Obesity is the most common nutritional disorder in companion animals nowadays(Reference German1). Studies conducted in different countries (e.g. England, Australia, USA) have estimated the incidence of overweight and obesity in the dog population between 22 and 40 %(Reference McGreevy, Thomson and Pride2). The cause of overweight and obesity is a chronic energy intake that exceeds energy expenditure. Dietary fibre may aid in the mitigation and prevention of obesity as it may increase and maintain satiety and prevent the feeling of hunger in the dogs. The feeling of hunger may result in an increase in begging and scavenging behaviour(Reference Weber, Bissot and Servet3), which may in turn encourage the owners to feed their pet more than the animal's physiological energy requirement(Reference Jewell and Toll4).
Several studies have evaluated the effect of dietary fibre on satiety in the dogs. Jewell & Toll(Reference Jewell and Toll4) and Jackson et al. (Reference Jackson, Laflamme and Owens5) showed a reduced daily energy intake when the dogs were fed high-fibre diets. In addition, voluntary food intake (VFI) of an additional meal 75 min after consumption of the morning meal was lower in the dogs fed high-fibre diets(Reference Jewell and Toll4). No effect of dietary fibre on VFI in the dogs was found by Butterwick & Markwell(Reference Butterwick and Markwell6). However, the dogs in the latter study were overweight and supplied with approximately 45 % of calculated maintenance energy requirements at target body weight (BW) to induce weight loss. This restriction in daily energy intake may have resulted in an increased feeding motivation to a level that nullified possible effects of dietary fibre on satiety(Reference Jackson, Laflamme and Owens5).
Several physical and chemical properties of dietary fibres may influence the duration of postprandial satiety. Fibre fermentability yielding SCFA may affect satiety through its actions on the production and secretion of gastrointestinal satiety hormones. Infusion of SCFA in the colon of rats(Reference Anini, Fu-Cheng and Cuber7) and oleic acid in the colon of dogs(Reference Greeley, Hashimoto and Izukura8) increased peripheral peptide tyrosine–tyrosine (PYY) concentrations. PYY can cross the blood–brain barrier and act on the arcuate nucleus of the hypothalamus, stimulating neurons that create a sensation of satiety and inhibiting neurons that stimulate feeding behaviour(Reference Batterham, Cowley and Small9). Stimulation of the secretion of glucagon-like peptide-1 (GLP-1), a proglucagon-derived peptide secreted by the enteroendocrine L-cells present in the distal part of the gastrointestinal tract(Reference Holst10), was increased by the inclusion of fermentable fibres in the diets of dogs during an oral glucose tolerance test(Reference Massimino, McBurney and Field11). Both PYY and GLP-1 contribute to the ileal brake and increase gastric emptying time and small intestinal transit time(Reference Wen, Phillips and Sarr12). This may prolong gastric distension and signals of satiation(Reference Pappas, Melendez and Debas13) and prolong the contact between nutrients and small intestinal receptors involved in maintaining satiety(Reference Houpt14). A delay in gastric emptying will also delay starch digestion and subsequent absorption of glucose(Reference Holt, Heading and Carter15), thereby maintaining more stable postprandial glucose and insulin concentrations in the blood(Reference Roberfroid16). Sows fed a diet high in sugarbeet pulp had more stable postprandial glucose concentrations compared with those fed a low-fibre diet that showed a drop in glucose concentration below basal levels. This was associated with an increase in physical activity possibly caused by the feelings of hunger(Reference de Leeuw, Jongbloed and Verstegen17). Fermentable fibres have also been found to affect peripheral ghrelin concentrations, a hormone correlated with hunger or appetite(Reference Cummings, Purnell and Frayo18). Rats fed diets supplemented with a short-chain oligofructose showed lower active ghrelin plasma concentrations 8 h after the last meal compared with those fed the diet without fructan supplementation(Reference Cani, Dewever and Delzenne19).
There is still little information available regarding the potency of various fermentable fibres to affect the satiety in dogs. The aim of the present study was therefore to investigate whether an increase in dietary fibre fermentability prolongs the duration of postprandial satiety as measured by VFI and physiological satiety metabolites when included in the diets of dogs.
Experimental methods
Animals
Sixteen (eight males and eight females) healthy adult beagle dogs aged between 2 and 6 years with an initial BW between 7·2 and 11·4 kg were individually housed in indoor pens at the Laboratory of Animal Nutrition of Ghent University (Merelbeke, Belgium). Dietary treatments were equally distributed among pens. The dogs were assigned to one of two dietary treatments (low-fermentable fibre (LFF) or high-fermentable fibre (HFF)) according to BW and sex (blocking factors) resulting in a mean BW of 9·7 (sem 0·5) and 9·7 (sem 0·4) kg for the LFF and the HFF groups, respectively. All the dogs were weighed before the start of the experiment and thereafter every 2 weeks until the end of the experiment. Each dog was fed individually to meet its maintenance energy requirement estimated at 415 kJ metabolisable energy/kg BW0·75(Reference Hesta, Roosen and Janssens20). The diets were fed twice daily in two equal portions at 08.30 and 18.30 hours after mixing with an equal amount of lukewarm water to increase palatability. Food intake was recorded during each meal throughout the entire experimental period and freshwater was provided ad libitum. All animal housing, care and experimental procedures were approved by and conformed to the requirements of the Ethical Committee of the Faculty of Veterinary Medicine of the Ghent University (Belgium, EC 2007/40).
Diets
The dogs were fed one of the two experimental diets formulated to be iso-nitrogenous and iso-energetic on a metabolisable energy basis, and iso-fibrous on a total dietary fibre (TDF) basis. Ingredient composition of both diets is shown in Table 1. The LFF diet contained cellulose as a fibre source, whereas the HFF diet contained a combination of sugarbeet pulp and inulin. Differences in fermentability between fibre sources used were based on the in vitro studies(Reference Bosch, Pellikaan and Rutten21, Reference Sunvold, Fahey and Merchen22). The content of molasses in the sugarbeet pulp was estimated to be 5 % and an identical amount of molasses was added to the LFF diet. TiO2 (2 g/kg diet) was included as an inert digestibility marker(Reference Jagger, Wiseman and Cole23).
Table 1 Composition of the low-fermentable fibre (LFF) and high-fermentable fibre (HFF) diets
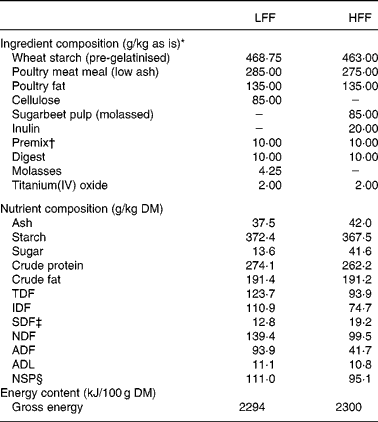
TDF, total dietary fibre; IDF, insoluble dietary fibre; SDF, soluble dietary fibre; NDF, neutral-detergent fibre; ADF, acid-detergent fibre; ADL, acid-detergent lignin.
* Wheat starch, Pregel Wheat Alpha (Meneba, Weert, The Netherlands); poultry meat meal, Meat Meal 63 (Sonac, Lingen, Germany); poultry fat (Sonac, Lingen, Germany); cellulose, Arbocel BWW40 (J. Rettenmaier Benelux, Zutphen, The Netherlands); sugarbeet pulp, molasses (Research Diet Services, Wijk bij Duurstede, The Netherlands); inulin, Beneo IPS (Orafti, Tienen, Belgium); premix (Twilmij B.V., Stroe, The Netherlands); digest, Luxus Digest N8008 (AFB International, Nuland, The Netherlands); titanium(IV) oxide (Sigma-Aldrich Chemie B.V., Zwijndrecht, The Netherlands).
† The premix provided per kilogram of diet: Ca, 0·41 g; P, 0·07 g; Mg, 0·05 g; K, 0·1 g; Na, 0·01 g; Cl, 0·09 g; linoleic acid, 0·15 g; PUFA, 0·17 g; lysine, 0·05 g; methionine, 0·02 g; methionine+cysteine, 0·04; threonine, 0·04 g; tryptophan, 0·02 g; retinol, 5·25 mg; vitamin D3, 50 μg; vitamin E, 100 mg; vitamin K3, 2 mg; vitamin B1, 10 mg; vitamin B2, 10 mg; niacin, 50 mg; pantothenic acid, 25 mg; vitamin B6, 7·5 mg; vitamin B12, 50 μg; biotin, 300 μg; choline chloride, 475 mg; folic acid, 1·25 mg; vitamin C, 100 mg; Fe, 75 mg; Mn, 35 mg; Cu, 5 mg; Zn, 75 mg; I, 1·75 mg; Co, 2 mg; and Se, 0·2 mg.
‡ Calculated by subtracting the IDF content from the TDF content.
§ Derived by subtracting the crude protein, crude fat, starch and sugar content from the organic matter content(Reference de Leeuw, Jongbloed and Verstegen17). As inulin was included in the analysed sugar content, the NSP content of the HFF diet is underestimated with approximately 18 g/kg DM (20 g/kg included in the diet with 90 % pure inulin).
Chemical analyses
The diets were analysed for DM, ash, starch, sugar, crude protein, crude fat, TDF, insoluble dietary fibre, neutral-detergent fibre (NDF), acid-detergent fibre (ADF), acid-detergent lignin and Ti. DM and ash contents were determined by drying to a constant weight at 103°C and combustion at 550°C, respectively. The starch content was analysed enzymatically(Reference Brunt24), while reducing sugars were extracted from the feed samples using 40 % ethanol and determined as described by Suárez et al. (Reference Suárez, Van Reenen and Beldman25). Crude protein (6·25 × N) was determined using the Kjeldahl method (ISO 5983-1, 2005) and crude fat was analysed according to the Berntop method (ISO 6492, 1999) with faecal samples being pre-digested with HCl. TDF and insoluble dietary fibre were analysed using the Association of Official Analytical Chemists methods(Reference Cunniff26, Reference Cunniff27). The soluble dietary fibre content was calculated by subtracting the insoluble dietary fibre content from the TDF content. Note that the inulin would not be recovered in the TDF fraction(Reference Prosky and Hoebregs28). NDF was analysed in defatted diet samples (fat extraction with petroleum-ether) according to a modified method of Van Soest et al. (Reference Van Soest, Robertson and Lewis29) described by Goelema et al. (Reference Goelema, Spreeuwenberg and Hof30). The ADF and acid-detergent lignin contents were determined according to Van Soest(Reference Van Soest31). Ti was analysed using a modified method based on the work by Short et al. (Reference Short, Gorton and Wiseman32) and Myers et al. (Reference Myers, Ludden and Nayigihugu33). The content of NSP was calculated by subtracting the starch, sugar, crude protein and crude fat content from the organic matter content(Reference de Leeuw, Jongbloed and Verstegen17). As inulin was included in the analysed sugar content, the NSP content of the HFF diet is underestimated with approximately 18 g/kg DM (20 g/kg included in the diet with 90 % pure inulin).
Apparent digestibility
After 10 d of adaptation to the experimental diets, a 3 d faecal collection was conducted for the determination of apparent digestibility of nutrients. On these days, all faeces produced by each dog were collected twice a day and weighed. The faeces were freeze-dried to a constant weight, pooled per dog and ground over a 1 mm sieve in a Retsch mill (ZM100, Retsch B.V., Ochten, The Netherlands). Then the faeces of each dog were analysed for DM, ash, crude protein, crude fat, NDF, ADF, acid-detergent lignin and Ti, according to the procedures described previously. Starch and sugar were not analysed as these were assumed to be completely digested and absorbed. The NSP content of faeces was calculated by subtracting the crude protein and fat contents from the organic matter content. The apparent digestibility coefficient (ADC) for the nutrients was calculated using the following equations:
where Nutrientflow is the nutrient flow (g/d), Nutrientf is the nutrient content of faeces (g/kg DM), Tii is the Ti intake (g), Tif is the Ti content of faeces (g/kg DM) and Nutrienti is the nutrient intake (g/d).
Faecal consistency and fermentation products
To evaluate colonic microbial fermentative activity for both dietary treatment groups, fresh faeces were collected from each dog during week 5 of the experiment within 15 min of defecation. Faeces consistency was scored using the following system(Reference Middelbos, Fastinger and Fahey34): 1 = hard, dry pellets – small, hard mass; 2 = hard, formed, dry stool – remains firm and soft; 3 = soft, formed moist – softer stool that retains shape; 4 = soft, unformed – stool assumes shape of container; 5 = watery – liquid that can be poured. Directly after faecal scoring, the faeces were collected and homogenised using two spoons whereafter the samples were taken for SCFA, NH3 and DM contents. All materials used for faeces collection and sampling were pre-sterilised using 70 % ethanol. For the determination of faecal SCFA and NH3 content, a sample of approximately 0·5–1·0 g was added to a 2 ml safe-lock tube (Eppendorf AG, Hamburg, Germany) containing 1·0 ml of 0·033 M-H3PO4 for SCFA analysis or 1·0 ml of 10 % TCA for NH3 analysis. After the addition of faeces, the contents of each tube were mixed on a vortex for 3 s, weighed and stored at − 20°C. For DM determination, approximately 1·5 g of faeces was added to a pre-weighed 2 ml safe-lock tube (Eppendorf AG), weighed and stored at − 20°C. For the determination of SCFA and NH3, the samples were thawed, mixed and centrifuged at 15 000 rpm for 5 min at 4°C (Centrifuge 5417R, Eppendorf AG). Concentrations of the SCFA (i.e. acetate, propionate, butyrate, iso-butyrate, valerate, iso-valerate) in the supernatant were determined as described by Bosch et al. (Reference Bosch, Pellikaan and Rutten21) Branched-chain proportion was calculated as the percentage of branched-chain fatty acids (iso-butyrate, valerate, iso-valerate) of total SCFA(Reference Awati, Williams and Bosch35). The faecal DM content was determined by freeze-drying to constant weight and used to calculate SCFA and NH3 content in the original faeces.
Blood sampling and plasma analyses
Blood sampling was performed in week 6 of the experiment. The dogs were sedated using 0·02 ml/kg BW methadone hydrochloride (Mephenon®, Denolin, Brussels, Belgium) and a central venous catheter (18G/20 cm Leaderflex®; Vygon, Écouen, France) was placed in the jugular vein. The catheters were flushed with 1 ml heparinised saline (0·1 mg heparin/ml saline solution) directly after catheter placement and just before the sampling procedure. Furthermore, at the time of placement of the catheter, 15 mg/kg BW amoxicillin (Clamoxyl LA®, GlaxoSmithKline N.V., Genval, Belgium) was administered subcutaneously. Blood samples (2·5–3·0 ml) were obtained from each dog 30 min prior to feeding and 20, 40, 60 and 90 min postprandial. Thereafter, blood was sampled from four dogs in each group at 120, 180, 240, 300, 360, 420, 480 and 540 min after feeding, while the other four dogs in each group were sampled at 150, 210, 270, 330, 390, 450, 510 and 570 min after feeding. The blood samples were collected in chilled collection tubes containing K3EDTA as an anticoagulant. After gentle mixing of the contents, each collection tube was opened and 25 μl dipeptidyl peptidase-IV inhibitor (Linco Research, MI, USA) and 125 μl Trasylol® (1·4 mg aprotinin/ml, Bayer AG, Leverkusen, Germany) were added. After gentle mixing of the contents, the tubes were temporarily stored on ice until centrifugation at 2500 g for 15 min at 4°C. After centrifugation, plasma was removed and stored in safe-lock tubes (Eppendorf AG) at − 20°C until analysis. Each blood sample was processed within 30 min after collection. Blood plasma was analysed for glucose, insulin, total PYY, total GLP-1 and total ghrelin concentration. Plasma glucose was analysed according to the hexokinase method using a commercial test kit (Human GmbH, Wiesbaden, Germany), while plasma insulin, total PYY and total ghrelin were analysed using commercial RIA kits (human-specific insulin RIA kit, Linco Research; rat/mouse PYY RIA kit, Linco Research; and total ghrelin RIA kit, Linco Research, respectively). Plasma GLP-1 was analysed using an RIA specific for the C-terminal of the amidated GLP-1(Reference Hvidberg, Nielsen and Hilsted36, Reference Ørskov, Rabenhøj and Wettergren37). The intra-assay CV for the assays were 7·1 % for insulin, 6·2 % for ghrelin, 14·8 % for PYY and 6 % for GLP-1. The values obtained at 120 and 150, 180 and 210, 240 and 270, 300 and 330, 360 and 390, 420 and 450, and 480 and 540 min postprandial were analysed together and are presented as time points 135, 195, 255, 315, 375, 435 and 495 min, respectively. The basal concentration was defined as the average of the level in the first and last samples (30 min before the morning feeding and 45 min before the evening feeding, respectively). For PYY, GLP-1 and ghrelin, the area under the curve (AUC) from basal until 195 min after the meal and the AUC from 195 to 495 min after the meal for each measured parameter were approximated using the trapezoidal summation. Trapezoids were calculated as the length of the base (interval time between consecutive samples in min) times the average of the heights of the two sides (concentrations of consecutive samples). The time intervals were selected based on a minimal orocaecal transit time of approximately 2·7 h in Standard Schnauzers with a BW of 12·9 (sem 2·1) kg(Reference Hernot, Dumon and Biourge38). From this time onwards, the digesta arrives in the large intestine and fermentable dietary fibre becomes available for the microbial population and SCFA may be produced.
Voluntary food intake
At the end of the study (week 7), each dog was offered 1 kg of the dry extruded control diet that dogs previously experienced as palatable (Hill's Science Plan Canine Adult with Beef, Hill's Pet Nutrition Inc., Topeka, KS, USA). The dogs were allowed to eat for 20 min, after which food intake was recorded. The diet was offered to each dog at precisely 6 h after the morning feeding (14.30 hours).
Statistical analyses
The dogs were randomly allocated to the two treatments according to the BW and sex. All data were analysed using the Statistical Analysis Systems statistical software package version 9.1 (SAS Institute, Cary, NC, USA). Differences in the ADC of nutrients, faecal characteristics (faecal score, DM, SCFA and NH3) and plasma metabolites (the basal concentrations of glucose, insulin, PYY, GLP-1 and ghrelin and AUC (0–195 and 195–495 min) of PYY, GLP-1 and ghrelin) between the dietary treatment groups were tested for significance using ANOVA by Proc GLM. The model used was Y = μ+D i+ɛij, where Y is the dependent variable, μ is the average intercept, D i is the effect of diet i and ɛij is the error term. For the VFI data, BW loss (as the percentage of initial BW) tended to be significant (P = 0·098) and was therefore included in the statistical model as a covariate. The effects of diet and time after feeding on plasma glucose, insulin, PYY, GLP-1 and ghrelin were tested for significance using ANOVA by Proc MIXED. The statistical model was Y = μ+D i+T j+(D × T)ij+ɛijk, where Y is the dependent variable, μ is the average intercept, D i is the effect of diet i, T j is the effect of time j, (D × T)ij is the interaction between diet and time and ɛijk is the error term. The basal concentrations were significant (P < 0·010) and included in the model as covariate. The correlations between VFI and plasma glucose and hormone concentrations were calculated using the Proc CORR statement. Differences were considered to be significant at P ≤ 0·05.
Results
All dogs remained healthy throughout the study, although a general decrease in the BW was observed for both groups (approximately 5 % BW loss for each dietary treatment). No significant differences were found between the dietary treatments in the BW at the start and end of the experiment and BW loss (P = 0·906, 0·909 and 0·927, respectively; data not shown). One dog in the LFF treatment group lost substantial BW during the trial and showed very high concentrations of ghrelin compared with the other dogs. The obtained physiological and VFI data from this dog were therefore excluded from the statistical analyses.
Apparent digestibility
The dogs fed the HFF diet showed higher ADC for DM and organic matter (P < 0·001), whereas the LFF-fed dogs had a higher ADC for crude fat (P < 0·001) and tended to have a higher crude protein digestibility (P = 0·099; Table 2). The NSP digestibility was higher for the HFF diet compared with the LFF diet (P < 0·001). In addition, the dogs fed the HFF diet showed higher ADC for NDF (P < 0·001) and ADF (P = 0·002) and tended to have a lower ADC for acid-detergent lignin (P = 0·082) compared with the dogs fed the LFF diet. Finally, the ADC for energy was higher for the HFF-fed dogs compared with the LFF-fed dogs (P < 0·001).
Table 2 Apparent digestibility coefficient (ADC; %) for nutrients and energy in the low-fermentable fibre diet (LFF) or the high-fermentable diet (HFF) fed to dogs
(Mean values with their standard errors for eight LFF-fed dogs and eight HFF-fed dogs)
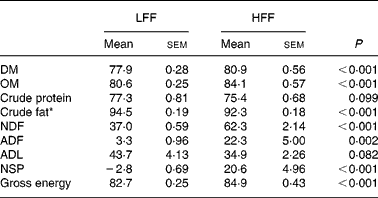
OM, organic matter; NDF, neutral-detergent fibre; ADF, acid-detergent fibre; ADL, acid-detergent lignin.
* Due to the limited amount of faecal material available for the analysis, the values presented were based on seven dogs for the LFF treatment and six dogs for the HFF treatment.
Faecal consistency and fermentation products
Significant differences in the faecal characteristics between the treatment groups were observed (Table 3). The faecal DM content was lower for the dogs fed the HFF than the LFF (P < 0·001) diet. Compared with the dogs fed the LFF diet, higher total SCFA, acetate and propionate concentrations were found for the dogs fed the HFF diet (P < 0·001). Moreover, butyrate concentrations tended to be higher in the HFF dogs (P = 0·060). The dogs fed the LFF diet showed a higher branched-chain ratio and NH3 concentration in the faeces compared with the dogs fed the HFF diet (P = 0·002 and 0·009, respectively). No treatment effect was found for faecal consistency score (P = 0·590).
Table 3 Characteristics of the faeces of the dogs fed the low-fermentable fibre (LFF) diet and the high-fermentable (HFF) diet
(Mean values with their standard errors for eight LFF-fed dogs and eight HFF-fed dogs)
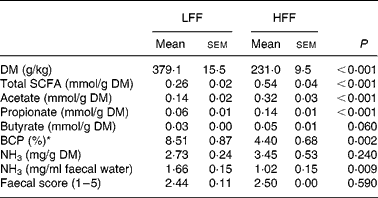
BCP, branched-chain proportion.
* Calculated as the percentage of branched-chain fatty acids (iso-butyrate, valerate, iso-valerate) of total SCFA(Reference Awati, Williams and Bosch35).
Plasma metabolites
Plasma glucose, insulin, PYY, GLP-1 and ghrelin parameters for both the dietary groups are shown in Table 4. The basal concentrations of the measured metabolites were not different between the treatments groups (P>0·05). For all the measured metabolites, postprandial concentrations changed after the meal (P < 0·01), but the concentrations were not affected by the dietary treatment (P>0·10 for diet and diet × time interaction, data not shown). No significant differences were found between the treatment groups in AUC0–195 min and AUC195–495 min of PYY, GLP-1 and ghrelin (P>0·10).
Table 4 Plasma glucose, insulin, peptide tyrosine–tyrosine (PYY), glucagon-like peptide-1 (GLP-1) and ghrelin parameters in the dogs fed a low-fermentable fibre diet (LFF) or a high-fermentable fibre diet (HFF)
(Least-squares means with their standard errors for seven LFF-fed dogs and eight HFF-fed dogs)
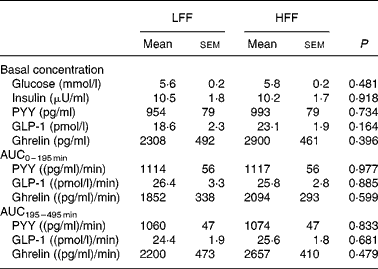
AUC, area under the curve.
Voluntary food intake
For each dog, the amount of food consumed at the end of the study was lower than the amount of food offered. The dogs fed the HFF diet tended to show a lower VFI compared with the dogs fed the LFF diet (P = 0·058, Fig. 1). No significant correlations were found between VFI and glucose, insulin, PYY, GLP-1 or ghrelin concentration in plasma at 6 h after the meal (P>0·05, data not shown).

Fig. 1 Voluntary food intake of the low-fermentable fibre (LFF) and high-fermentable diet (HFF)-fed dogs. Dogs had ad libitum access for 20 min to the control diet that was presented 6 h following their morning meal (experimental diet). Values are means for seven dogs fed the LFF and eight dogs fed the HFF, with their standard errors represented by vertical bars (P = 0·058).
Discussion
The present study evaluated the impact of dietary fibre fermentability on the duration of postprandial satiety as measured by the hormones involved in satiation and VFI in dogs. The selection of fibre sources was based on the in vitro fermentation studies(Reference Bosch, Pellikaan and Rutten21, Reference Sunvold, Fahey and Merchen22), that showed a low microbial degradability for cellulose and moderate and rapid fermentability for, respectively, sugarbeet pulp and inulin using the faeces from the dogs as inoculate. The difference in fibre degradability between the two diets was also shown in the present study. The dogs fed the HFF diet showed a higher ADC for NDF, ADF and NSP compared with the LFF-fed dogs, indicating a higher intestinal microbial degradability of those fibre sources used in the HFF diet. The higher microbial fibre degradation in the HFF-fed dogs resulted in a higher SCFA production, also reflected in a higher SCFA concentration in the faeces of these dogs. In the case of low availability of fermentable fibre (as with the LFF diet), the microbial population will probably resort to more proteolytic fermentation(Reference Williams, Verstegen and Tamminga39). This was observed in the present study where the LFF-fed dogs showed a higher faecal NH3 concentration and BCP, both being the indicators of microbial protein degradation(Reference Macfarlane, Gibson and Beatty40). Finally, the ADC for crude protein tended to be lower for the dogs on the HFF diet, which is in agreement with the similar studies evaluating fermentable dietary sources(Reference Hesta, Roosen and Janssens20, Reference Flickinger, Schreijen and Patil41, Reference Verlinden, Hesta and Hermans42). It is suggested that this decrease should not be attributed to a lower true protein digestibility(Reference Flickinger, Schreijen and Patil41), but is related to an increased microbial proliferation and to a higher faecal bacterial protein excretion(Reference Hesta, Roosen and Janssens20, Reference Verlinden, Hesta and Hermans42). From these summarised results, it can be concluded that compared with the LFF diet, the HFF diet resulted in higher large intestinal dietary fibre fermentation. This would consequently lead to higher SCFA concentrations in the large intestine.
The increased large intestinal fermentation was expected to have an impact on host satiety and appetite through its effect on the secretion of the gastrointestinal satiety-related hormones PYY, GLP-1 and ghrelin. Concerning the feelings of satiety and appetite, the dogs in the HFF treatment group tended to have a lower VFI compared with the LFF-fed dogs (P = 0·058). This suggests that dogs fed the HFF diet were less motivated to consume food when freely available. The amount of food consumed was however not correlated with any of the measured physiological metabolites. The causal relationship between the postprandial satiety-related hormone concentrations and the feelings of satiety or hunger varies between studies. For example, several recent studies found an association in the human subjects of changes in self-reported hunger or satiety after a test meal with changes in the concentrations of postprandial PYY(Reference Stoeckel, Weller and Giddings43, Reference Stock, Leichner and Wong44), whereas Weickert et al. (Reference Weickert, Spranger and Holst45) reported blunted postprandial PYY and ghrelin responses in healthy women without alterations in hunger scores. Furthermore, Smeets et al. (Reference Smeets, Soenen and Luscombe-Marsh46) found that a high-protein lunch increased satiety but without increasing the plasma GLP-1 response, whereas a lunch adequate in protein but with a high carbohydrate content resulted in lower satiety rating but with increased GLP-1 response. Based on these findings, it was therefore suggested that the concentrations in the satiety-related hormones may be related to the nutrient-induced satiety without being directly and mathematically related to satiety(Reference Veldhorst, Smeets and Soenen47). This relationship may also indicate differences in interactions with other hormones or central sensitivity for these hormones(Reference Smeets, Soenen and Luscombe-Marsh46).
It can be questioned whether the dietary contrasts in the present study were sufficient to evoke differences in the secretion of measured hormones. The HFF contained 85 g/kg as-fed sugarbeet pulp and 20 g/kg as-fed inulin which was slightly higher compared with the HFF diet used by Massimino et al. (Reference Massimino, McBurney and Field11) (60 g/kg sugarbeet pulp, 20 g/kg gum arabic, 15 g/kg fructo-oligosaccharide on as-fed basis). The dogs fed the latter diet showed enhanced GLP-1 production and plasma GLP-1 concentrations after an oral load of glucose compared with the dogs fed a diet containing 70 g/kg as-fed cellulose(Reference Massimino, McBurney and Field11). The dietary contrast in the present experiment can therefore be considered to have the potential to affect at least GLP-1 production and secretion.
The present study aimed to induce a contrast in large intestinal SCFA concentrations that would affect the secretion of PYY and GLP-1 by the enteroendocrine L-cells. These specialised cells are present predominantly in the canine distal gastrointestinal tract(Reference Onaga, Zabielski and Kato48). It has been suggested that SCFA (mainly acetate and propionate) activate the GPR43 receptor expressed by the L-cells that are consequently stimulated to release PYY(Reference Karaki, Mitsui and Hayashi49). Several studies reported increased PYY release after the large intestinal infusion of SCFA in rats(Reference Anini, Fu-Cheng and Cuber7) and oleic acid in dogs(Reference Greeley, Hashimoto and Izukura8). In addition, inclusion of fermentable fibre in a diet increased large intestinal PYY gene expression(Reference Keenan, Zhou and McCutcheon50) and PYY concentrations in rats(Reference Keenan, Zhou and McCutcheon50, Reference Delzenne, Cani and Daubioul51). Gee & Johnson(Reference Gee and Johnson52) reported similar effects of a single meal of fermentable fibre on plasma PYY concentrations in rats, but in the human subjects the observed effects were less. In addition to the production of PYY, the L-cells produce GLP-1 derived from the precursor molecule proglucagon(Reference Holst10). Several studies reported enhanced expression of the proglucagon gene by SCFA(Reference Tappenden, Drozdowski and Thomson53) or inclusion of fermentable fibre in the diet(Reference Massimino, McBurney and Field11, Reference Keenan, Zhou and McCutcheon50). Moreover, fermentable fibre increased the number of L-cells in the proximal colon of rats(Reference Cani, Hoste and Guiot54). On the other hand, GLP-1 release was not stimulated after large intestinal SCFA infusion in rats(Reference Anini, Fu-Cheng and Cuber7). Interactions between satiety-related hormones may have contributed to the observed effects on satiety. For example, Neary et al. (Reference Neary, Small and Druce55) observed additive effects of PYY and GLP-1 in the inhibition of appetite and induction of satiety. Similarly, in obese rats a combination of intraperitoneal injection of amylin and PYY was found to reduce food intake more than amylin or PYY alone(Reference Roth, Coffey and Jodka56).
Other mechanisms underlying the feelings of satiety or hunger may also have contributed to the observed differences between the treatment groups. Although for both experimental diets postprandial glucose concentrations were equally stable and no large fall below basal glucose level was found in sows as observed by de Leeuw et al. (Reference de Leeuw, Jongbloed and Verstegen17), small transient declines in blood glucose concentrations could still be present and different between treatments. A transient decline in blood glucose preceded meal initiation in rats(Reference Louis-Sylvestre and Le Magnen57) and a meal request in the human subjects(Reference Campfield, Smith and Rosenbaum58). Similar observations could only be performed when blood was sampled more frequently for glucose determination or continuous monitoring of blood glucose concentrations. Furthermore, the SCFA mainly produced in the HFF-fed dogs can be used as a source of energy (mainly acetate) at times when glucose supply from the small intestine is decreasing(Reference Bergman59, Reference Bleiberg, Beers and Persson60). Bleiberg et al. (Reference Bleiberg, Beers and Persson60) estimated that large intestinal acetate production could contribute in excess of 5 % of the total energy needs of dogs. Whether the SCFA from large intestinal fermentation and used as a source of energy will lead to pronounced differences in the feelings of satiety remains to be investigated.
In conclusion, the present study showed that the dogs fed the HFF diet had an increased large intestinal fibre degradation and the production of SCFA than the dogs fed the LFF diet. The HFF-fed dogs consumed less food during a challenge meal, which may be related to increased feelings of satiety. Postprandial plasma PYY, GLP-1, ghrelin and glucose responses did not differ between the treatment groups and could not be linked to the observed lowered voluntary food consumption of the dogs fed the HFF diet. It is likely that other satiety-related hormones and/or mechanisms controlling the feelings of satiety or hunger may have been involved in the observed decrease in VFI in the present study. Finally, inclusion of fermentable fibre in canine diets may contribute to the prevention or mitigation of obesity through its effects on satiety.
Acknowledgements
The present study was supported by the Wageningen Institute of Animal Sciences and the Laboratory of Animal Nutrition, Ghent University. George Fahey Jr is acknowledged for his advices concerning the composition of the experimental diets. The authors sincerely thank Steven Galle, Rebekka Hollebosch, Mariëtte Kooper, Yvonne Paijmans, Georgios Papadopoulos and Herman De Rycke involved in the caretaking of the dogs and/or sample collection. All authors contributed fundamentally to the present study. G. B. contributed to all facets including research design, data collection, analyses, interpretation and manuscript preparation; W. H. H., M. H., G. P. J. J. and A. F. B. v. d. P. contributed to research design, data interpretation and manuscript preparation; A. V. and M. H. contributed to animal expertise, blood-collection protocol, blood collection and manuscript preparation; J. J. H. contributed to GLP-1 analyses, data interpretation and manuscript preparation. The authors declare that there is no conflict of interest.







