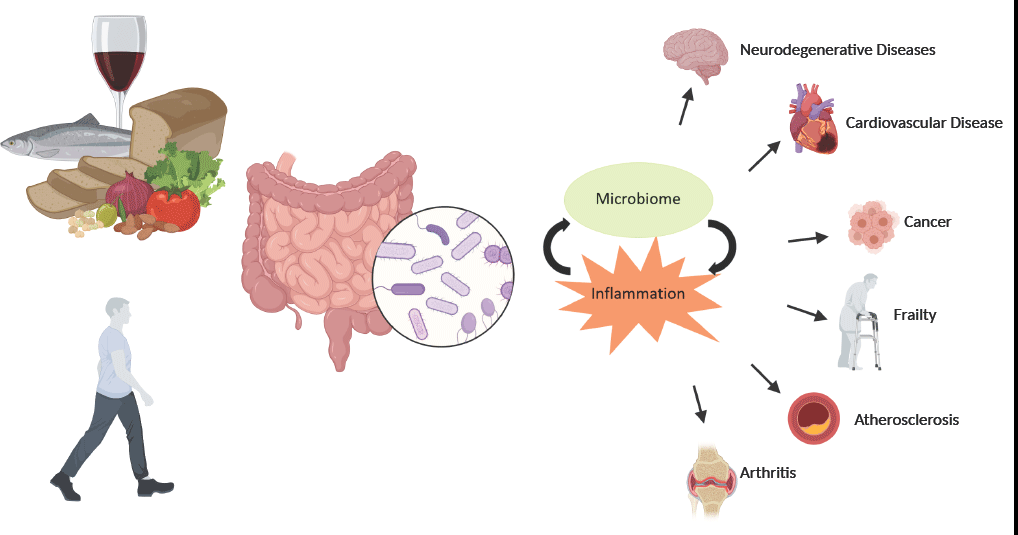
With current projections placing 1·5 billion people globally over the age of 65 by the year 2050, it has never been more important to identify strategies that promote the maintenance of health, well-being and functionality through a person’s older years. Chronic inflammation has been identified as a predominant factor contributing to the onset and progression of many age-related diseases(Reference Michaud, Balardy and Moulis1). The acute inflammatory response is an essential function of a healthy immune system that is triggered in response to chemical or physical stimuli. This process is tightly regulated as inadequate resolution of inflammation or a prolonged exposure to the inflammatory trigger may lead to a state of chronic inflammation that may contribute to the aetiology of diseases such as cancers, type 2 diabetes, atherosclerosis and cardiovascular and neurodegenerative diseases(Reference Chen, Deng and Cui2,Reference Medzhitov3) .
As humans age, there is a notable dysregulation of the immune system which involves a marked decline in the adaptive responses and mild increases in innate activity. This process, called immunosenescence, can impair a person’s ability to fight infection, weaken vaccination response and lead to higher levels of adiposity, and changes in the gut microbiota composition. These outcomes can lead to a state of chronic low-grade inflammation termed inflammageing(Reference Franceschi, Bonafe and Valensin4). This immune dysregulation is accompanied by increased levels of pro-inflammatory cytokines, such as IL-6, TNF-α and C-reactive protein, particularly in older people(Reference Forsey, Thompson and Ernerudh5,Reference Wei, Xu and Davies6) . Increased inflammation, specifically levels of C-reactive protein, IL-6 and TNF-α, has been associated with many age-related diseases including sarcopenia, osteoporosis and fracture, malnutrition, cognitive decline, increased risk of frailty and increased all-cause mortality(Reference Michaud, Balardy and Moulis1,Reference Rea, Gibson and McGilligan7) .
There are many common lifestyle factors that can contribute to chronic inflammation, for instance smoking, alcohol consumption, chronic stress and importantly, poor diet and lack of physical activity (PA). The Mediterranean Diet (MedDiet), a centuries-old dietary pattern evolving from the traditional eating patterns of the countries surrounding the Mediterranean basin, has long been touted as one of the most favourable dietary patterns for promoting good health and longevity(Reference Sofi, Abbate and Gensini8). The diet is predominantly plant based containing high amounts of fruits and vegetables, extra virgin olive oil (EVOO), wholegrains and legumes with moderate consumption of poultry, dairy foods and wine, and low intakes of discretionary foods and red and processed meats(Reference Davis, Bryan and Hodgson9). Early research by Ancel Keys and the Seven Countries Study highlighted the protective effect of the MedDiet against CVD(Reference Menotti and Puddu10,Reference Hatzis, Papandreou and Patelarou11) , and follow-up studies have continued to show the protective effects of a MedDiet across a range of other chronic diseases and overall mortality(Reference Kromhout, Menotti and Alberti-Fidanza12,Reference Fidanza, Alberti and Lanti13) . While this strong evidence exists, some mechanisms supporting these outcomes are not completely understood. One proposed mechanism of action is via the gut microbiota and the reduction of chronic inflammation, a state which underpins many chronic diseases(Reference Tosti, Bertozzi and Fontana14,Reference Thorburn Alison, Macia and Mackay Charles15) .
The aim of this narrative review is to provide an overview of the MedDiet diet and lifestyle, summarise the current evidence on how both a MedDiet and PA influence the human gut microbiota and discuss the relationship between the gut microbiota and healthy ageing, with a focus on inflammation.
Mediterranean diet and lifestyle
The MedDiet has existed for centuries, evolving from the traditional eating patterns of people native to countries surrounding the Mediterranean basin. It is not just one pattern, but rather numerous variations of a similar way of eating, determined by local food availability and cultural and religious practices(Reference Trichopoulou and Lagiou16). Despite these differences, the region’s dietary pattern consists of the same core principles and a focus on fresh wholefoods with limited processing. The diet is predominantly plant based with high intakes of fruits and vegetables, wholegrains, legumes, nuts and seeds and includes a moderate to high consumption of high-quality dietary fats, primarily from EVOO. There is a preference for fish over red meat, limited sweets and a moderate consumption of red wine around mealtimes and social settings(Reference Davis, Bryan and Hodgson9). However, the MedDiet is more than just the food consumed in these regions; the dietary pattern is heavily underpinned with physical activity, adequate rest, culinary activities, frugality and a high degree of socialisation and community(Reference Bach-Faig, Berry and Lairon17).
Research focusing on the MedDiet began in the 1950s when Ancel Keys and the team of the Seven Countries Study investigated the relationship between CVD and various lifestyle factors after noticing the differences in CVD between American businessmen and those living in post-war Europe(Reference Menotti and Puddu10). Multiple follow-up studies have demonstrated the association between dietary patterns, like those seen in the cohorts from Italy and Greece, and a reduced risk of CHD(Reference Menotti and Puddu10,Reference Hatzis, Papandreou and Patelarou11) . Interest in the MedDiet’s effects on health outcomes has only grown in the past few decades. A recent umbrella review by Dinu et al.(Reference Dinu, Pagliai and Casini18) meta-analysed sixteen randomised controlled trials and thirteen meta-analyses of observational studies, involving 12·8 million participants, investigating the relationship between the adherence to a MedDiet and health outcomes. The review provides strong evidence for the protective nature of the MedDiet with an 11 % reduced risk in overall mortality (95 % CI 0·89, 0·93), 33 % reduced risk of CVD (95 % CI 0·58, 0·77) and a 21 % reduced risk of neurodegenerative diseases (95 % CI 0·70, 0·90)(Reference Dinu, Pagliai and Casini18). An increasing number of studies are also focusing on the MedDiet’s impact in maintaining the physical functioning of older people demonstrating an association between higher adherence to a MedDiet and reduced risk of frailty, sarcopenia and development of a mobility disabilities and increased skeletal muscle retention and handgrip strength(Reference Barrea, Muscogiuri and Di Somma19–Reference Milaneschi, Bandinelli and Corsi21).
Traditionally, the populations of the Mediterranean region had high incidental levels of PA due to the laborious nature of their occupational status. For instance, in 1960’s Crete, many inhabitants were shepherds or farmers who would travel up to 20 and 8 km on foot per day, respectively, often on uneven or rugged terrain(Reference Christakis, Severinghaus and Maldonado22). This is a stark contrast to the current lifestyles of those living in developed nations where sedentary behaviours have been increasing for decades due to technological advances and the rise of less active occupations(Reference Owen, Sparling and Healy23). The health benefits of PA are widely accepted in the literature, and it has been associated with reducing the risk of premature mortality and multiple chronic health conditions including some forms of cancer, CVD, metabolic conditions and risk of stroke(Reference Warburton and Bredin24).
Human gut microbiota through life
The gut microbiota consists of approximately 100 trillion microbial cells that form a symbiotic relationship with its human host, exerting many beneficial physiological effects that are protective of disease including maintaining the integrity of the intestinal epithelium, energy extraction from undigestible material, prevention of colonisation by pathogenic microbes and involvement in immune system development and regulation(Reference Turnbaugh, Ley and Hamady25,Reference Huttenhower, Gevers and Knight26) . It is comprised of several bacterial phyla, with Firmicutes and Bacteroidetes being identified as the two most dominant. The relative abundance of these two phyla appears to be an important determinant of human health(Reference Huttenhower, Gevers and Knight26). However, many members of these phyla are capable of exerting different effects through the individual genes they carry, and therefore their ability to produce metabolites like SCFA and bioactive compounds(Reference Wang and Zhao27), suggesting that microbiota diversity and relative abundance of particular taxa, along with their functional capability, may be more important(Reference Claesson, Jeffery and Conde28).
Microbiota colonisation begins at birth and continues to transform rapidly during the early years of life, stabilising at approximately 2–5 years of age where its composition resembles that of an adult microbiota(Reference Rodríguez, Murphy and Stanton29). From this point, the stability and resilience of an individual’s core microbiota increase and temporary external factors, like short-term dietary changes or the use of antibiotics, generate only transient changes in composition. As humans age, there is a gradual and continual shift in lifestyle that includes decreases in nutritional quality and PA, increased intestinal transit time and higher use of medications, which produce a compounding effect on the gut microbiota, and composition begins to alter(Reference Vemuri, Gundamaraju and Shastri30). These alterations present as a loss of microbial diversity, increased inter-individual variability and shifts in the relative abundance of numerous species(Reference Biagi, Nylund and Candela31,Reference Claesson, Cusack and Sullivan32) . Specific changes that have been reported are decreases in Clostridium cluster IV, Faecalibacterium prausnitzii and bifidobacterial members, which are all known for their ability to produce SCFA through the fermentation of fibre(Reference Biagi, Nylund and Candela31,Reference Zwielehner, Liszt and Handschur33–Reference Mueller, Saunier and Hanisch35) . One of the largest studies conducted in an elderly cohort analysed the microbiota composition of participants who were living in different circumstances/environments. The authors reported significant decreases in the microbiota diversity of the elderly participants in long-stay care compared with that of community-dwelling participants, and that this loss of diversity was significantly associated with an increase in frailty. Further investigation found the microbiota shift found in these populations was primarily driven by dietary intake(Reference Claesson, Jeffery and Conde28). Recently, research has investigated the microbiota composition on centenarians, individuals who demonstrate healthy ageing and longevity. Numerous studies have reported a decrease in the core taxa and increases in sub-dominant species including opportunistic pathogens and usually transient bacteria including Methanobrevibacter and Escherichia (Reference Wu, Zeng and Zinellu36,Reference Kim, Choi and Shin37) . Similar to elderly cohorts, decreases in the relative abundance of SCFA producing Faecalibacterium have been reported in centenarians; however, the relative abundance of health promoting bacteria Akkermansia, Bifidobacterium and Christensellaceae is also commonly reported, perhaps promoting health benefits through functional redundancy(Reference Kim, Choi and Shin37,Reference Biagi, Franceschi and Rampelli38) . Wu et al. also reported that the functional profile of a centenarian gut microbiota was distinctly different than that of elderly and young adult participants, including increased SCFA pathways and altered amino acid metabolism(Reference Wu, Zeng and Zinellu36). Similar shifts in taxonomic and functional profiles were reported in a 2021 study in an elderly population(Reference Wilmanski, Diener and Rappaport39). The authors reported an increasingly unique microbiota composition with age, with a decrease in the relative abundance of Bacteroides, and increases in Christensellaceae, Methanobrevibacter and Desulfibrio in healthy participants, with these changes correlating with a decreased risk of mortality in participants over 85 years of age. Further, genus level uniqueness was associated with altered amino acid metabolism, with increases in phenylalanine/tyrosine and tryptophan metabolism(Reference Wilmanski, Diener and Rappaport39). Unfortunately, the cross-sectional nature of studies investigating the gut microbiota of elderly individuals and centenarians makes it impossible to ascertain if the certain taxa seen in healthy ageing are present in the earlier years of life and contribute to the longevity observed, or if the microbiota itself is a consequence of reaching extreme ageing.
Dietary intake and the gut microbiota
As research into the gut microbiota has developed, it has been widely accepted that an individual’s diet and lifestyle is one of the strongest modifiable factors that influences microbial richness, diversity and composition(Reference Singh, Chang and Yan40,Reference Conlon and Bird41) . The influence of diet has been studied over the past two decades, providing an understanding of how nutrition can promote the growth of certain microbial taxa by providing their preferred substrate, but many studies have focused on particular foods or select nutrients(Reference Creedon, Hung and Berry42,Reference So, Whelan and Rossi43) . There is an abundance of research identifying dietary fibre as a key modifier of gut microbiota composition by increasing the relative abundance of beneficial SCFA producers(Reference So, Whelan and Rossi43). As research continues to strengthen the role of the microbiota in host health and disease, additional foods and food components have begun to be investigated. Dietary fats have become of interest due to their ability to traverse the large intestine and colon intact, where they are metabolised by the local gut bacteria and potentially influence microbial composition. Until recently, the effects of high dietary fat intakes on the gut microbiota have failed to differentiate between the fatty acid profiles of meat-based Westernised diets and a plant-centred diet like the MedDiet, which includes high intakes of EVOO, n-3 fatty acids and nuts. While human studies have been scarce thus far, there is emerging evidence that due to its high concentration of phenolic compounds, EVOO can exert both prebiotic and antibacterial effects on the gut microbiota, resulting in increases in beneficial Lactobacillus and Bifidobacterium and decreases in the potentially pathogenic Staphylococcus (Reference Rodríguez-García, Sánchez-Quesada and Algarra44–Reference Luisi, Lucarini and Biffi46). Recent intervention studies have also highlighted the beneficial impacts of n-3 fatty acids on the gut microbiota, showing that they are capable of increasing the relative abundance of SCFA producers in a similar fashion to that of fibre(Reference Watson, Mitra and Croden47,Reference Vijay, Astbury and Le Roy48) . Further, multiple studies investigating the effect of nuts on the gut microbiota have shown that almonds, pistachios and walnuts are all capable of exerting effects on the relative abundance of various gut bacteria. While differences in fatty acid composition between the three types of nuts present difficulties in providing conclusive results, the intake of high PUFA containing walnuts produced significant shifts in β-diversity in all three studies(Reference Bamberger, Rossmeier and Lechner49–Reference Holscher, Guetterman and Swanson51). Additional foods that constitute a MedDiet that have been shown to modulate gut microbiota are dairy foods like milk and yogurt, and polyphenol-rich fruits, vegetables and red wine(Reference Aslam, Marx and Rocks52–Reference Nash, Ranadheera and Georgousopoulou55). A sample of studies detailing the impacts of these foods on the gut microbiota can be found in Table 1.
Table 1. Summary of studies assessing the impact of Mediterranean diet component and a whole Mediterranean diet intervention on the human gut microbiota

MD, Mediterranean diet; MetS, metabolic syndrome.
Recently, there has been a shift towards investigating the role that whole dietary patterns and other lifestyle factors, including PA, play in establishing and modifying the human microbiota. So far, a number of studies have shown PA to be a novel influencer of microbiota composition; however, the mechanisms are not well understood(Reference Mailing, Allen and Buford56).
Effects of a Mediterranean diet and lifestyle on the gut microbiota
Research into the effect of a MedDiet and the microbiota is still in its infancy, with only a limited amount studies being published in the last 5 years. With its emphasis on wholegrains, fruits, vegetables and legumes, the MedDiet is an excellent source of complex carbohydrates and non-digestible fibre, preferentially used by certain microbes as a substrate to produce SCFA (e.g. butyrate) that are beneficial to host health(Reference Chambers, Preston and Frost57). In one of the earliest studies, De Filippis et al. compared the habitual diet of Italian vegans, vegetarians and omnivores regarding their MedDiet adherence and microbiota composition. While they found no significant difference in diversity across diet groups or adherence levels, those who showed higher adherence to a MedDiet reported higher levels of faecal SCFA(Reference De Filippis, Pellegrini and Vannini58). The relationship between MedDiet adherence and faecal SCFA has been corroborated in three subsequent cohort studies where researchers also found changes in the relative abundance of certain microbes(Reference Gutierrez-Diaz, Fernandez-Navarro and Sanchez59–Reference Garcia-Mantrana, Selma-Royo and Alcantara61). These changes included an increase in F. prausnitzii, a known butyrate producer considered to have anti-inflammatory properties(Reference Li, van Esch and Henricks62), and lower levels of Escherichia coli, a well-known gut coloniser with pathogenic and inflammatory potential(Reference Kittana, Gomes-Neto and Heck63).
A recent observational study by Gallè and colleagues collected the habitual dietary habits, PA levels and microbiota composition of 140 Italian university students. A higher adherence to a MedDiet was associated with higher relative abundance of lactic acid bacteria(Reference Galle, Valeriani and Cattaruzza64), which have been previously demonstrated to be beneficial to human health through their ability to enhance metabolism, protect against infections in the gastrointestinal tract and the ability to modulate both allergy and inflammatory responses(Reference Pessione65). This relationship was also demonstrated in a 3-month intervention study where participants followed a MedDiet supplemented with 40 g of EVOO per day showed an increase in the relative abundance of lactic acid bacteria. The magnitude of this effect demonstrated greater significance in overweight and obese participants(Reference Luisi, Lucarini and Biffi46). Additional studies have highlighted the impact that participant obesity classification and metabolic status have on the gut microbiota’s response to a MedDiet. A 2019 study in elderly obese women following a hypoenergetic MedDiet for 15 d showed a significant increase in α-diversity in participants with obesity class II (35–39·9 kg/m2)(Reference Cancello, Turroni and Rampelli66), and in a subset of patients from the CORDIOPREV Study, where Haro and colleagues reported increases in the relative abundance of a number of species including F. prausnitzii and Bifidobacterium longum in the participants with the metabolic syndrome exclusively(Reference Haro, Garcia-Carpintero and Alcala-Diaz67).
Recently, a large intervention study by Ghosh et al. was part of the NU-AGE 12-month randomised clinical trial and investigated the effects of a tailored MedDiet in relation to the gut microbiota and frailty in elderly participants (n 612) across five European countries(Reference Ghosh, Rampelli and Jeffery68). While the authors reported no change in microbial diversity, there were changes in microbial composition that were positively associated with improved cognition and reduced frailty markers and negatively associated with inflammatory biomarkers C-reactive protein and IL-17. Many of the bacterial taxa associated with both adherence and inflammatory biomarkers were known SCFA producers. Similarly, the CARDIVEG study compared the gut microbiotas of participants after 3 months of either a Mediterranean or vegetarian diet and found significant changes in the relative abundance of several taxa in both groups. Interestingly, changes in the SCFA profile of the gut were observed exclusively in the MedDiet group(Reference Pagliai, Russo and Niccolai69). These changes were also negatively associated with levels of pro-inflammatory biomarkers (IL-17 and IL-12)(Reference Pagliai, Russo and Niccolai69), again highlighting the relationship between the gut microbiota, SCFA production and inflammation.
Smaller, more short-term intervention studies have highlighted that beneficial changes in the gut microbiota can even be achieved over a short period of time. Messiler et al. found changes in microbiota composition, and increases in gene richness and faecal SCFA levels within the first 4 weeks on the MedDiet intervention(Reference Meslier, Laiola and Roager70), while Zhu and colleagues reported an increase in bacteria capable of producing SCFA in just 4 d of a MedDiet(Reference Zhu, Sawrey-Kubicek and Beals71). While these studies give evidence that alterations in the relative abundance of certain species of bacteria can be achieved in a limited time frame, these changes are likely transient in nature. Dietary studies with a cross-over design or those including follow-up analyses have shown that the microbiota reverts to pre-study composition a few weeks after the cessation of the dietary intervention(Reference Burton, Rosikiewicz and Pimentel72–Reference Shin, Jung and Kim74). A summary of the relevant studies can be found in Table 1.
Physical activity and the gut microbiota
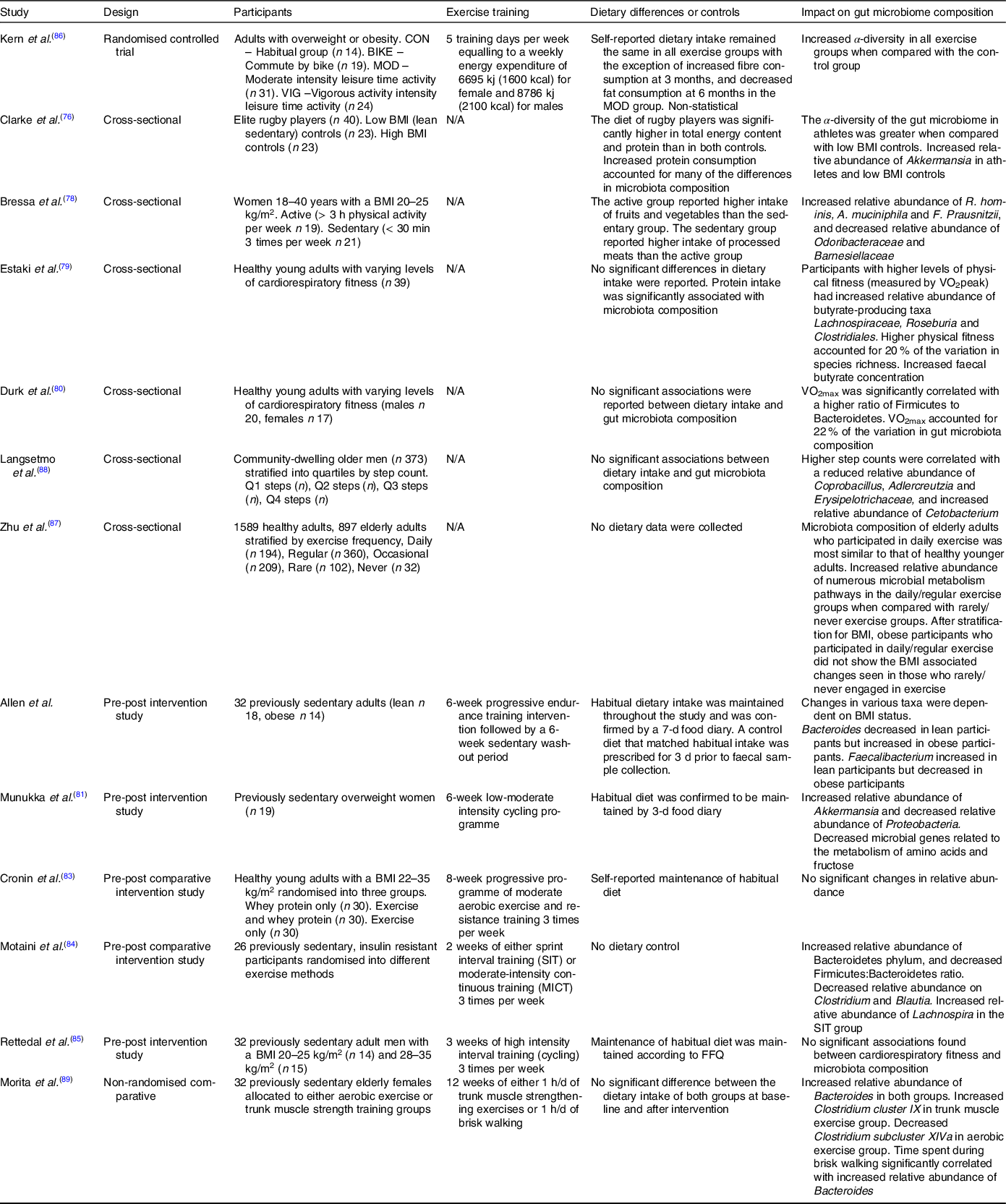
Although there are numerous studies in murine models, research into the effects of PA on the gut microbiota of humans is limited to within the last decade. In the earliest study, Clarke et al. compared the gut microbiota of elite rugby players with that of healthy and high BMI control groups and the authors found increased microbiota diversity within the individual samples (α-diversity) of the rugby players, along with an increase in the relative abundance of several bacterial taxa, notably Akkermansia muciniphila (Reference Clarke, Murphy and O’Sullivan75), a mucin degrading bacteria from the Verrucomicrobia phylum involved in maintaining the integrity of the intestinal barrier, is negatively associated with impaired metabolic function and inflammation(Reference Derrien, Belzer and de Vos76). A. muciniphila abundance was also increased in the active participants of a 2017 study in premenopausal women, along with known butyrate producers F. prausnitzii and Roseburia hominis (Reference Bressa, Bailén-Andrino and Pérez-Santiago77). Other observational studies have also found correlations between levels of cardiovascular fitness and microbiota composition. Estaki et al. found increased α-diversity in participants with greater VO2 max (Reference Estaki, Pither and Baumeister78), while Durk et al. reported significant correlation between VO2 max and changes in the ratio of the two dominant phyla, Firmicutes and Bacteroidetes in a comparable population(Reference Durk, Castillo and Márquez-Magaña79). It should be noted that two of these studies reported significant dietary differences between groups, with the active participants having higher protein and fibre intakes than that of the controls. Consequently, the contribution of PA to the changes in the microbiota cannot be completely ascertained.
The small number of longitudinal studies on the topic so far have reported varying results. In a study conducted by Manukka and colleagues previously sedentary and overweight female participants (n 17) underwent 6 weeks of endurance exercise resulting in shifts in microbiota composition, including an increase in the relative abundance of the Akkermansia genus, but also noted that not all participants responded equally to the intervention(Reference Munukka, Ahtiainen and Puigbó80). A similarly designed study by Allen et al. that included lean and obese participants also reported changes in composition after the 6-week exercise period in the lean participants, with authors noting that changes were dependent on obesity status(Reference Allen, Mailing and Niemiro81). Contradictory evidence was found in another study looking at the effects of exercise and whey protein on the gut microbiota in overweight and obese participants (n 90), where no significant changes in composition were reported, potentially due to the varied obesity status of participants(Reference Cronin, Barton and Skuse82). More recent and shorter longitudinal studies have also failed to provide definitive evidence on the subject. Motaini et al. investigated the effect that 2 weeks of sprint interval training or moderate-intensity continuous training had on the microbiota of twenty-six previously sedentary participants and reported a decreased Firmicutes: Bacteroidetes ratio by an increase of the relative abundance of that both training modalities of Bacteroidetes. Interestingly, the two methods of training had distinctive effects on the relative abundance of certain species, with sprint interval training and moderate-intensity continuous training resulting in an increase in Lachnospira and F. prausnitzii, respectively(Reference Motiani, Collado and Eskelinen83). Conflicting results were reported in a 2020 study that found no significant changes in microbiota diversity or composition after 3 weeks of high intensity interval training in thirty-two male participants(Reference Rettedal, Cree and Adams84).
While there are several observational and longitudinal studies investigating the effects of PA on the microbiota, there is a paucity of evidence from randomised controlled trials outside of animal models. In one of the only randomised controlled trials conducted, Kern et al. implemented a 6-month trial comparing habitual PA and three exercise interventions: commute to work by bike, leisure time moderate exercise and leisure time vigorous exercise (n 88)(Reference Kern, Blond and Hansen85). While all three exercise groups had proportionate exercise-induced energy expenditure, increases in cardiorespiratory fitness and decreases in fat mass, only the vigorous exercise group showed a significant increase in α-diversity independent to fat mass and cardiorespiratory fitness. However, a potential limitation of this study as reported by the authors is that a change in dietary intake due to increased exercise cannot be ruled out as a contributing factor to microbiota composition changes.
Currently, there is limited research investigating the effects of PA on the elderly microbiota and studies so far have yielded varying results. In a 2017 cross-sectional study utilising data from the American Gut Project, Zhu et al. discovered a significant association between higher levels of self-reported PA and a decrease in microbiota α-diversity in overweight elderly (BMI > 25 kg/m) participants(Reference Zhu, Jiang and Du86). Interestingly, the authors also reported a positive association between α-diversity and increasing age, which contradicts numerous studies in the area(Reference Claesson, Jeffery and Conde28,Reference Biagi, Nylund and Candela31,Reference Claesson, Cusack and Sullivan32) . In contrast, a smaller cross-sectional study (n 373) of elderly men found no association between α-diversity and PA levels; however, changes in microbial composition were reported(Reference Langsetmo, Johnson and Demmer87) and Morita and colleagues compared 12 weeks of daily core muscle training or aerobic exercise on the gut microbiota of thirty-two elderly Japanese women, reporting a no change in α-diversity. However, significant increase in the relative abundance of Bacteroides was observed in the aerobic exercise group(Reference Morita, Yokoyama and Imai88). Studies detailing the impact of PA can be found in Table 2.
Table 2. Summary studies assessing the impact of physical activity status or an exercise intervention on the human gut microbiota
Gut microbiota in immune regulation and inflammation
The gut microbiota plays a crucial role in the development, maturation and regulation of the immune system. This intricate relationship begins at birth, where the immature immune system allows for the colonisation and expansion of the microbiota without inflammatory consequence, and immunological sensing of microbial molecules and metabolites allows for the development of food antigen tolerance and protective defences against pathogens concomitantly(Reference Belkaid and Hand Timothy89,Reference Penders, Stobberingh and Brandt90) . Perturbations in the development of the early relationship can have detrimental impacts on long-term health(Reference Arrieta, Stiemsma and Amenyogbe91). Given this interdependent relationship, age-related modifications of the gut microbiota may play a crucial role in the inflammation of ageing.
Studies directly investigating the effect of microbiota composition on inflammatory status have mostly been limited to animal models but have provided evidence that the aged microbiota can increase intestinal inflammation and permeability, and promote infiltration of microbes and microbial products into systemic circulation, which in turn can elevate pro-inflammatory biomarkers such as TNF-α and IL-6(Reference Spychala, Venna and Jandzinski92–Reference Thevaranjan, Puchta and Schulz94). A human study analysing the gut microbiota of three age groups of Italian individuals reported major changes in the centenarian microbiota. Compared with younger cohorts, centenarians showed increases in the phyla Proteobacteria, which have been linked to increased inflammation and the onset of human disease and decreased relative abundance of F. prausnitzii and other SCFA producers. These changes were associated with increases in pro-inflammatory cytokines IL-6 and IL-8(Reference Biagi, Nylund and Candela31,Reference Rizzatti, Lopetuso and Gibiino95) .
While the 2020 study by Ghosh and colleagues focused on the impact of a MedDiet on the gut microbiota, frailty and health outcomes, it also highlights the relationship between the microbial composition of elderly participants and their inflammatory status. The diet positive taxa identified were negatively associated with the levels of C-reactive protein and IL-17(Reference Ghosh, Rampelli and Jeffery68). These results strengthen the theory that gut microbiota composition has an impact on inflammatory outcomes and demonstrate the ability of a MedDiet to induce favourable changes in the ageing gut microbiota.
Microbiota-mediated impacts on inflammation can occur in multiple pathways through various systems, molecules, receptors and cells. The high dietary fibre content of the MedDiet gives rise to an increase in SCFA, the most investigated microbial metabolites, which are capable of acting both locally and systemically. In the gut, they play a critical role in maintaining the integrity of the intestinal barrier as they provide an energy source for colonocytes and have a regulatory role in the expression of tight junction proteins(Reference Peng, Li and Green96). As seen in murine models, if the intestinal barrier is compromised, potentially harmful microbes and microbial products can infiltrate systemic circulation, inducing the inflammatory response and contributing to immune dysregulation(Reference Fransen, van Beek and Borghuis93,Reference Thevaranjan, Puchta and Schulz94) . Further, SCFA also promote the conversion of naïve T cells into forkhead box P3 (Foxp3+) and IL-10 secreting regulatory T cells (Tregs) and interact with intestinal and peripheral innate immune cells via inhibitory effects on histone deacetylase and NF-κB, leading to down-regulation of gene expression for pro-inflammatory cytokines like TNF-α, IL-6 and IL-1(Reference Li, van Esch and Henricks62,Reference Vinolo, Rodrigues and Nachbar97) . Recent work has begun to investigate the microbial metabolites of additional substrates including dietary polyphenols and amino acids. The majority of dietary polyphenols, known for their antioxidant and anti-inflammatory potential, reach the large intestine intact where they undergo transformation into bioavailable compounds by the gut microbiota(Reference Man, Zhou and Xia98,Reference Pandey and Rizvi99) . These compounds, including protocatechuic acid and urolithins, are able to enter circulation and influence the inflammatory response through mitogen-activated protein kinase and NF-κB pathways(Reference González-Sarrías, Larrosa and Tomás-Barberán100,Reference Zheng, Li and He101) . Additionally, the gut microbiota plays a central role in the metabolism of the amino acid tryptophan into various catabolites including kynurenine, indolic compounds, tryptamine and serotonin(Reference Bosi, Banfi and Bistoletti102). The majority of dietary tryptophan is fed into the kynurenine pathway, leading to the creation of various end products that are involved in a host of physiological responses including neurotransmission, and immune system and inflammatory regulation(Reference Bosi, Banfi and Bistoletti102,Reference Agus, Planchais and Sokol103) . One mechanism by which this is achieved is through the activation of the aryl hydrocarbon receptors that are highly expressed in immune cells. The activation of aryl hydrocarbon receptors plays an essential role in T cell differentiation into immunomodulatory Tregs, leading to the down-regulation of inflammation(Reference Man, Zhou and Xia98,Reference Mezrich, Fechner and Zhang104) . The relationship between the kynurenine pathway, gut microbiota and the immune system is complex, with inflammatory mediator and microbiota signalling impacting the production of kynurenine pathway end products(Reference Kennedy, Cryan and Dinan105,Reference Dehhaghi, Kazemi Shariat Panahi and Guillemin106) . This tri-directional communication warrants further research to fully elucidate clinical implications.
In addition to the direct interaction with immune cells, the gut microbiota also engages in bidirectional communication with the brain via the gut–brain axis that is comprised of neural, endocrine, immune and metabolic pathways. Initial research focused on the role of the gut–brain axis in hunger and satiety signalling but has now expanded to exploring its role in conditions including irritable bowel syndrome, depression and anxiety, autism spectrum disorder and neurodegenerative diseases(Reference Dinan and Cryan107–Reference Meguid, Yang and Gleason109). One way in which the gut–brain axis can influence inflammation is through the cholinergic anti-inflammatory pathway(Reference Pavlov and Tracey110). The vagus nerve, which is comprised of both afferent and efferent nerve fibres, has fibres that innervate the intestinal wall. While these fibres do not extend into the intestinal lumen, microbial compounds and metabolites can diffuse across the gut barrier where they can activate the vagus nerve, with the resulting signal is processed by the central nervous system(Reference Bonaz, Bazin and Pellissier111). The proceeding efferent signalling of the vagus nerve results in decreased cytokine secretion by immune cells, including macrophages, dendritic cells and T-cells, through the binding of the neurotransmitter acetylcholine to their nicotinic receptors, heavily influencing intestinal homoeostasis and immune responses(Reference Bonaz, Bazin and Pellissier111,Reference Breit, Kupferberg and Rogler112) .
Knowledge gaps and future directions
The current breath of evidence suggests that both a MedDiet and PA may modulate the gut microbiota composition in a way that is beneficial to host health. However, the majority of studies have been cross-sectional or longitudinal in design and have had wide variation in duration, participant characteristics and outcome measurements, making conclusions regarding causality challenging. There is a definitive need for further well-designed RTC to elucidate a sufficient understanding of the topic. There is also a current gap in the literature investigating the combined effect of these interventions, and to identify any synergistic effects this combination may have on microbiota composition.
Changes in the composition and function of the ageing microbiota have been well demonstrated in the literature so far, and further investigation into the association of these perturbations and the chronic inflammation seen in ageing is warranted. So far, only the NU-AGE project has evaluated the effect of the MedDiet, microbiota and healthy ageing outcomes and additional studies are required to build a body of knowledge capable of influencing healthy ageing strategies.
Conclusion
There is an abundance of literature demonstrating the health benefits of a MedDiet and lifestyle through various stages of life. Comprised of high intakes of fibre, polyphenols and beneficial fatty acids, it is plausible that some of these benefits can be attributed to gut microbiota modulation. As research continues, the role of the gut microbiota in human health is being further elucidated and the intricate relationship between the gut microbiota and the immune system has been brought to the forefront. Although the aetiology of age-related diseases is multi-factorial in nature, involving a multitude of molecular mechanisms, we propose that the beneficial modification of the ageing gut microbiota by utilising a combination of a MedDiet and PA may be a powerful strategy in attenuating age-related inflammation and improving health during a person’s later years of life.
Acknowledgements
We would like to thank the team at APC Microbiome, University College Cork, Ireland for their advice and guidance regarding the microbiome content of this review. This research received no external funding but was completed as part of a Research Training Program (RTP) and postgraduate degree at the University of South Australia.
J. S. C., K. J. M. and B. S. S. contributed to the concept of the review, J. S. C. performed the review and J. S. C., K. J. M. and B. S. S. edited draft and approved the final version.
The authors declare no conflicts of interest.