Inhibitory control, the cognitive ability of controlling attention and behavior to suppress a dominant response in favor of a subdominant response, plays an important role in children’s cognitive and socioemotional development, including the development of psychopathology (e.g., Olson et al., Reference Olson, Tardif, Miller, Felt, Grabell, Kessler, Wang, Karasawa and Hirabayashi2011; Yavuz et al., Reference Yavuz, Dys and Malti2022). The development of inhibitory control is influenced by biological and experiential factors. Increasing evidence building on the developmental origins of health and disease (DOHaD) framework has established the role of in-utero experiences in shaping children’s developmental outcomes (Doyle & Cicchetti, Reference Doyle and Cicchetti2018), including both inhibitory control and psychopathology. Although the link between variations in prenatal SES and mood and children’s risk for psychopathology has been established, the developmental pathways linking these prenatal risk factors to later socioemotional development remain not well understood (e.g., Shackman & Gee, Reference Shackman and Gee2023). One possible pathway is linked to the neural correlates of inhibitory control. Neural correlates of inhibitory control index the neurobiological mechanisms underlying the development of inhibitory control and may provide a unique perspective on the developmental pathway linking prenatal risk to children’s psychopathology that cannot be captured with behavioral measures (Buzzell et al., Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020). In the present study, we examined the roles of ERP components and time-frequency measures of inhibitory control in the longitudinal relations between prenatal risk factors (i.e., SES and maternal psychopathology) and children’s psychopathology.
Neurophysiological correlates of inhibitory control
ERPs
The most studied neurophysiological correlates of inhibitory control are N2 and P3 ERP components, which have been found to be involved in children’s inhibitory control and have also been related to children’s psychopathology (Hoyniak & Petersen, Reference Hoyniak and Petersen2019; Riggins & Scott, Reference Riggins and Scott2020). The N2 component is the second negative deflection in the waveform and occurs approximately 200–400 ms post-stimulus in children over frontal areas (Bruin & Wijers, Reference Bruin and Wijers2002). This component has been associated with conflict monitoring and response inhibition (Hoyniak, Reference Hoyniak2017). Specifically, the literature has mostly examined N2 amplitude and N2 latency to peak as measures of the N2 component. Elicited by tasks that require inhibitory control (e.g., the Go/No-go task), N2 amplitude is significantly larger in magnitude to stimuli that require inhibition compared to stimuli that require a prepotent response (Conejero et al., Reference Conejero, Rico-Picó, Moyano, Hoyo and Rueda2023; Hoyniak & Petersen, Reference Hoyniak and Petersen2019). As children grow older, they become better at ignoring distracting information, so decreases in the difference between the N2 amplitude of stimuli that require inhibition and the N2 amplitude of stimuli that require a prepotent response could indicate a decrease in their processing of irrelevant information (Lo, Reference Lo2018). However, recent studies with young children have not found a significant difference between N2 amplitudes of the two types of stimuli (Algarín et al., Reference Algarín, Nelson, Peirano, Westerlund, Reyes and Lozoff2013; Andreu et al., Reference Andreu, García-Rubio, Melcón, Schonert-Reichl and Albert2023; Hosch et al., Reference Hosch, Swanson, Harris, Oleson, Hazeltine and Petersen2024; Lamm et al., Reference Lamm, White, McDermott and Fox2012; Sullivan et al., Reference Sullivan, Xie, Conte, Richards, Shama, Haque, Petri and Nelson2022), suggesting inconsistencies in the presence of the differences in N2 amplitudes in early development (Hoyniak, Reference Hoyniak2017). Besides N2 amplitude, N2 latency to the peak is another measure, which is defined as the interval between the onset of the stimulus and the most negative point of N2 (Jodo & Kayama, Reference Jodo and Kayama1992). Shorter N2 latency to peak may represent more efficient response inhibition in response to the inhibition stimuli compared to the activation stimuli (Smith et al., Reference Smith, Smith, Provost and Heathcote2010).
The P3 component is a positive-going wave peaking between 300 and 600 ms for adults and is maximal over central-parietal areas (Riggins & Scott, Reference Riggins and Scott2020). Similar to the N2 component, the literature has focused on the P3 amplitude and P3 latency to peak as measures of the P3 component. Children’s P3 latency to peak is delayed or longer than adults, and as children age, their latency to peak can decrease by 3.6–18.4 ms per year (Riggins & Scott, Reference Riggins and Scott2020). The P3 is primarily elicited in tasks that engage attention, memory, and problem solving (Polich, Reference Polich2007). Similar to the N2 component, the P3 could be elicited by response inhibition tasks like the Go/No-go task due to the P3’s direct or indirect relations with inhibition (Huster et al., Reference Huster, Enriquez-Geppert, Lavallee, Falkenstein and Herrmann2013; Luijten et al., Reference Luijten, Machielsen, Veltman, Hester, de Haan and Franken2014). However, compared to the N2, the P3 is thought to be more closely related to cognitive evaluation processes related to decision making, rather than conflict monitoring (Polich, Reference Polich2007). The P3 amplitude is larger in magnitude in response to the inhibition stimuli (No-go) compared to stimuli that require a prepotent response (Go) (Conejero et al., Reference Conejero, Rico-Picó, Moyano, Hoyo and Rueda2023; Eimer, Reference Eimer1993; Kopp et al., Reference Kopp, Mattler, Goertz and Rist1996). More efficient response inhibition could also be represented by shorter P3 latency to peak in response to the inhibition stimuli compared with the prepotent stimuli (Johnstone et al., Reference Johnstone, Pleffer, Barry, Clarke and Smith2005).
Time-frequency analyses
While ERPs have proven to be productive for our understanding of inhibitory control across development, they have several important limitations. ERP analyses do not fully utilize the information contained in the EEG signal, as they do not distinguish different frequencies of oscillations and assume the component of interest is temporally consistent across trials (Cohen, Reference Cohen2014; Morales & Bowers, Reference Morales and Bowers2022). In contrast, time-frequency measures separately measure signal strength (EEG power) and signal consistency across trials (intertrial phase synchrony; ITPS). Importantly, it has been well documented that signal consistency increases with age across childhood, such that low consistency may be a characteristic of immature neural systems (Bowers et al., Reference Bowers, Buzzell, Bernat, Fox and Barker2018; DuPuis et al., Reference DuPuis, Ram, Willner, Karalunas, Segalowitz and Gatzke- Kopp2015; Morales et al., Reference Morales, Bowers, Leach, Buzzell, McSweeney, Yoder, Fifer, Elliott and Fox2022, Reference Morales, Bowers, Leach, Buzzell, Fifer, Elliott and Fox2023). Moreover, given that neuronal oscillations are a fundamental property of brain function (Buzsaki & Draguhn, Reference Buzsaki and Draguhn2004), time-frequency measures have been proposed to provide a neurophysiological mechanism underlying the processes captured by the EEG data like inhibitory control (Cavanagh & Frank, Reference Cavanagh and Frank2014). In this way, compared with ERP measures, time-frequency measures provide a more direct measure of the neural mechanisms underlying inhibitory control, linking early prenatal risk to later psychopathology.
Although time-frequency analyses and ERPs are complementary as measures of inhibitory control, most research to date on inhibitory control has exclusively focused on ERPs. The relatively few time-frequency analyses of children’s inhibitory control have primarily focused on the theta frequency band (4–8 Hz; Adam et al., Reference Adam, Blaye, Gulbinaite, Delorme and Farrer2020; Nigbur et al., Reference Nigbur, Ivanova and Stürmer2011). Theta oscillations have been related to the N2 component (Cavanagh & Frank, Reference Cavanagh and Frank2014). Signal strength (power) in the theta band in midfrontal areas is increased when children and adults engage in cognitive control to detect and signal the need for control to other brain systems that are involved in attention or action selection (Adam et al., Reference Adam, Blaye, Gulbinaite, Delorme and Farrer2020; Meyer et al., Reference Meyer, Endedijk, Van Ede and Hunnius2019; Nigbur et al., Reference Nigbur, Ivanova and Stürmer2011; Watson & Bell, Reference Watson and Bell2013). Moreover, similar to ERPs, the latency to peak of midfrontal theta power in response to inhibitory control has been found to be faster among older children, potentially indicating increased efficiency in information processing and inhibitory control implementation. In addition, signal consistency (ITPS) in the theta band has been found to be higher for conditions that require inhibition (e.g., No-go) compared with other conditions (e.g., Go) (Papenberg et al., Reference Papenberg, Hämmerer, Müller, Lindenberger and Li2013), suggesting that theta consistency could also be a time-frequency measure of inhibitory control in childhood.
Similar to theta oscillations, delta (1–4 Hz) oscillations have also been studied as an electrophysiological measure related to inhibitory control (Prada et al., Reference Prada, Barceló, Herrmann and Escera2014). Theta oscillations are thought to contribute to the N2 component, and delta oscillations are thought to contribute to the P3 component (Cavanagh & Frank, Reference Cavanagh and Frank2014; Prada et al., Reference Prada, Barceló, Herrmann and Escera2014). Using Go/No-go and stop trial tasks, researchers found that enhanced signal strength (power) in the delta band could index response inhibition (Schmiedt-Fehr & Basar-Eroglu, Reference Schmiedt-Fehr and Basar-Eroglu2011). In addition, delta signal consistency (ITPS) has been found to be higher for conditions that require inhibition (e.g., No-go) compared with other conditions (e.g., Go) (Papenberg et al., Reference Papenberg, Hämmerer, Müller, Lindenberger and Li2013; Schmiedt-Fehr & Basar-Eroglu, Reference Schmiedt-Fehr and Basar-Eroglu2011), suggesting that consistency in the delta band could also be a time-frequency measure related to inhibitory control.
Relations between risk factors and children’s inhibitory control
Although there has been limited work linking prenatal risk factors to inhibitory control, several risk factors, including both SES and maternal psychopathology, have been well established as important predictors of children’s inhibitory control. For instance, SES and maternal psychopathology are thought to impact children’s self-regulation through several mechanisms, including parenting behaviors (Warnock et al., Reference Warnock, Craig, Bakeman, Castral and Mirlashari2016) and stress-related pathways in the postnatal period (Evans & Kim, Reference Evans and Kim2013). Mothers with psychopathology may find parenting challenging, particularly during children’s early years, resulting in lower supportive parenting behaviors like sensitivity (NICHD Early Child Care Research Network, 1999). Parenting behaviors, in turn, could predict children’s inhibitory control (Geeraerts et al., Reference Geeraerts, Endendijk, Deković, Huijding, Deater-Deckard and Mesman2021). For SES, greater financial resources can reduce stress in the household and increase parental investment, providing a more cognitively enriching environment for children to develop inhibitory control (Brody et al., Reference Brody, Murry, Kim and Brown2002; Hoff et al., Reference Hoff, Laursen, Tardiff and Bornstein2002). The relatively few studies examining prenatal mechanisms support the DOHaD framework by suggesting potential prenatal long-term influences (Lewis et al., Reference Lewis, Austin, Knapp, Vaiano and Galbally2015; Monk et al., Reference Monk, Lugo-Candelas and Trumpff2019). Specifically, prenatal and perinatal maternal psychopathology and distress could influence child development via fetal brain development and programing effects, including hormonal priming effects, alteration of placental function and perfusion, and epigenetic mechanisms (Lewis et al., Reference Lewis, Austin, Knapp, Vaiano and Galbally2015; Monk et al., Reference Monk, Lugo-Candelas and Trumpff2019). Although prenatal psychopathology has been found to predict children’s inhibitory control at 6–9 years (Buss et al., Reference Buss, Davis, Hobel and Sandman2011), to our knowledge, no studies have examined prenatal effects on electrophysiological markers of inhibitory control. Instead, existing studies have documented relations between SES and maternal psychopathology and electrophysiological markers of inhibitory control from infancy to adolescence. For instance, SES had positive and concurrent associations with children’s N2 and P3 amplitudes (John et al., Reference John, Finch and Tarullo2019; Kishiyama et al., Reference Kishiyama, Boyce, Jimenez, Perry and Knight2009). Theta power evoked in response to errors was smaller in toddlers from low-SES families, compared with toddlers from high-SES families (Conejero et al., Reference Conejero, Guerra, Abundis-Gutiérrez and Rueda2016). Maternal education was longitudinally and positively related to the resting theta power at 10 and 24 months (Bernier et al., Reference Bernier, Calkins and Bell2016). For findings on maternal psychopathology, Connell et al. (Reference Connell, Danzo, Magee and Uhlman2019) found that compared with adolescent girls (10–14 years) of non-depressed mothers, daughters of depressed mothers exhibited a less negative N2 amplitude and a smaller P3 amplitude. Although it has been documented that prenatal risk factors (e.g., maternal SES and distress) is related to electrophysiological markers in children (e.g., Hernandez et al., Reference Hernandez, Sania, Bowers, Leach, McSweeney, Yoder, Fifer, Elliott, Shuffrey, Rauh, Him, Fox and Morales2024; Pierce et al., Reference Pierce, Thompson, Gharib, Schlueter, Reilly, Valdes, Roberts, Conroy, Levitt and Nelson2019), these studies have been done using EEG at rest and have mostly collected data during infancy. To our knowledge, few studies have examined the longitudinal predictions from earlier SES and maternal psychopathology to later electrophysiological markers of children’s inhibitory control, especially when SES and maternal psychopathology were measured during the prenatal period to support the DOHaD framework. To fill this gap in the literature and better understand the development of children’s inhibitory control, we investigated relations between early risk factors, including prenatal SES and maternal psychopathology, and children’s later event-related electrophysiological measures, including ERP components and power and consistency in the theta and delta bands.
Inhibitory control’s relation to psychopathology
Children’s inhibitory control has been found to be an important predictor of children’s psychopathology (see Lipszyc & Schachar, Reference Lipszyc and Schachar2010 for a meta-analytic review). Regarding associations with ERP measures, the magnitude of N2 amplitude has been found to be negatively associated with externalizing psychopathology in childhood (see Hoyniak & Petersen, Reference Hoyniak and Petersen2019 for a meta-analytic review). Additionally, children with a smaller P3 amplitude displayed more aggression symptoms (Petersen et al., Reference Petersen, Hoyniak, Bates, Staples and Molfese2018). Similarly, children with high internalizing problems had a smaller P3 amplitude than children with low internalizing problems, and their P3 amplitude was the smallest when they had both internalizing and externalizing problems (Hill et al., Reference Hill, Shen, Locke, Steinhauer, Konicky, Lowers and Connolly1999). For time-frequency measures, reduced mediofrontal theta is thought to be an endophenotype for externalizing disorders for adolescents and young adults (Gilmore et al., Reference Gilmore, Malone and Iacono2010; Kamarajan et al., Reference Kamarajan, Pandey, Chorlian, Manz, Stimus, Anokhin, Bauer, Kuperman, Kramer, Bucholz, Schuckit, Hesselbrock, Porjesz and Abulseoud2015).
Moreover, because earlier factors like SES and maternal psychopathology have both been found to be important predictors of children’s psychopathology (e.g., see Goodman et al., Reference Goodman, Rouse, Connell, Broth, Hall and Heyward2011 and Peverill et al., Reference Peverill, Dirks, Narvaja, Herts, Comer and McLaughlin2021 for meta-analytic reviews), inhibitory control could act as a mediator in the prediction from earlier factors to children’s psychopathology. For example, data from the Bucharest Early Intervention Project suggest that the development of event-related mediofrontal theta across the adolescent period (12–16 years) was negatively predicted by the early neglect children experienced in Romanian institutions (Buzzell et al., Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020). Moreover, changes in mediofrontal theta development were related to adolescents’ psychopathology during the same period, and importantly, reduced theta power acted as a mediator in the positive relation between neglect and adolescents’ psychopathology (Buzzell et al., Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020). This finding is similar to studies with rodents that revealed that repeated exposure to maternal neglect during a sensitive period of rodent growth is related to reduced mediofrontal theta power during the juvenile period (Reincke & Hanganu-Opatz, Reference Reincke and Hanganu-Opatz2017). In the current study, we would like to extend the existing literature by examining the mediating role of both the ERP components and time-frequency measures of children’s inhibitory control in the relations between prenatal risk factors and children’s psychopathology. Supporting the DOHaD framework (Doyle & Cicchetti, Reference Doyle and Cicchetti2018), prenatal risk factors, including maternal mood and SES, have been widely investigated in their predictions of children’s internalizing and externalizing problems (Doyle & Cicchetti, Reference Doyle and Cicchetti2018). Besides other potential biological mechanisms related to hormones and the immune system (Tien et al., Reference Tien, Lewis and Liu2020), neurophysiological indices of children’s inhibitory control could serve as a developmental pathway in the associations between prenatal risk factors and children’s internalizing and externalizing problems.
The current study
In the current study, we used children’s ERPs and time-frequency measures of inhibitory control to examine their associations with maternal prenatal risk factors and examine if they predicted children’s behavioral problems. Specifically, we had three aims in the current study. The first aim was to examine if individual differences in ERP measures and time-frequency measures of inhibitory control could be predicted by prenatal maternal factors. Based on prior research, we hypothesized that higher prenatal SES would be related to better inhibitory control skills, indexed by larger N2 magnitude, P3 amplitude, and theta/delta power and ITPS, as well as shorter latency to peak for all the ERPs and time-frequency measures. We also expected that higher prenatal maternal psychopathology would be related to lower levels of inhibitory control, indexed by smaller N2 magnitude, P3 amplitude, and theta/delta power and ITPS and longer latency to peak for all the ERPs and time-frequency measures.
The second aim was to examine if inhibitory control and children’s emotional and behavioral problems had concurrent associations. We hypothesized that higher N2 magnitude, P3 amplitude, and theta/delta power and ITPS would be related to less internalizing and externalizing problems, as well as longer latency to peak for all the ERP measures and time-frequency measures would be related to more internalizing and externalizing problems.
The third aim directly builds on the first two aims. We investigated if any of the ERPs and time-frequency measures of inhibitory control could act as mediators in the relations between prenatal risk factors and children’s behavioral problems. We hypothesized that both ERPs (i.e., N2 amplitude and latency to peak, P3 amplitude and latency to peak) and time-frequency measures (i.e., theta/delta power, consistency across trials, and latency to peak) would act as mediators of the negative relations between SES and children’s internalizing and externalizing problems, and the positive relations between maternal psychopathology and children’s internalizing and externalizing problems.
Method
Participants
Children (N = 560; age range = 4–11 years (M age = 7.13 years); 51.75% females) and their biological mothers were recruited as part of a large longitudinal study that examined how early environmental exposures could influence children’s development. Participants included in this analysis were originally recruited as part of the Safe Passage Study (Dukes et al., Reference Dukes, Burd, Elliott, Fifer, Folkerth, Hankins, Hereld, Hoffman, Myers, Odendaal, Signore, Sullivan, Willinger, Wright and Kinney2014) and are now participants in the NIH-funded Environment Influences on Child Health Outcomes network (Gillman & Blaisdell, Reference Gillman and Blaisdell2018). Data were collected in two sites in South Dakota. Most participants were White (81.23%), followed by American Indian (12.98%), and other (5.79%). Most participants were non-Latinx (95.79%). Data on prenatal risk factors, including SES and maternal psychopathology, were reported by mothers during the prenatal period. Mothers reported on average 14.87 years of education (Range = 7–17 years) and $3297 as average monthly income (Range = $250–$5000) during the prenatal period. Data on children’s EEG were collected at the following ages: 4 (n = 70; M age = 4.23; SD age = .15), 5 (n = 122; M age = 5.23; SD age = .15), 7 (n = 185; M age = 7.22; SD age = .14), 9 (n = 97; M age = 9.24; SD age = .13), and 11 (n = 86; M age = 11.24; SD age = .14). Internalizing and externalizing behaviors were collected concurrently with the EEG assessment or at a later timepoint. EEG and children’s psychopathology were assessed concurrently for most children (75%), while children’s psychopathology was assessed two (18%) or three (7%) years later for some children. Four-year-old children had EEG data but did not have data on parent reports of internalizing and externalizing behaviors. Participants with and without data on internalizing and externalizing behaviors did not differ in any indicators of prenatal SES and prenatal maternal psychopathology nor by children’s race and ethnicity. The only difference was in children’s sex ratio. Participants with data on internalizing and externalizing behaviors had a higher percentage (56%) of females than participants without data on internalizing and externalizing behaviors (44%), χ 2(1) = 7.18, p = .007.
Procedure
All mothers provided informed consent, and children, ages eight and older, provided assent before data collection. During the prenatal period, mothers self-reported their depression and anxiety symptoms as well as demographic information, including SES (i.e., income, education, and information about insurance). When children were 4–11 years old, they came to the laboratory with their primary caregivers for health (e.g., anthropometrics and spirometry) and cognitive assessments, including the Go/No-Go task called the “Zoo Game,” which was administered while acquiring EEG. E-Prime 2.0.10 (Psychology Software Tools, Pittsburgh, PA) was used to present the task. The code and stimuli for the task are publicly available (see https://github.com/ChildDevLab/Tasks). Children sat approximately 70 cm in front of the presentation computer during task administration. The Zoo Game commenced after children completed a 3-minute baseline and a three-stimulus auditory oddball paradigm (Morales et al., Reference Morales, Bowers, Leach, Buzzell, McSweeney, Yoder, Fifer, Elliott and Fox2022) as part of a separate protocol. Primary caregivers reported children’s internalizing and externalizing problems while children completed the EEG and/or other assessments. At the end of the visit, families were compensated, and children were provided a small toy. All tasks and procedures were approved by the Avera Institutional Review Board.
EEG data acquisition
EEG data were obtained through a 64-channel HydroCel Geodesic Sensor Net, sampled at 500 Hz and with a gain of 12 dB via EGI software (Net Station Version 5.4; Electrical Geodesics, Inc., Eugene, OR). We removed four face channels (E61–E64) of the nets because they were not used to collect EEG data but other psychophysiological data (e.g., heart rate). Before data collection, the research staff checked impedance values for all EEG channels and confirmed them to be below 50 kΩ.
Zoo game (Go/No-Go task)
ERP components and event-related delta/theta power were collected through the Zoo Game. As previously described (Morales et al., Reference Morales, Bowers, Leach, Buzzell, McSweeney, Yoder, Fifer, Elliott and Fox2022), the Zoo Game is a computer-based Go/No-go task developed by Fox and colleagues (He et al., Reference He, Degnan, McDermott, Henderson, Hane, Xu and Fox2010; Morales et al., Reference Morales, Miller, Troller-Renfree, White, Degnan, Henderson and Fox2020; Troller-Renfree et al., Reference Troller‐Renfree, Buzzell, Bowers, Salo, Forman‐Alberti, Smith, Papp, McDermott, Pine, Henderson and Fox2019) based on the design of Durston et al. (Reference Durston, Thomas, Yang, Ulug, Zimmerman and Casey2002). In the task, children were asked to help a zookeeper catch animals that escaped from cages in a zoo. They were told to catch all animals except the orangutans because the orangutans were the zookeeper’s assistant, helping to catch other animals. Children who were four years old were presented with only one picture of an orangutan, while children who were older than four years were presented with three different pictures of orangutans. In Go trials, after children saw pictures of animals that were not orangutans, they needed to press a button to catch them. In No-go trials, after children saw pictures of orangutans, they needed to withhold their response without pressing any button. On each trial, an animal stimulus was presented on the screen for 750 ms, followed by a blank screen for 500 ms or until the child pressed the button (whichever occurred first). The intertrial interval was 500–1000 ms. Children first practiced the task until the experimenter thought they understood the task procedures. After the practice trials, children completed up to 320 trials, including eight blocks with 40 trials in each block. The task had 75% Go trials and 25% No-go trials. For both Go and No-go trials, children needed to finish at least half of the trials and were accurate in 60% or higher rate of the trials to be included in subsequent analyses. The average numbers of Go trials were 118.03, 140.06, 158.58, 190.58, and 204.72 for 4-, 5-, 7-, 9-, and 11-year-old children, respectively. The average numbers of No-go trials were 32.26, 36.02, 39.25, 49.13, and 55.93 for 4-, 5-, 7-, 9-, and 11-year-old children, respectively. The number of trials were related with measures of SES and EEG (see supplementary materials; Table S1). After controlling for age, number of trials were related to children’s theta power and externalizing problems (rs = .08−.26, ps < .05). Importantly, a sensitivity analysis showed that controlling for the number of trials showed the same pattern of results, including a significant mediation (see supplementary materials; Table S5).
EEG preprocessing
The preprocessing of the data has been previously described in Morales et al. (Reference Morales, Bowers, Leach, Buzzell, McSweeney, Yoder, Fifer, Elliott and Fox2022). In brief, we conducted the EEG preprocessing using the EEGLAB toolbox (Delorme & Makeig, Reference Delorme and Makeig2004) with custom MATLAB scripts (The MathWorks, Natick, MA) using the Maryland Analysis of Developmental EEG pipeline (Debnath et al., Reference Debnath, Buzzell, Morales, Bowers, Leach and Fox2020). Continuous EEG data were high-pass filtered at 0.3 Hz and low-pass filtered at 49 Hz. We identified and removed bad channels using the EEGLAB plug-in FASTER (Nolan et al., Reference Nolan, Whelan and Reilly2010). To remove ocular and other stereotyped artifacts, we utilized independent component analysis (ICA). Artifactual independent components were removed from the original dataset using the Adjusted-ADJUST algorithm (Leach et al., Reference Leach, Morales, Bowers, Buzzell, Debnath, Beall and Fox2020; Mognon et al., Reference Mognon, Jovicich, Bruzzone and Buiatti2011). The data were then segmented into three-second epochs for two additional steps of artifact rejection. First, to capture the presence of residual ocular activity which was kept after ICA, we rejected any epochs in which ocular channel (E1, E5, E10, and E17) voltages exceeded ± 150 μV. Second, we interpolated non-ocular channels that exceeded ± 125 μV at the epoch level. However, if over 10% of the channels (not considering globally rejected channels) exceeded ± 125 μV, we rejected the epoch instead. Then, we interpolated any remaining missing channels using the spherical spline method (Perrin et al., Reference Perrin, Pernier, Bertrand and Echallier1989). The data was then referenced to the average reference. All epochs were time-locked to the presentation of the stimuli (i.e., animal) using only correct trials. Moreover, we excluded trials with anticipatory responses (<150 ms).
ERPs
ERPs for each child were averaged separately for each of the two conditions (Go and No-go). We baseline corrected the average voltage in the −200 to 0 ms pre-stimulus period, using mean amplitude measures for each child and each condition. The time windows and electrode clusters for each ERP component (N2 and P3) were selected based on previous publications with developmental populations (John et al., Reference John, Finch and Tarullo2019; Sullivan et al., Reference Sullivan, Xie, Conte, Richards, Shama, Haque, Petri and Nelson2022) and visual inspection. For the N2, we focused on the frontocentral electrode cluster (FCz; E4, E7, and E54) 250–500 ms after the stimulus. For the P3, we focused on the parietal electrode cluster (E33, E34, E36, and E38) 400–700 ms after the stimulus. A figure of the electrode clusters is shown in the supplementary materials (Figure S2).
Time-frequency (TF) power
We computed TF power in each epoch of interest using custom MATLAB scripts (Morales & Bowers, Reference Morales and Bowers2022), adapted from Cohen (Reference Cohen2014). First, we applied a surface Laplacian filter to the epoched data to mitigate volume conduction over the scalp by filtering out spatially broad features of the data (Cohen, Reference Cohen2014), so that both spatial and functional specificity of brain activity were improved (Tenke & Kayser, Reference Tenke and Kayser2012). Each epoch was convolved with Morlet wavelets, which estimated spectral power in the frequency range of 1 − 30 Hz (in 60 steps spaced logarithmically). To optimize the TF resolution and balance the tradeoff between frequency and time resolution, we set three wavelet cycles at the lowest frequency (1 Hz) and gradually increased the number of wavelet cycles to 10 for the highest frequency (30 Hz). We calculated power separately for correct trials in each of the Go and No-go conditions for all channels. For each condition, we normalized power using a (dB) transform (dB power = 10 × log 10[power/baseline]). In the transform, the baseline was the average power for the condition from − 300 to −100 ms before the stimulus was presented (Delorme & Makeig, Reference Delorme and Makeig2004). Finally, to facilitate data manipulation, we downsampled data to 50 Hz following TF decomposition.
ITPS
We computed ITPS using custom MATLAB scripts (Morales & Bowers, Reference Morales and Bowers2022), adapted from Cohen (Reference Cohen2014). ITPS measures the consistency of phase oscillations across trials for each frequency and each timepoint. ITPS values range from zero, which indicates completely random phase alignment at a certain time point, to one, which indicates perfect phase alignment at a certain time point. We computed ITPS by first taking the phase angle difference across trials, and then averaging the phase angle differences. When we estimated ITPS, we used a subsampling procedure to eliminate biases associated with having different numbers of trials per condition (Cohen, Reference Cohen2014). We computed ITPS using 10 trials randomly selected per condition. This subsampling procedure was performed 50 times to make sure that all the data were used, and then all subsamples were averaged to calculate the final ITPS. ITPS was baseline corrected per condition based on − 300 to − 100 ms before stimulus onset. This process created ITPS surfaces per condition with the same dimensions as the TF measures for each electrode for each participant. Finally, to facilitate data manipulation, we downsampled data to 50 Hz following TF decomposition and computation of ITPS.
TF regions of interest selection
We selected the frequencies and time windows for the regions of interest (ROIs) a priori based on previous literature on developmental populations (Buzzell et al., Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020) and confirmed with visual inspection. In line with previous literature on young children (3–6 years; e.g., Canen & Brooker, Reference Canen and Brooker2017; DuPuis et al., Reference DuPuis, Ram, Willner, Karalunas, Segalowitz and Gatzke- Kopp2015), we defined the theta frequency band to be 4–8 Hz and delta to be 1–4 Hz with overlapping boundary. We focused on delta and theta power and ITPS over the frontocentral electrodes (FCz; E4, E7, and E54). To determine the time windows of interest unbiased for condition effects, we averaged each measure over all conditions throughout the interval and created time windows around the peak while ensuring most of the peak were captured. For theta and delta power, we selected from 200 to 700 ms (theta) and from 250 to 1000ms (delta) post stimuli. For theta and delta ITPS, we selected from 0 to 500 ms (theta) and from 0 to 750 ms (delta) post stimuli.
Prenatal maternal depressive symptoms
Mothers reported their depressive symptoms during the prenatal period using the Edinburgh Postnatal Depression Scale (EPDS; Cox et al., Reference Cox, Holden and Sagovsky1987), which has 10 items rated on a 4-point scale (1= not at all/almost never to 4 = as much as I always could/most of the time). Example items include “I have felt sad or miserable” and “I have been so unhappy that I have been crying.” EPDS has been validated for mothers during the pregnancy with high test-retest reliability and high concurrent validity (Bergink et al., Reference Bergink, Kooistra, Lambregtse-van den Berg, Wijnen, Bunevicius, van Baar and Pop2011). The 10 items were first standardized as z-scores and then averaged to create a composite score of prenatal maternal depression. For mothers who completed the EPDS more than one time during pregnancy, we took the average across all their reports in pregnancy. This measure also showed high internal consistency (Cronbach’s α = .82) with the sample of the current study.
Prenatal maternal anxiety
Mothers reported their trait and state anxiety symptoms during the prenatal period using the State-Trait Anxiety Inventory (STAI; Spielberger et al., Reference Spielberger, Gonzalez-Reigosa, Martinez-Urrutia, Natalicio and Natalicio1971). It differentiates between “state anxiety” which refers to the temporary condition (e.g., “I am presently worrying over possible misfortunes.”) and “trait anxiety” which refers to the more general and long-standing quality (e.g., “I am included to taking things hard.”). Each of the two conditions has 20 items rated on a 4-point scale (1= not at all/almost never to 4 = very much so/almost always). STAI has been validated for mothers during the pregnancy with high construct and content validity (Gunning et al., Reference Gunning, Denison, Stockley, Ho, Sandhu and Reynolds2010). It also showed high internal consistency (Cronbach’s αs = .90 and .88, for state anxiety and trait anxiety, respectively) with the sample of the current study.
Child behavioral problems
Mothers reported children’s internalizing and externalizing problems using the Strengths and Difficulties Questionnaire (SDQ; Goodman, Reference Goodman1997). SDQ contains 25 items to form 4 difficulties subscales–conduct problems (e.g., “often has temper tantrums”), hyperactivity/inattention (e.g., “constantly fidgeting”), emotional problems (e.g., “many fears, easily scared”), and peer problems (e.g., “rather solitary, prefers to play alone”), and a prosocial subscale (e.g., “kind to younger children”). Parents rated statements on a 4-point scale (0 = not true, 1 = somewhat true, or 2 = certainly true). SDQ has been validated for parent reports on school-aged children with high construct, concurrent, discriminate, and predictive validity (Stone et al., Reference Stone, Otten, Engels, Vermulst and Janssens2010). In the current study, we used the four difficulties subscales to measure children’s behavioral problems. We combined emotional and peer problems to create the internalizing problem subscale and combined conduct problems and hyperactivity/inattention to create the externalizing problem subscale. The two types of behavioral problems showed adequate internal consistency (Cronbach’s αs = .67 and .84, for internalizing and externalizing problems, respectively) with the sample of the current study.
Prenatal socioeconomic status (SES)
For SES, we included mothers’ reported household income, education level, and insurance type (i.e., public or private insurance) during the prenatal period. We included information on insurance as a measure of SES because accessing to health care is an important aspect of SES (Adler & Newman, Reference Adler and Newman2002). Moreover, insurance information has been found to be strongly correlated with other SES measures, including income and education level (Casey et al., Reference Casey, Pollak, Glymour, Mayeda, Hirsch and Schwartz2018), as was the case in our sample.
Analytic approach
First, in order to examine differences in all ERPs and TF measures between Go and No-Go conditions, we used t-tests to examine the main effect of conditions (Go vs. No-Go). If the effect of interest was significant, indicating that the ERP or TF measures successfully captured a correlate of inhibitory control, we then conducted correlations with other study variables. Second, we examined the partial correlations between prenatal predictors (maternal psychopathology and SES), neurophysiological measures, and children’s psychopathology, while controlling for age. In order to examine the mediating role of ERPs and TF measures in the prediction from prenatal SES and maternal psychopathology to children’s internalizing and externalizing problems, we first examined if there were significant relations from at least one prenatal predictor to an EEG-based mediator and from the same mediator to at least one outcome. Third, after identifying potential mediators based on the partial correlations, we performed a structural equation model (SEM) using the lavaan package in R (Rosseel, Reference Rosseel2012) to examine potential mediation effects using the bootstrap method to define the confidence intervals for mediation effects using 10,000 bootstrap samples (Mackinnon et al., Reference MacKinnon, Lockwood and Williams2004). Because we did not have specific hypotheses regarding individual components of SES or types of maternal psychopathology, we created latent measures of prenatal SES (education level, income, and insurance) and psychopathology (state and trait anxiety and depression). This model utilized full information maximum likelihood estimation to account for missing data and MLR estimator to deal with non-normality and non-independence of the variables. For evaluating model fit, we followed Hu and Bentler’s (Reference Hu and Bentler1999) guidelines.
Results
Descriptive analyses and differences between Go and No-Go conditions
Descriptive statistics and partial correlations controlling for age among all the study and control variables are presented in Table 1. Correlations among all study variables, including age, can be found in the supplementary materials. As expected, as age increased, accuracy in the Go (r = .61) and No-Go (r = .29) conditions increased, while reaction time in both conditions decreased (Go, r = −.77, No-Go, r = −.69). In addition, behavioral indices of inhibitory control were related with indices of SES and children’s externalizing behaviors. Higher SES predicted higher accuracy and slower reaction times, and higher accuracy and slower reaction times predicted lower externalizing behaviors (Table S1). However, behavioral indices were not related to either SES or children’s psychopathology after controlling for child age and sex.
Table 1. Means, standard deviations, and partial correlations of all study variables and covariates controlling for age
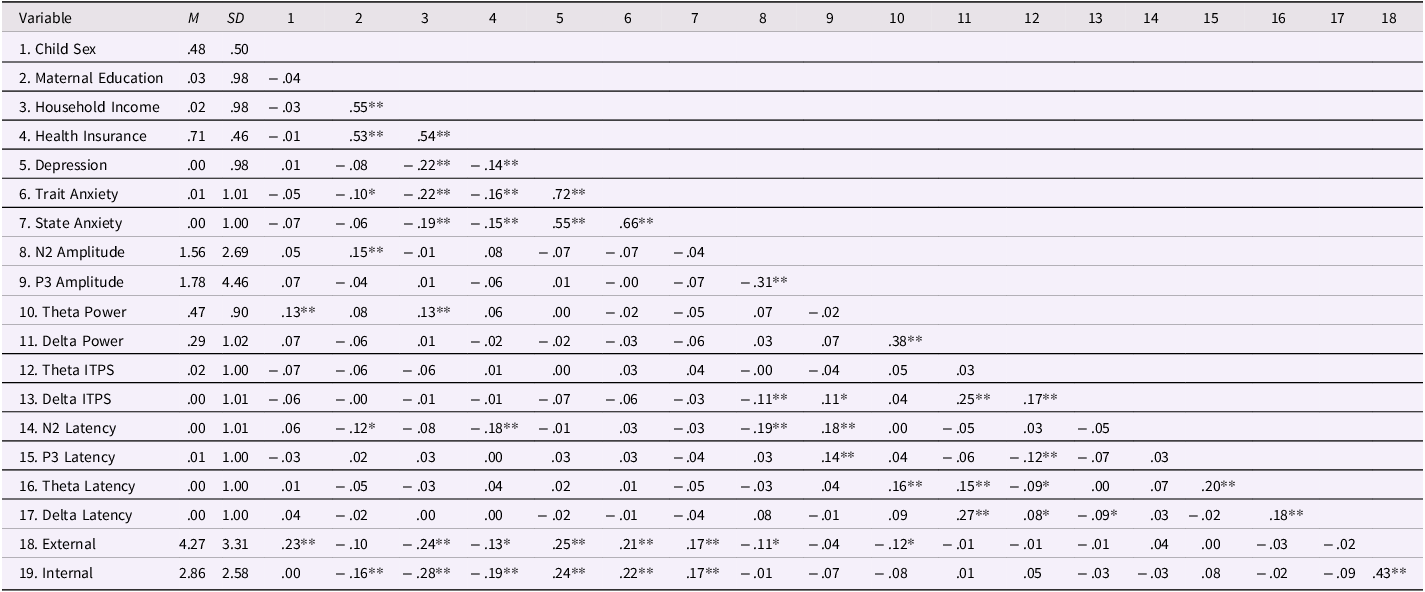
Note. M = mean; SD = standard deviation; External = children’s externalizing problems; Internal = children’s internalizing problems; For Child Sex, 0 = female, 1 = male; For Health Insurance, 0 = public assistance, 1 = commercial health insurance. Maternal education, household income, maternal depression, maternal trait anxiety, maternal state anxiety, theta ITPS, delta ITPS, and all latency measures were standardized by their means and standard deviations.
*p < .05. **p < .01.
Differences between Go and No-Go conditions
ERP amplitudes
As shown in Figure 1, in contrast to the hypothesis, we found a more positive amplitude deflection between 250 and 500 ms in frontocentral electrodes in the No-go condition (M = −9.72), compared to the Go condition (M = −11.46), t(559) = 13.74, p < .001, d = .58. Because of this, we did not explore further the role of the N2 in its relations with prenatal predictors or children’s psychopathology. As expected, we observed a clear P3 component, indexed by a more positive amplitude deflection between 400 and 700 ms in parietal electrodes in the No-Go condition (M = 24.35), compared to the Go condition (M = 22.57), t(559) = 9.45, p < .001, d = .40.
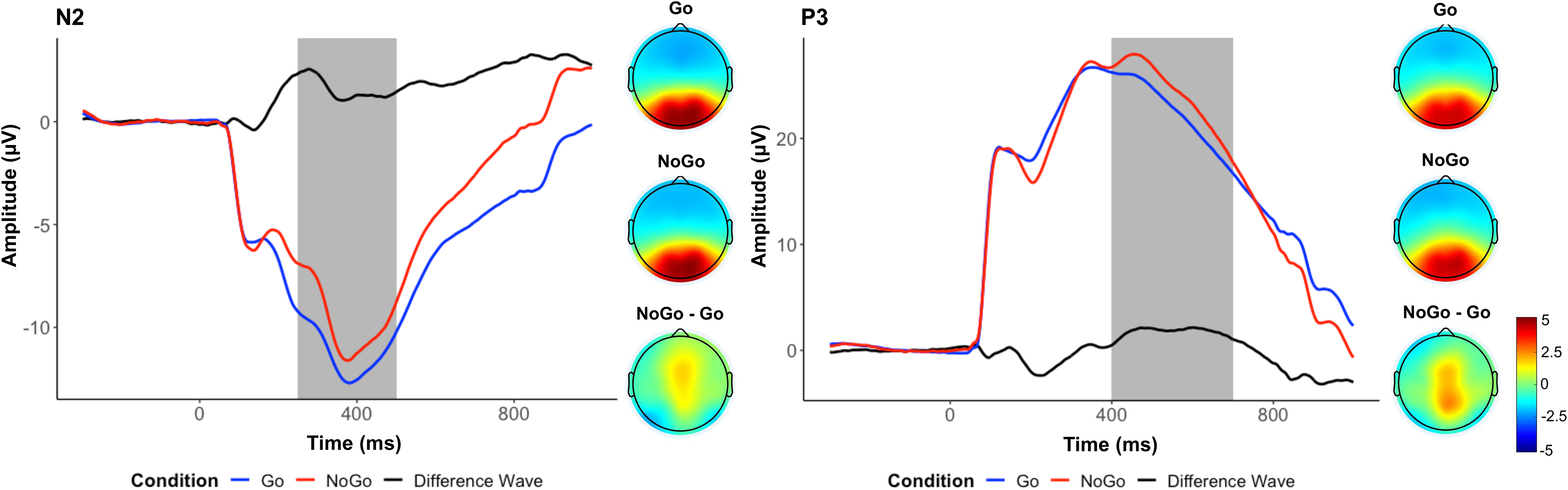
Figure 1. Event-related potential measures: N2 and P3. Average waveforms across all participants for the N2 (left) and the P3 (right), and topographs for each condition and their difference during the selected time window (shaded area).
TF power
As shown in Figure 2, between 250 and 750 ms in frontocentral electrodes, we observed a higher theta power in the No-Go condition (M = 1.23), compared to the Go condition (M = .76), t(559) = 12.27, p < .001, d = .52. Between 250 and 1000ms in frontocentral electrodes, we observed a higher delta power in the No-Go condition (M = .79), compared to the Go condition (M = .50), t(559) = 6.74, p < .001, d = .29.
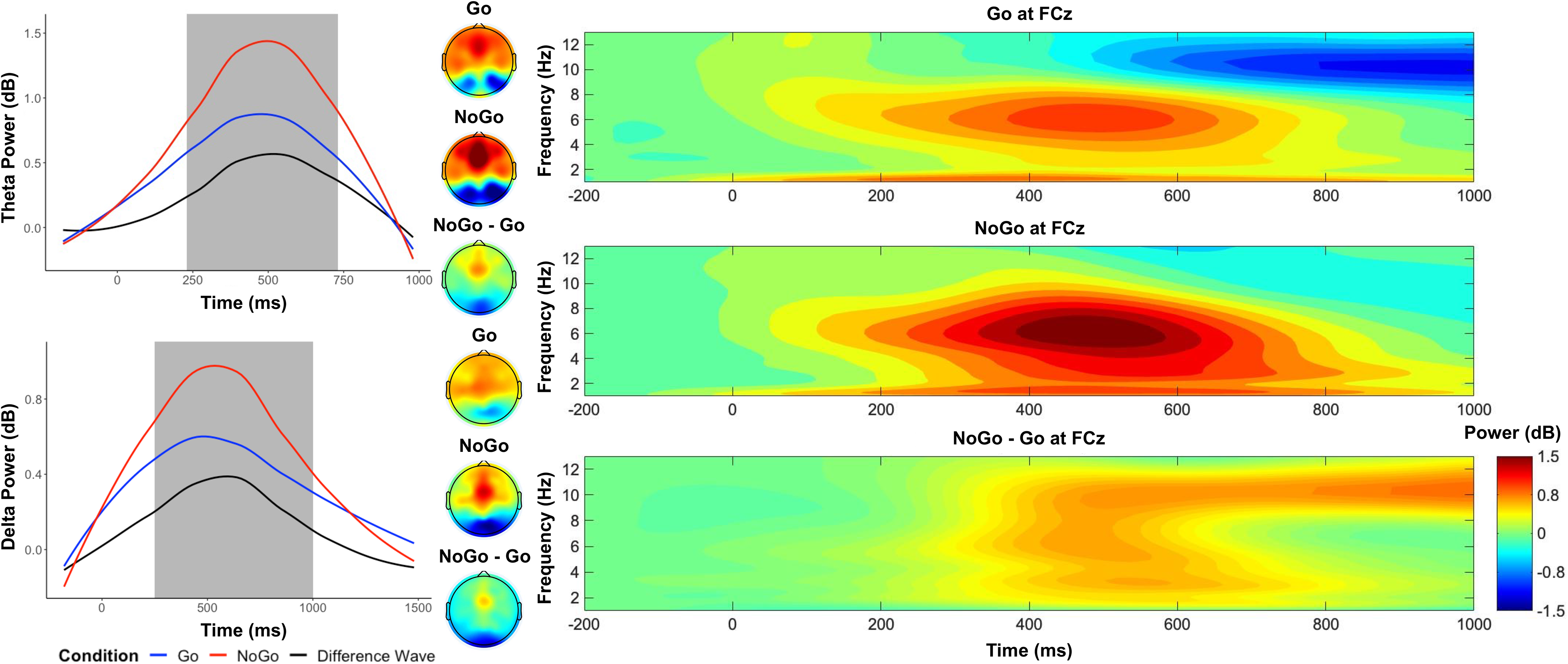
Figure 2. Time–frequency dynamics of theta and delta power of Go and No-go trials at the FCz cluster. Plots show time–frequency power for each condition for theta (top left panel) and delta (bottom left panel), and topographs for each condition and their difference during the selected time window (shaded area). The right panels display the time-frequency surfaces of power by condition for frontocentral cluster.
TF ITPS
As shown in Figure 3, between 250 and 750 ms in frontocentral electrodes, we observed a higher theta ITPS in the No-Go condition (M = .05), compared to the Go condition (M = .04), t(559) = 4.60, p < .001, d = .19. Between 250 and 1000ms in frontocentral electrodes, we observed a higher delta ITPS in the No-Go condition (M = .11), compared to the Go condition (M = .08), t(559) = 11.04, p < .001, d = .47.
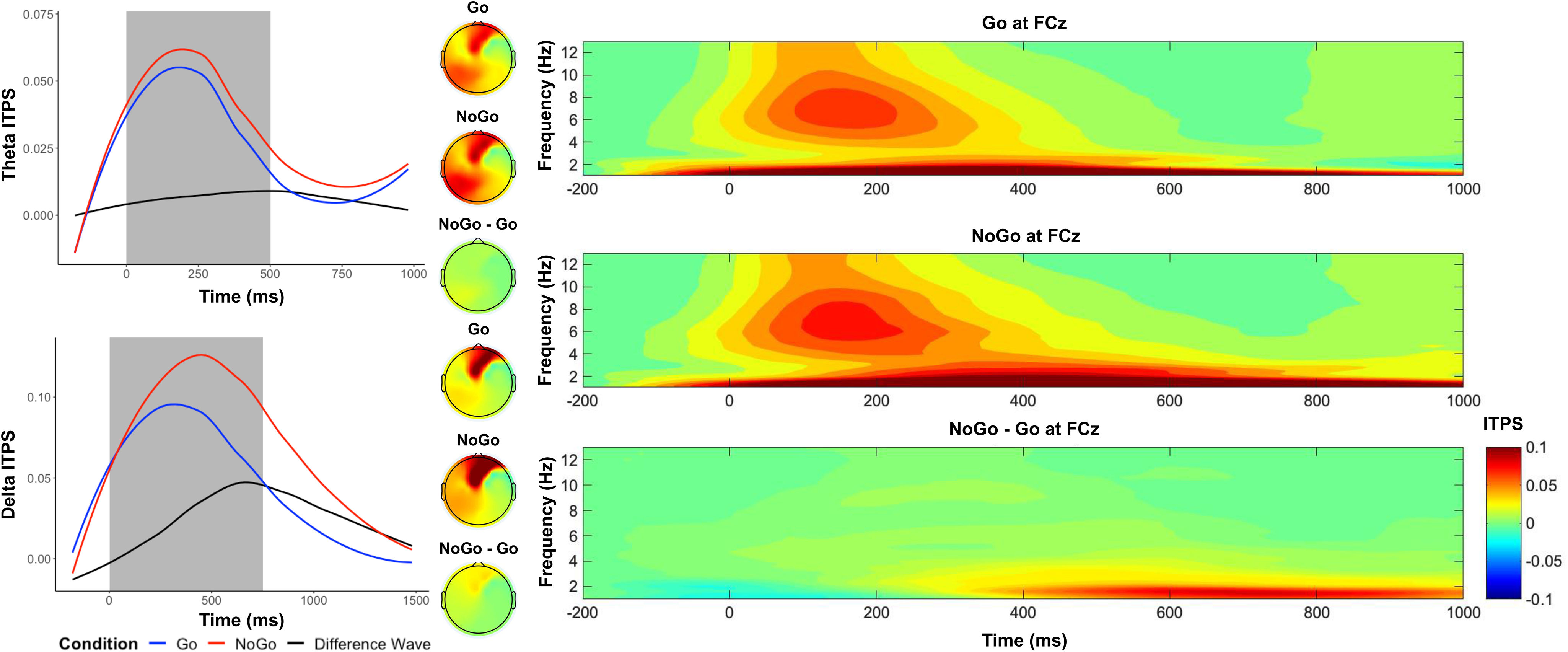
Figure 3. Time–frequency dynamics of theta and delta intertrial phase synchrony (ITPS) of Go and No-go trials at the FCz cluster. Plots show time–frequency ITPS for each condition for theta (top left panel) and delta (bottom left panel), and topographs for each condition and their difference during the selected time window (shaded area). The right panels display the time-frequency surfaces of ITPS by condition for frontocentral cluster.
Primary analyses
Based on the correlations shown in Table 1, theta power was the only potential mediator that was related to at least one of the prenatal predictors (i.e., prenatal SES and maternal psychopathology) and at least one of children’s behavioral problems (i.e., internalizing and externalizing behaviors). Thus, we only examined the mediating role of theta power in the relationships between prenatal SES and children’s externalizing problems because both prenatal SES and children’s externalizing problems were correlated with theta power. Theta power as the mediator was represented by the difference score between the Go and No-go trials. In the SEM model, SES, child gender, and child age were specified as predictors to predict the mediator and externalizing problems, which was specified as the outcome to be predicted by both the predictors and the mediator. Specifically, maternal SES was a latent variable created by the three aspects of SES: education level, income, and insurance type because the scores on the three subscales were moderately and positively correlated. The standardized factor loadings for education level, income, and insurance type were .71, .78, and .72, respectively (ps < .001). The model demonstrated good fit for the data, χ 2(9) = 19.44, p = .02; CFI = .98; TLI = .94; SRMR = .03; RMSEA = .05; 95% CI [.02, .08]. The model indicated that maternal SES was positively related to theta power. Theta power and maternal SES were negatively related to children’s externalizing problems. Further, we found support for a significant mediation, and the mediation estimated effect was −.069, such that theta power significantly mediated the relation between maternal SES and children’s externalizing problems with 95% CIs [−.196, −.005] after 10,000 times of bootstrapping. Significant results are summarized in Figure 4, and all other results (nonsignificant results and results of control variables) are displayed in Table S2 of supplementary materials.
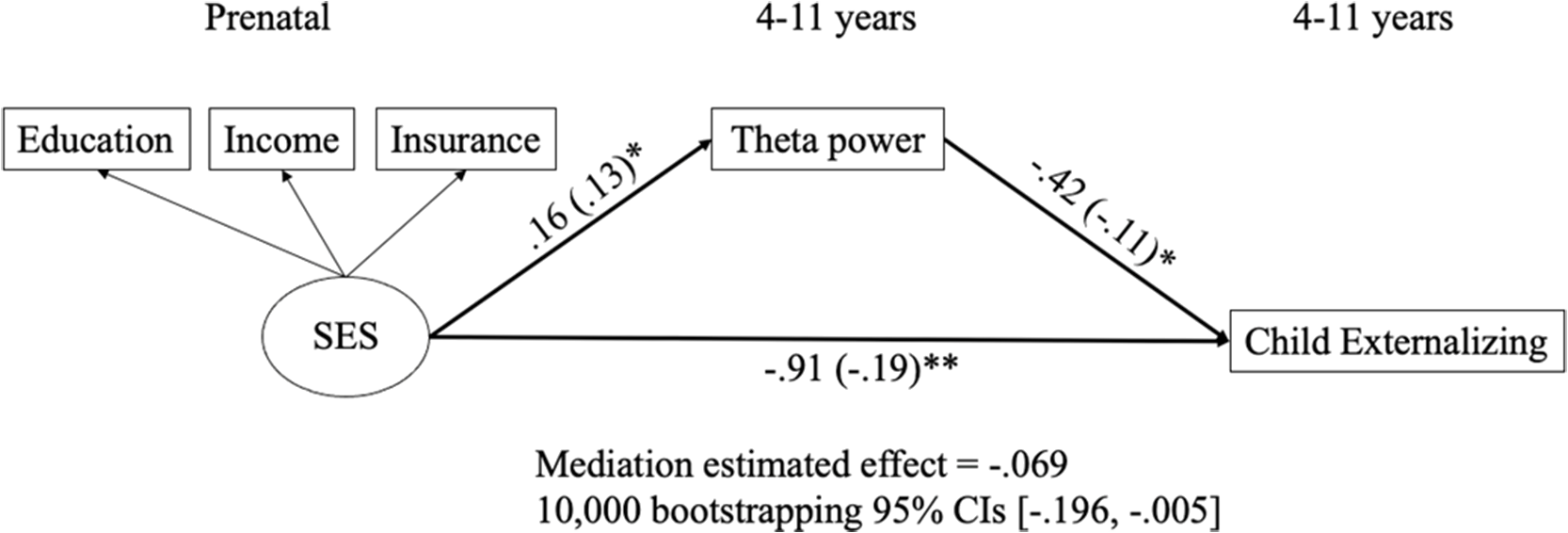
Figure 4. Relations of prenatal SES, theta power, and children’s externalizing behaviors. All predictions were controlled for child sex and child age. Unstandarized and standardized (in parentheses) estimates were presented. See Table S2 for results of nonsignificant results and results of control variables. *p < .05. **p < .01.
Sensitivity analyses were conducted as some participants in our current sample (four-year-old children) did not have parental assessments of internalizing and externalizing behaviors. Results of the models with and without four-year-old children yielded the same results; thus, we decided to include four-year-old children in models to increase the overall sample size. In addition, because not all children had psychopathology data collected at the time of their EEG measures, we conducted an analysis that included children with only concurrent measures. As shown in the supplementary materials (Table S4), results from this sensitivity analysis revealed a similar pattern of results. Although the effect sizes were similar, the relation between theta and externalizing was only marginally significant (p = .08), likely due to the reduction in sample size. Finally, a sensitivity analysis was conducted while controlling for the number of trials, finding an analogous pattern of results. Although the relation between SES and theta power was marginally significant (p = .06), the effect size was similar, and the indirect effects of the mediator were still significant leading to the same conclusions (see supplementary materials; Table S5).
Discussion
In the current study, we examined if ERPs and time-frequency measures of inhibitory control were significant mediators in the relation between prenatal risk factors and children’s psychopathology. We found that children’s theta power mediated the negative relation between prenatal SES and children’s externalizing problems. Our findings support the DOHaD framework by highlighting the role of prenatal risk factors on the development of children’s neurophysiological correlates of inhibitory control and psychopathology. Moreover, consistent with previous findings (Buzzell et al., Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020), the results further support the role of mediofrontal theta power as a developmental pathway linking early risk factors to later children’s psychopathology.
In support of the use of neurophysiological measures of inhibitory control, most of the ERP and time-frequency measures, including P3 amplitudes, theta and delta power, theta and delta consistency across trials, and latency to peak of all ERPs and time-frequency measures, were found to represent children’s inhibitory control as indexed by a larger magnitude in the inhibition condition (No-go trials) than in the activation condition (Go trials). It suggests that P3 measures and event-related theta and delta measures could be used to measure children’s inhibitory control from preschool years to early adolescence. The only exception was N2 amplitude, exhibiting a more negative magnitude in the activation condition (Go trials) than the inhibition condition (No-go trials). Similarly, Hosch et al. (Reference Hosch, Swanson, Harris, Oleson, Hazeltine and Petersen2024) and Sullivan et al. (Reference Sullivan, Xie, Conte, Richards, Shama, Haque, Petri and Nelson2022) both found a more negative magnitude of N2 amplitudes in the activation condition (Go trials) than in the inhibition condition (No-go trials) in children whose average age was around 5 years. In a systematic review and meta-analysis of children’s No-go N2 component, Hoyniak (Reference Hoyniak2017) found several studies that reported more negative magnitudes of N2 amplitudes in the activation condition (Go trials) than the inhibition condition (No-go trials) in children (e.g., Algarín et al., Reference Algarín, Nelson, Peirano, Westerlund, Reyes and Lozoff2013; Trinkl et al., Reference Trinkl, Greimel, Bartling, Grünewald, Schulte-Körne and Grossheinrich2015). One possibility for these inconsistent findings in the literature is that the N2 changes in morphology, making it difficult to know if the same component is being captured across ages or if the changes in amplitude represent different components. Similarly, it is possible that earlier components (e.g., P2) or later components (e.g., P3) overlap with the N2 differently at different ages. In addition, N2 of the No-go condition could be more variable in children, making it smaller in averaged data. This may be especially salient for child-friendly Go/No-go tasks, which use more engaging stimuli to keep children’s attention instead of more controlled stimuli (e.g., simple Xs and Os commonly used with adults). This highlights one of the challenges of ERPs, which rely on correctly identifying the components. In contrast, time-frequency analyses represent the EEG data as oscillations, providing a more direct representation of the neurophysiological mechanisms without having to correctly identify changing or overlapping ERP components (Morales & Bowers, Reference Morales and Bowers2022). Similarly, in this study, we observed theta and delta power and consistency effects between conditions, while the N2 effects were less clear. Moreover, individual differences in theta power were related to prenatal risk and children’s psychopathology.
When examining potential developmental pathways, children’s theta power mediated the negative relation between prenatal SES and children’s externalizing problem. Specifically, prenatal SES negatively and longitudinally predicted children’s theta power, which also had a negative and concurrent relation with their externalizing problems. Similarly, Lecheile et al. (Reference Lecheile, Spinrad, Xu, Lopez and Eisenberg2020) found SES predicted children’s later effortful control, which includes inhibitory control, and was measured by parent report survey and behavioral tasks. In addition, theta power evoked in response to errors and resting theta power have both been related to measures of SES (Bernier et al., Reference Bernier, Calkins and Bell2016; Conejero et al., Reference Conejero, Guerra, Abundis-Gutiérrez and Rueda2016). Thus, the finding in the current study supports the emerging EEG literature linking SES and children’s self-regulation, as indexed by theta power. The present study was the first to identify the mediating role of event-related theta power in the longitudinal negative relation between prenatal SES and children’s externalizing behavior. Furthermore, our findings support reports that reduced mediofrontal theta may be an endophenotype for externalizing disorders for adolescents and young adults (Gilmore et al., Reference Gilmore, Malone and Iacono2010; Kamarajan et al., Reference Kamarajan, Pandey, Chorlian, Manz, Stimus, Anokhin, Bauer, Kuperman, Kramer, Bucholz, Schuckit, Hesselbrock, Porjesz and Abulseoud2015). Similarly, our findings extend Buzzell et al. (Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020), which found that theta power acted as a mediator in the positive relation between neglect in institutional rearing and adolescents’ psychopathology. The prediction from prenatal SES to theta power supported the DOHaD framework that prenatal factors could predict children’s developmental outcomes (Doyle & Cicchetti, Reference Doyle and Cicchetti2018). In addition, because SES is stable and is related to parenting starting from early childhood (Azad et al., Reference Azad, Blacher and Marcoulides2014), the effects of prenatal SES on inhibitory control could also be partly mediated by social interactions with parents (Hackman et al., Reference Hackman, Gallop, Evans and Farah2015). Social interactions with caregivers have been associated with increases in theta power in both children (Wass et al., Reference Wass, Noreika, Georgieva, Clackson, Brightman, Nutbrown, Covarrubias, Leong and Menon2018) and rodent pups (Courtiol et al., Reference Courtiol, Wilson, Shah, Sullivan and Teixeira2018; Sarro et al., Reference Sarro, Wilson and Sullivan2014), and are thought to be one of the mechanisms shaping theta development. Thus, it is possible that parent-child interactions lead to increases in theta power, which are, in turn, protective for psychopathology. Future studies should examine this potential developmental pathway, as well as investigate the type of parenting behaviors that lead to increases in theta power during inhibitory control.
The mediation model findings showed that prenatal SES was an important predictor of children’s brain development related to inhibitory control, convergent with previous findings of a positive relation between SES and self-regulation (Blair & Raver, Reference Blair and Raver2012; Lecheile et al., Reference Lecheile, Spinrad, Xu, Lopez and Eisenberg2020). In addition, although not as frequently implemented as surveys and behavioral measures, electrophysiological measures of inhibitory control were also related to children’s psychopathology. Interestingly, behavioral indices of inhibitory control did not mediate the relation between early risk factors and children’s psychopathology in the current study. Because theta power was the only electrophysiological assessment of inhibitory control that mediated the prediction from early risk factors to children’s psychopathology, the finding highlights the importance of time-frequency measures of inhibitory control. In the current study, we found a longitudinal association of prenatal SES with children’s theta power, but not with theta consistency across trials. These results showed the uniqueness of different time-frequency measures and the importance of differentiating the distinct aspects of the EEG signal (strength and consistency). Another strength of time-frequency analyses pertains to the convergence of findings across species (Narayanan et al., Reference Narayanan, Cavanagh, Frank and Laubach2013; Tsujimoto et al., Reference Tsujimoto, Shimazu and Isomura2006; Womelsdorf et al., Reference Womelsdorf, Johnston, Vinck and Everling2010). In line with our current findings, both rodents and humans who experienced early neglect, an extreme type of deprivation, exhibited reductions in mediofrontal theta power during the juvenile period (Buzzell et al., Reference Buzzell, Troller-Renfree, Wade, Debnath, Morales, Bowers, Zeanah, Nelson and Fox2020; Reincke & Hanganu-Opatz, Reference Reincke and Hanganu-Opatz2017). Although the mediation role of inhibitory control in the developmental pathway from early risk factors to children’s psychopathology depended on the measure of inhibitory control, we still found evidence to support the importance of neural measures of inhibitory control.
In contrast to SES, prenatal maternal psychopathology did not predict children’s inhibitory control. Moreover, children’s inhibitory control did not mediate the positive associations of maternal psychopathology with children’s internalizing and externalizing behaviors. One explanation could be that we did not examine symptoms of maternal psychopathology on a clinical sample, so the level of maternal psychopathology was relatively low with low variance so that it was not predictable to children’s inhibitory control. In addition, instead of inhibitory control, other types of children’s characteristics, like emotion regulation and temperament, that were not examined in the study could be mediators of the developmental pathways between prenatal risk factors to children’s psychopathology.
Limitations and future directions
The current study has several limitations. First, 4-year-old participants did not have data on internalizing and externalizing problems. That is, in the mediation models, data on early risk factors and electrophysiological correlates of inhibitory control were available for the 4-year-old group, but not for internalizing and externalizing problems. Second, the electrophysiological measures of inhibitory control and children’s internalizing and externalizing problems were not collected concurrently for all participants. Future longitudinal studies should measure children’s internalizing and externalizing problems and inhibitory control both concurrently and longitudinally to better address the directionality. Third, we examined the role of prenatal SES and maternal psychopathology on children’s inhibitory control in early/middle childhood without considering the effects of other related factors in the postnatal environment (e.g., postnatal SES and maternal psychopathology). Although SES and maternal psychopathology are relatively stable from the prenatal period to the postnatal period, changes in these risk factors from pregnancy to early childhood have been found to be differentially related to child executive function outcomes (Morales et al., Reference Morales, Bowers, Shuffrey, Ziegler, Troller-Renfree, Hernandez, Leach, McGrath, Ola, Leve, Nozadi, Swingler, Lai, Schweitzer, Fifer, Camargo, Khurana Hershey, Shapiro, Keating, Hartert, Deoni, Ferrara and Elliott2024; Park et al., Reference Park, Brain, Grunau, Diamond and Oberlander2018). Fourth, because maternal depression and anxiety are both internalizing behaviors, we did not examine the predictions of prenatal maternal externalizing behaviors, which could be a focus for future research. Fifth, our study only examined prenatal risk factors. Future studies should examine and control for the effects of risk factors during the postnatal period to better understand prenatal mechanisms. Sixth, the internal consistency was low for internalizing behaviors, potentially due to relatively low levels of children’s internalizing behavior in the sample. Further, based on the findings from the mediation models in the current study, future research could explore the mediating role of electrophysiological measures of inhibitory control with other important behavioral measures of inhibitory control (e.g., Lecheile et al., Reference Lecheile, Spinrad, Xu, Lopez and Eisenberg2020; Spinrad et al., Reference Spinrad, Eisenberg, Silva, Eggum, Reiser, Edwards, Iyer, Kupfer, Hofer, Smith, Hayashi and Gaertner2012), and other EEG paradigms (e.g., oddball).
Conclusion
The current study utilized ERPs and time-frequency measures of inhibitory control to examine if these measures could mediate the relation between prenatal risk factors and children’s psychopathology. Children’s theta power mediated the negative association between prenatal SES and children’s externalizing problems, suggesting the importance of incorporating time-frequency measures of inhibitory control. These findings confirm that prenatal SES and maternal psychopathology play important roles in children’s brain development and are related to inhibitory control and children’s psychopathology. The negative relation between children’s inhibitory control and their psychopathology was supported through electrophysiological measures of inhibitory control. Thus, interventions that improve families’ SES and reduce maternal psychopathology during the prenatal period could help improve children’s self-regulation skills and psychological well-being.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579424000816.
Acknowledgments
We thank the many research assistants involved in collecting the data and the participating families without whom the study would not have been possible.
Funding statement
This research was supported by grants from the National Institutes of Health (UH3 OD023279) to Amy Elliott.
Competing interests
None.








