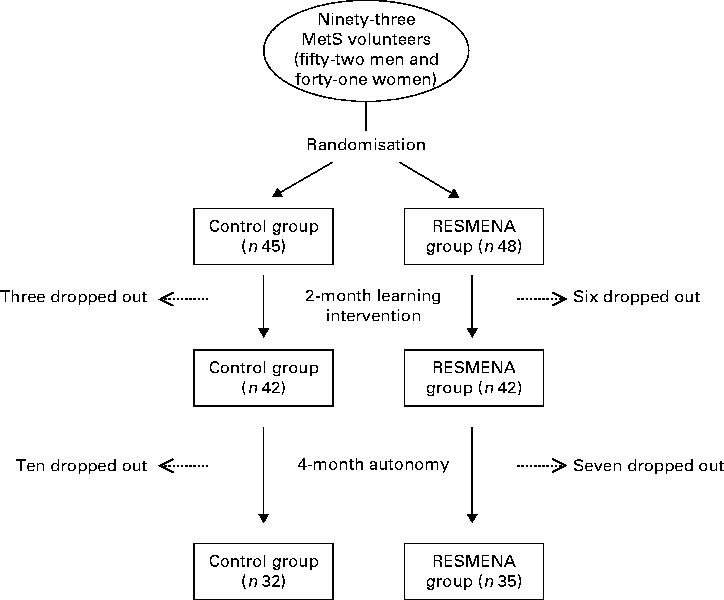
Fig. 1 Flow diagram of the participants of the study. MetS, metabolic syndrome; RESMENA, MEtabolic Syndrome REduction in NAvarra.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the University of Navarra (065/2009). Written informed consent to participate in the intervention trial (www.clinicaltrials.gov; NCT01087086) was obtained from all the subjects.
Study protocol
The study was designed as a randomised, controlled trial to compare the effects of two dietary strategies on the co-morbidities of the MetS during 4 months of autonomy, after 2 months of nutritional advice. The participants were randomly assigned to consume the control or the experimental diet (Control and RESMENA groups, respectively). The study lasted for a total of 6 months divided into two sequential periods: an 8-week nutritional-learning intervention period, during which a nutritional assessment was carried out for the participants every 15 d(Reference Lopez-Legarrea, de la Iglesia and Abete16), and a 4-month self-control period, during which the participants followed the previously acquired dietary habits. The present article focuses on the self-control period information.
The CONSORT (CONsolidated Standards Of Reporting Trials) 2010 guidelines(Reference Moher, Hopewell and Schulz17) were followed by taking into account the design of the present study, two-group longitudinal intervention, except for blinding. The participants were asked to maintain their normal physical activity during the study, which was checked using a 24 h physical activity questionnaire(Reference Navas-Carretero, Perez-Granados and Schoppen18) at the beginning and at the end of both the intervention and autonomous periods.
During the 2-month nutritional-learning intervention period, the participants visited the Metabolic Unit at the University of Navarra every 2 weeks for anthropometric measurements and bioimpedance body composition analysis carried out by trained nutritionists following validated protocols(Reference Zulet, Bondia-Pons and Abete3). Moreover, the nutritionists asked the participants about the feelings and sensations that they were experiencing with the new diet to determine their well-being. Finally, different advice was given to the participants in each situation as well as recipes, general information about food and the importance of dietary adherence. Before and after the 4-month-long self-control period, body composition was measured using dual-energy X-ray absorptiometry, and fasting blood and 24 h urine samples were collected in addition to the anthropometric and bioimpedance assessments.
Diets
In the trial, two energy-restricted diets ( − 30 % energy of the studied requirements) were prescribed and compared. The Control diet was based on the AHA guidelines(Reference Grundy, Cleeman and Daniels14), including three to five meals/d, a macronutrient distribution of 55 % total energy value from CHO, 15 % from proteins and 30 % from lipids, a healthy FA profile and a cholesterol content of less than 300 mg/d. The RESMENA diet was characterised by a higher meal frequency, consisting of seven meals/d (including breakfast, lunch, dinner and two snacks in the morning and two snacks in the afternoon), and by a different macronutrient distribution, 40 % total energy value from CHO, 30 % from proteins and 30 % from lipids(Reference Zulet, Bondia-Pons and Abete3). Furthermore, this pattern tried to reinforce high n-3 PUFA and high natural antioxidant food consumption and promoted low GL CHO intake. It also maintained a healthy FA profile and a cholesterol content of less than 300 mg/d as the Control diet.
The RESMENA participants were prescribed a 7 d menu plan, while the Control group was prescribed a previously described(Reference Abete, Parra and De Morentin19) food exchange system plan. A 48 h weighed food record was collected at the beginning and at the end of both the nutritional-learning and autonomous periods in order to assess the participants' adherence to the prescribed nutritional patterns. The composition of the designed diets, as well as the different dietary records, was analysed using the DIAL (Alce Ingenieria) software(20).
The sum of EPA and DHA (EPA+DHA) intake obtained using the DIAL program(20) was used to estimate n-3 PUFA consumption. For the calculation of the healthy eating index score, the DIAL program gives different values ranging from 0 to 100 taking into account the daily servings of cereals, vegetables, fruits, dairy products and meat and the percentage of energy provided by total and saturated fats, the amount of cholesterol and Na per d and the variety of diets expressed by the number of different foods consumed during each of the 3 d. The final score is classified into five categories: >80 points indicate an ‘excellent diet’; 71–80 points, a ‘very good diet’; 61–70 points, a ‘good diet’; 51–60 points, an ‘acceptable diet’; a final score between 0 and 50 points indicates an ‘inadequate diet’(20). Total antioxidant capacity was calculated using the Carlsen et al. (Reference Carlsen, Halvorsen and Holte21) data, considering raw or cooked preparations(Reference Carlsen, Halvorsen and Holte21, Reference Puchau, Zulet and de Echavarri22). Finally, the GL was obtained from the international updated website database based in the Human Nutrition Unit, School of Molecular Biosciences from the University of Sydney(23).
Clinical and biochemical assessments
Anthropometric measurements were taken in fasting conditions as described previously(Reference Pérez, Martínez and de Morentín24). Body weight was assessed to the nearest 0·1 kg using bioimpedance (TANITA SC-330; Tanita Corporation) equipment. BMI was calculated as the body weight divided by the squared height (kg/m2). Waist and hip circumferences were measured using a commercial measure tape following validated protocols as described previously(Reference Zulet, Bondia-Pons and Abete3). Total body fat mass, android fat mass, lean mass and fat-free mass were determined using dual-energy X-ray absorptiometry (Lunar iDXA™, software version 6.0; GE Healthcare). Systolic blood pressure and diastolic blood pressure were assessed using a digital monitor (Medisana AG, MTC) following the WHO criteria.
Serum total cholesterol, HDL-C, TAG, NEFA, glucose, homocysteine, uric acid, total protein, creatinine, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were measured using an autoanalyser Pentra C-200 (Horiba ABX) with specific kits. Insulin concentrations were determined using an ELISA kit (Mercodia) in a Triturus autoanalyser (Grifols SA). Insulin resistance was estimated using the homeostasis model assessment index (HOMA-IR), which was calculated using the following formula(Reference Aller, Abete and Astrup25):
LDL-cholesterol (LDL-C) levels were calculated using the Friedewald formula(Reference Friedewald, Levy and Fredrickson26):
ApoB values were measured with a specific kit (Tina-quant Apolipoprotein B version 2) using a Roche/Hitachi autoanalyser (Model 904 Modular) (Roche Diagnostics).
Statistical analyses
Based upon previous studies(Reference Konig, Deibert and Frey27, Reference Katcher, Legro and Kunselman28), the sample size (forty per group) was calculated to detect a difference of 4·3 cm with a variation of ± 6·8 cm between the groups in the reduction of waist circumference with a P< 0·05 and a power of 80 %. The estimated dropout rate was 25 %, and the initial number of the recruited subjects was 109. However, twelve subjects did not present the MetS according to the International Diabetes Federation criteria when the study began and another four volunteers decided not to undergo the dietary treatment after signing the written informed consent. Therefore, the intervention trial was started with ninety-three subjects presenting the MetS (n 45 Control group and n 48 RESMENA group).
Mean values and standard errors are reported for the measured variables. Differences between the beginning and the end of the autonomous period were analysed by a paired t test. Differences between both the groups (RESMENA v. Control) were assessed using a multivariate ANOVA adjusted for sex and age.
A linear regression analysis was used to assess the potential relationships and associations among the different components of the diet and the variations in anthropometric and biochemical parameters. Comparisons between median body-weight loss and median dietary fibre intake categories were made using a multivariate ANOVA adjusted for sex and age. Analyses were carried out using the SPSS 15.1 software for Windows (SPSS, Inc.). Values of P< 0·05 were considered to be statistically significant.
Results
Dietary records
As expected, the dietary records after the self-control period revealed that the RESMENA group had higher protein intake (P= 0·001), PUFA levels (P= 0·017), total antioxidant capacity (P= 0·043) and meal frequency intake (P< 0·001) than the Control group, but both the groups consumed the same amount of energy. However, no significant differences were found regarding fibre, GL and EPA+DHA intake. Furthermore, the quality score based on the healthy eating index values indicated no differences between the dietary groups (Table 1).
Table 1 Comparison of Control and MEtabolic Syndrome REduction in NAvarra (RESMENA) dietary records after the self-control period (Mean values with their standard errors)
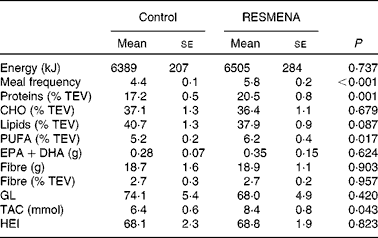
TEV, total energy value; CHO, carbohydrates; GL, glycaemic load; TAC, total antioxidant capacity; HEI, healthy eating index.
Anthropometric and biochemical parameters
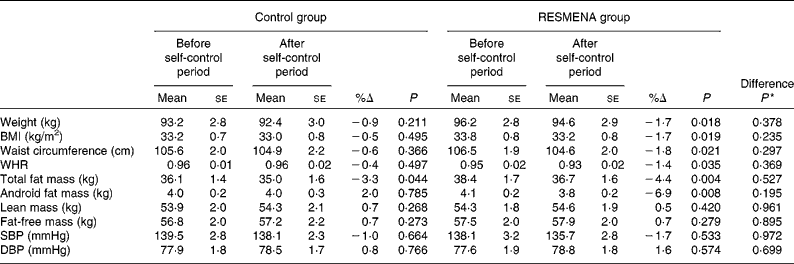
After 4 months of autonomy, both the Control and RESMENA groups exhibited a significant decrease in total fat mass ( − 3·3 %; P= 0·044 and − 4·4 %; P= 0·004, respectively). However, only the RESMENA group exhibited a significant decrease in body weight (P= 0·018), BMI (P= 0·019), waist circumference (P= 0·021), waist:hip ratio (P= 0·035) and android fat mass (P= 0·008). No significant differences were found in either of the experimental groups concerning lean mass, fat-free mass and blood pressure (Table 2).
Table 2 Changes in anthropometric, body composition and blood pressure parameters in both the experimental groups (Control and MEtabolic Syndrome REduction in NAvarra (RESMENA)) after a 4-month self-control period (Mean values with their standard errors)

%Δ, percentage of change; WHR, waist:hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Comparison between the dietary groups.
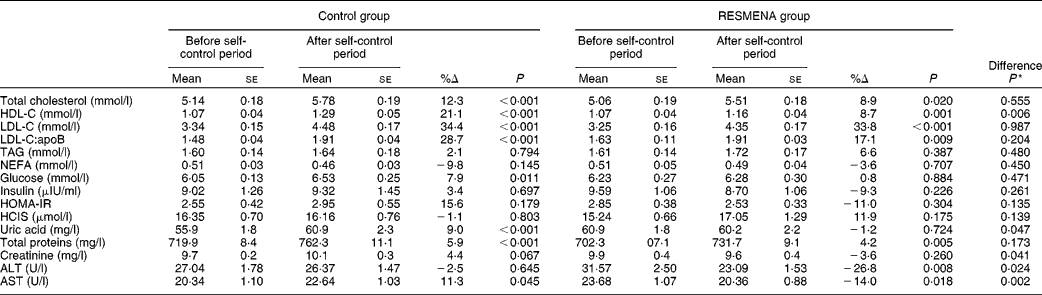
At the end of the study, both the Control and RESMENA groups had significantly increased total cholesterol levels (P< 0·001 and P= 0·020, respectively), LDL-C levels (P< 0·001), LDL-C:apoB ratio (P< 0·001 and P= 0·009, respectively) and HDL-C levels (P< 0·001 and P= 0·001, respectively) (Table 3), as well as total proteins levels (P< 0·001 and P= 0·005). Interestingly, only the Control group showed a significant increase in glucose (P= 0·011), AST (P= 0·045) and uric acid (P< 0·001) concentrations. However, the RESMENA group had significantly decreased concentrations of both transaminases, ALT (P= 0·008) and AST (P= 0·018). Significant differences were found between the groups concerning changes in uric acid, ALT and AST concentrations (P= 0·047, 0·024 and 0·002, respectively). Finally, creatinine concentrations were increased in the Control group and decreased in the RESMENA group, resulting in significant differences between them (P= 0·041).
Table 3 Changes in biochemical parameters in both the experimental groups (Control and MEtabolic Syndrome REduction in NAvarra (RESMENA)) after a 4-month self-control period (Mean values with their standard errors)

%Δ, percentage of change; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; HCIS, homocysteine; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
* Comparison between the dietary groups.
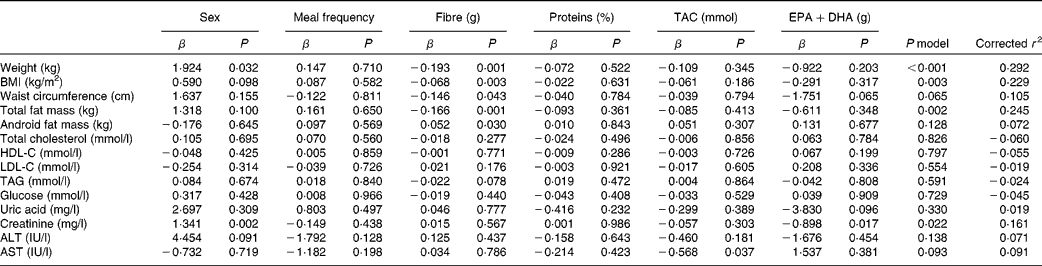
A linear regression was modelled to evaluate individual nutritional factors potentially responsible for the variations in anthropometric and biochemical parameters (Table 4). Total antioxidant capacity values seemed to influence AST concentrations (P= 0·037), EPA+DHA intake played a role in creatinine depletion (P= 0·017) and sex appeared to influence body-weight (P= 0·032) and creatinine concentration (P= 0·002) changes. However, the main influential dietary factor was the dietary fibre, exhibiting a positive potential effect on body composition parameters, by reducing body weight (P= 0·001), BMI (P= 0·003), waist circumference (P= 0·043), total fat mass (P= 0·001) and android fat mass (P= 0·030). This resulted in a model P value significant for body weight (P< 0·001), BMI (P= 0·003) and total fat mass (P= 0·002), independently of the dietary group. In this context, the population was categorised, considering the median value fibre consumption ( ≤ 18·3 and >18·3 g), and body composition parameter changes were compared between the groups (Table 5). Subjects consuming >18·3 g fibre exhibited a significant decrease in body weight (P= 0·001), BMI (P= 0·006), waist circumference (P= 0·010), total fat mass (P= 0·002) and android fat mass (P= 0·001) and a trend towards significance to reduce the waist:hip ratio (P= 0·057). Significant differences were found between the groups concerning weight (P= 0·008), BMI (P= 0·035) and android fat mass (P= 0·008).
Table 4 Results of regression analyses, considering the change in anthropometric and biochemical parameters as the dependent variable and different dietary components evaluated as the independent ones

TAC, total antioxidant capacity; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 5 Effects of fibre consumption on anthropometric and body composition parameters (Mean values with their standard errors)
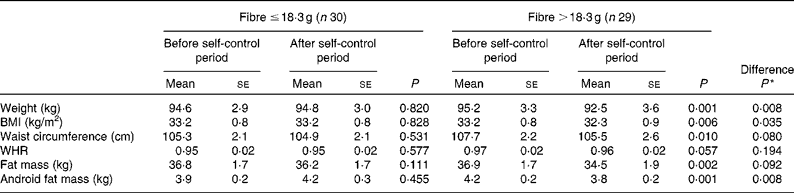
WHR, waist:hip ratio.
* Comparison between the dietary groups.
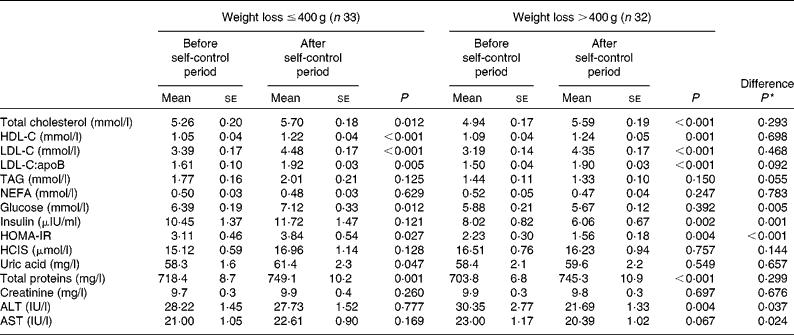
As no nutritional factors potentially responsible for the variations in blood biochemical parameters were found, the influence of body-weight reduction on them was analysed separately. Therefore, the population was categorised by body-weight loss median, ≤ 0·400 kg (non-responders to weight loss; NR) and >0·400 kg (responders to weight loss; R), as cut-offs and biochemical parameters were compared between the groups (Table 6). Both the experimental sets exhibited a significant increase in total cholesterol levels (NR: P= 0·012 and R: P< 0·001), LDL-C levels (P< 0·001), HDL-C levels (NR: P< 0·001 and R: P= 0·001), LDL-C:apoB ratio (NR: P= 0·005 and R: P< 0·001) and total protein levels (NR: P= 0·001 and R: P< 0·001) without significant differences between the groups. However, subjects who had lost >0·400 kg presented, as expected, a higher decrease in glucose (P= 0·005), insulin (P= 0·001), HOMA-IR (P< 0·001), ALT (P= 0·037) and AST (P= 0·024) levels compared with subjects who had lost ≤ 0·400 kg of body weight regardless of the dietary group. However, no significant differences were found between both the experimental sets concerning the remaining biochemical measurements.
Table 6 Effects of body-weight reduction on biochemical parameters (Mean values with their standard errors)

HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; HCIS, homocysteine; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
* Comparison between the dietary groups.
Discussion
As the prevalence of the MetS is reaching epidemic rates, and since the maintenance of acquired healthy dietary habits is still a pending subject for clinical nutrition research, the present study reports a new dietary strategy to combat the co-morbidities of the MetS during a self-control period after a nutritional-learning intervention period.
Under the same energy restriction ( − 30 % total energy value), this new dietary strategy (RESMENA diet) showed more effectiveness by continuing to maintain/improve some anthropometric measurements than the diet based on the AHA guidelines (Control diet), although statistical significance between the dietary groups was not reached. These results are consistent with those of other studies concerning moderately high-protein content diets(Reference Larsen, Dalskov and van Baak29). The positive effects of the RESMENA diet were specifically highlighted by the loss of body weight at the expense of android fat mass and reduction of waist circumference, waist:hip ratio and BMI. Since it has been demonstrated that central obesity is associated with increased risks of diabetes mellitus, hypertension, CVD(Reference Hermsdorff, Zulet and Puchau30, Reference Isomaa, Almgren and Tuomi31) and MetS manifestations in general(Reference Isomaa, Almgren and Tuomi31), the effects of the RESMENA dietary strategy described herein should be considered in future nutritional intervention research.
Unexpectedly, with regard to biochemical values independently of the dietary treatment or absolute weight loss, subjects had increased total cholesterol and LDL-C levels, even when the diets were based on a healthy FA profile, especially the RESMENA diet, where n-3 FA intake was also reinforced. These results are in agreement with different systematic reviews that did not report the clear effects of hypoenergetic diets on LDL-C depletion(Reference Clifton32, Reference Souto-Gallardo Mde, Bacardi Gascon and Jimenez Cruz33) and with studies that stated that in some cases LDL-C values may increase despite weight loss(Reference Clifton and Keogh34). Moreover, the LDL:apoB ratio that predicts the LDL-C particle size(Reference Tani, Saito and Anazawa35) increased significantly in all the participants irrespective of the dietary treatment or weight loss reduction, which indicates an increase in LDL particle size and a lower risk of ischaemic cardiac events(Reference Tzotzas, Evangelou and Kiortsis36). HDL-C concentrations were increased in both the dietary groups, but the increment was higher in the Control group than in the RESMENA group. This outcome seems logical, since the Control diet is specifically based on the AHA guidelines, which are mainly focused on CVD care and, therefore, on lipid profile management.
Since insulin resistance has been proposed to be related to the development of the MetS(Reference Reaven37), one of the main aims of the dietary treatment of the MetS is the improvement of related parameters such as serum glucose concentrations and HOMA-IR. In the present study, in participants following the Control diet, Serum glucose values significantly increased; HOMA-IR also increased although not significantly. This could be explained by the fact that central obesity usually precedes insulin resistance, being a risk factor for the development of type 2 diabetes(Reference Reaven37, 38), and the RESMENA group was the only dietary group that exhibited a significant decrease in android fat mass. Furthermore, these results are in accordance with those of other investigations that have shown a positive role of low-CHO diets in insulin resistance syndromes(Reference Abete, Astrup and Martinez39).
Although uric acid has been proposed to be able to function as an antioxidant(Reference Ames, Cathcart and Schwiers40), some studies have reported an association of the increase in the end product levels of this purine with gout and uric acid kidney stones(Reference Johnson, Lanaspa and Gaucher41) and, more importantly, with adverse effects in obesity(Reference Masuo, Kawaguchi and Mikami42), diabetes(Reference Kodama, Saito and Yachi43) hypertension(Reference Sundstrom, Sullivan and D'Agostino44), CVD(Reference Jeemon and Prabhakaran45), fatty liver(Reference Lonardo, Loria and Leonardi46) and with the prevalence of the MetS in general(Reference Gao, Qi and Qiao47). Based on the results of the present study, the RESMENA diet can be considered as a better option for patients with high uric acid concentrations than the diet based on the AHA guidelines, as uric acid levels were significantly increased in the Control group, while they remained almost unchanged in the RESMENA group.
High serum creatinine levels are well known as an index of renal function(Reference Jabary, Martin and Munoz48) and are associated with obesity(Reference Agnani, Vachharajani and Gupta49). In the present study, it was found that the end product levels of serum creatinine were increased in the Control group and decreased in the RESMENA group, despite a higher protein intake.
Transaminases, mainly ALT, are markers of hepatocyte injury that have been reported to show a correlation with insulin resistance and later development of diabetes, liver lipid content and histological features of non-alcoholic fatty liver disease, which is increasingly being regarded as the main hepatic manifestation of the MetS(Reference Hamaguchi, Kojima and Takeda50, Reference Larson-Meyer, Newcomer and Heilbronn51). ALT transaminase has also been reported to be correlated with the levels of C-reactive protein, a marker of low-grade inflammation associated with the MetS(Reference Kerner, Avizohar and Sella52). Dietary weight loss has been reported to be associated with a depletion of these liver enzymes(Reference Straznicky, Lambert and Grima53), but irrespective of the type of diet(Reference Rodriguez-Hernandez, Cervantes-Huerta and Rodriguez-Moran54). However, in the present study, the RESMENA diet showed better benefits in the treatment of participants with the MetS with regard to these markers, as the levels of both ALT and AST transaminases were significantly decreased in the RESMENA group, while AST concentrations were significantly increased in the Control group.
Several health benefits of dietary fibre have been described, including the prevention and mitigation of type 2 diabetes mellitus, CVD and colon cancer by reducing the risk of hyperlipidaemia, hypercholesterolaemia and hyperglycaemia(Reference Kaczmarczyk, Miller and Freund55). Moreover, diverse clinical studies have examined the role of this dietary component in body-weight reduction, and a strong relationship has been established(Reference Du, van der A and Boshuizen56, Reference Kristensen, Toubro and Jensen57). Different mechanisms by which dietary fibre intake can influence body weight have been proposed. Recently, the role of dietary fibre in gut microbiota in the development of obesity and its associated co-morbidities has come to the forefront(Reference Parnell and Reimer58). Data suggest that fibre can reduce the risk of obesity by promoting satiety and reducing energy intake(Reference Brownawell, Caers and Gibson59, Reference Burton-Freeman60), and numerous studies have been carried out to determine the effects of dietary fibre on satiety(Reference Pereira and Ludwig61–Reference Wanders, van den Borne and de Graaf63). Many different mechanisms have been suggested, such as a lower metabolisable energy content of fibre than of other nutrients(Reference Livesey64), a relatively constant meal intake volume(Reference Poppitt and Prentice65), a decreased total energy intake by consuming foods rich in fibre, and the increased chewing activity or oral exposure time to foods after a high dietary fibre intake, which may result in earlier satiation(Reference Zijlstra, de Wijk and Mars66). Furthermore, fibre can slow down gastric emptying and consequently increase stomach distension, which also leads to satiation(Reference de Graaf, Blom and Smeets67). In the present study, when the impact of this dietary component on anthropometric and body composition measurements was studied, the results obtained are in agreement with those of the studies mentioned above, as fibre consumption showed positive effects on the improvement of these measurements in individuals affected by the MetS.
As most individuals with the MetS are overweight, the dietary treatment of this syndrome might be primarily focused on body-weight and abdominal fat reduction. Moreover, obesity is considered to be the main cause of insulin resistance and type 2 diabetes, important co-morbidities of the MetS(38). Therefore, body-weight reduction should also be a main target for improving related parameters such as glucose and HOMA-IR. Serum TAG levels, correlated with insulin sensitivity(Reference Lemieux, Pascot and Couillard68), have also been reported to be associated with the MetS(Reference Crepaldi69) as the International Diabetes Federation uses them for the diagnosis of the MetS(Reference Eckel, Grundy and Zimmet1). Furthermore, since high ALT and AST concentrations are correlated with non-alcoholic fatty liver disease and as obesity is frequently associated with non-alcoholic fatty liver disease, it is clear that body-weight loss can involve a reduction in transaminase levels. In the present study, when participants were categorised by the weight loss median as a cut-off, it could be noted that participants who had lost more body weight exhibited a significantly higher decrease in TAG, glucose, insulin, HOMA-IR, ALT and AST levels compared with individuals who had lost less body weight. Only participants consuming the RESMENA diet significantly lost body weight and yielded the best results regarding glucose, HOMA-IR, ALT and AST. Nutritional factors potentially responsible for the variations in blood biochemical parameters were not found. Therefore, it can be hypothesised that body-weight loss and waist circumference reduction were the main factors contributing to the improvement of these biochemical parameters in the RESMENA group.
The dietary records collected after the self-control period showed the expected differences between the designed dietary pattern compositions, except for fibre, GL and EPA+DHA intake. This could be explained by the fact that although the RESMENA diet was specially enriched in high-fibre food, the Control group consumed 15 % total energy value from CHO more than the RESMENA group did. Moreover, the dietary records analysed in the present study were collected at the end of the study. Therefore, the participants might not have completed them with the same thoroughness as for the former dietary records, which were provided before and after the nutritional-learning period. In addition, as both diets were designed following a healthy pattern, it is logical that the quality score based on the healthy eating index indicated similar values between the dietary groups.
Conclusion
In summary, the present study suggests a new dietary treatment, the so-called RESMENA dietary pattern, to combat the MetS during an autonomous period. This dietary pattern showed more beneficial effects than a diet based on the AHA guidelines concerning body composition, especially central obesity, and regarding several biochemical parameters, by reducing transaminase levels and maintaining uric acid and serum glucose concentrations. Therefore, the RESMENA diet might be a good option as a long-term dietary treatment of the co-morbidities of the MetS.
Acknowledgements
The authors thank the participants of the present study and Blanca E. Martínez de Morentín, physician, Salomé Pérez, nurse, and Verónica Ciaurriz, technician, for their excellent technical assistance at the Metabolic Unit of the University of Navarra.
The present study was supported by the Health Department of the Government of Navarra (48/2009) and the Línea Especial about Nutrition, Obesity and Health (University of Navarra LE/97). The support from CIBERobn and RETICS schemes is gratefully acknowledged. Carlos III Health Institute provided a predoctoral grant to R.I (no. FI10/00587). None of the funding institutions and frameworks had a role in the design and analysis of the study or in writing of the article.
The authors' contributions were as follows: R. I. contributed to the design and fieldwork, data collection, analysis and writing of the manuscript; P. L.-L. and I. A. were involved in the design and fieldwork; I. B.-P. contributed to sample collection, interpretation and critical reading of the final version of the manuscript; S. N.-C. and L. F. were involved in the recruitment and selection of participants; M. A. Z. was responsible for general coordination, follow-up, design and financial management; J. A. M., project co-leader, was responsible for follow-up, design, financial management and editing of the manuscript. All the authors actively participated in the manuscript preparation as well as read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.