INTRODUCTION
Following Marshall (Reference Marshall1954), Cyclothone (Gonostomatidae, Stomiiformes) is often claimed to be the most abundant of all vertebrate genera. Most of its 13 species have depth ranges centred in the mesopelagic zone, where what little they eat comprises vertically migrant calanoid copepods, with lesser quantities of ostracods, plus trace amounts of other mesozooplankton (De Witt & Cailliett, Reference De Witt and Cailliett1972; Gorelova, Reference Gorelova1980; Maynard, Reference Maynard1982; Mauchline & Gordon, Reference Mauchline and Gordon1983; Roe & Badcock, Reference Roe and Badcock1984; Gordon et al., Reference Gordon, Nishida and Nemoto1985; Palma, Reference Palma1990; Yoon et al., Reference Yoon, Nival, Choe, Picheral and Gorsky2007; McClain-Counts, Reference McClain-Counts2010). In contrast, the circumglobal Cyclothone microdon (Günther 1878), amongst the most abundant of all fish species, is largely bathypelagic, being found primarily at 500–2700 m depth (Badcock, Reference Badcock, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984). Since Cyclothone spp. show little evidence of vertical migration, C. microdon might be expected to sustain itself on the scarce resources available below the depths reached by mesopelagic diel migrants. To date, however, only Mauchline & Gordon (Reference Mauchline and Gordon1983) have provided substantial information on the diet of that species, which they derived from 68 stomachs containing identifiable prey, taken from specimens caught in the Rockall Trough at unreported depths. Those individuals had consumed similar prey to that of their shallower-dwelling congeners.
From 2007 to 2010, four midwater-trawl surveys were conducted in The Gully (Figure 1), a very large submarine canyon incised into the continental margin immediately east of Sable Island, Nova Scotia, Canada (Kenchington et al., Reference Kenchington, Best, Bourbonnais-Boyce, Clement, Cogswell, MacDonald, MacEachern, MacIsaac, MacNab, Paon, Reid, Roach, Shea, Themelis and Kenchington2009, Reference Kenchington, Benjamin, Best, Cogswell, Cook, DeVaney, Lirette, MacDonald, MacIsaac, Mallam, McIntyre, McMillan, Moors-Murphy, Morton, Paon, Roach, Shea, Themelis and Kenchington2014b). The canyon supports an endangered population of northern bottlenose whales, Hyperoodon ampullatus (Forster 1770), which feed there at depths of 500–1500 m. They are thought to eat primarily armhook squid, Gonatus spp. (Hooker et al., Reference Hooker, Iverson, Ostrom and Smith2001, Reference Hooker, Whitehead, Gowans and Baird2002), living at the whales’ feeding depths, and one particular focus for the trawl surveys was the prey available to Gonatus spp. in The Gully. Hence, the surveys used an International Young Gadoid Pelagic Trawl (‘IYGPT’), the meshes of which are too large for efficient sampling of Cyclothone spp. Those were the most abundant fish in the catches nonetheless. Limited additional sampling with a fine-mesh Tucker trawl took many C. microdon, plus a few Cyclothone pseudopallida Mukhacheva 1964 (Kenchington et al., Reference Kenchington, Cochrane, Gjerdrum, Greenan, Lirette, Moors-Murphy and Thompson2014c), suggesting that most of the Cyclothone spp. in the canyon live and feed at the same depths to which the whales dive. Sampling limitations notwithstanding, we therefore examined the stomach contents of individuals in selected catches taken by the IYGPT for evidence of the prey available to the Cyclothone spp. at bathypelagic depths – and hence potentially at the base of the food chain supporting the whales. In the event, most of the Cyclothone spp. found with food in their stomachs had fed above 750 m depth and we therefore terminated the work.
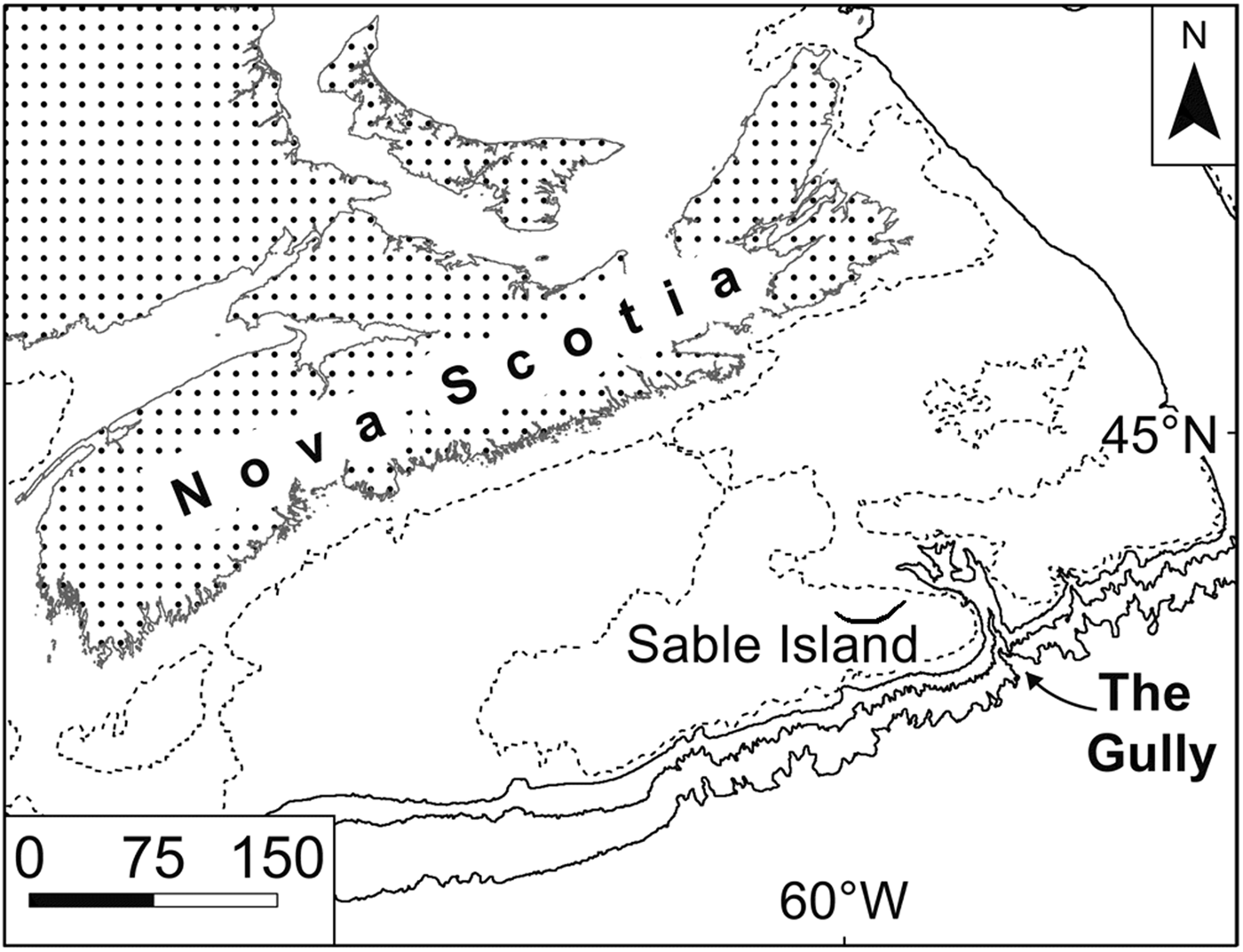
Fig. 1. Map of the Scotian Shelf, showing the location of The Gully. Contours shown at 100 (dashed), 200, 1000 and 2000 m depths. Scale bar in kilometres.
We here present the results from those samples which were examined, as a confirmation of Mauchline & Gordon's (Reference Mauchline and Gordon1983) observations on C. microdon and a doubling of the limited available information on the diet of this extremely abundant species. To provide a context for the prey data, we first examine the spatio-temporal distribution of Cyclothone spp. in The Gully and outline the sizes, sexes and maturation stages of the specimens.
MATERIALS AND METHODS
Study site
The Gully is a focus for multidisciplinary studies of canyon ecology and much is now known (e.g. Greenan et al., Reference Greenan, Petrie and Cardoso2014; Kenchington et al., Reference Kenchington, Cogswell, MacIsaac, Beazley, Law and Kenchington2014a; MacIsaac et al., Reference MacIsaac, Kenchington, Kenchington and Best2014; Moors-Murphy, Reference Moors-Murphy2014; Shan et al., Reference Shan, Sheng and Greenan2014). Of present importance, within the depth range of Cyclothone spp., the canyon is flooded by either Labrador Sea Water or else North Atlantic Central Water (Kenchington et al., Reference Kenchington, Cochrane, Gjerdrum, Greenan, Lirette, Moors-Murphy and Thompson2014c). Below rim depth, there is a slow net up-canyon flow, the water either upwelling over the rim or passing through a valley that links the canyon head to a mid-shelf basin (Greenan et al., Reference Greenan, Petrie and Cardoso2014; Shan et al., Reference Shan, Sheng and Greenan2014). Time-specific physical conditions during the trawl surveys have been presented by Kenchington et al. (Reference Kenchington, Best, Bourbonnais-Boyce, Clement, Cogswell, MacDonald, MacEachern, MacIsaac, MacNab, Paon, Reid, Roach, Shea, Themelis and Kenchington2009, Reference Kenchington, Cochrane, Gjerdrum, Greenan, Lirette, Moors-Murphy and Thompson2014c).
Field methods
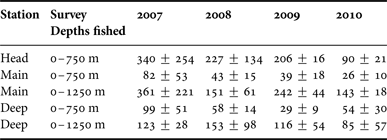
Full details of the four surveys have been presented by Kenchington et al. (Reference Kenchington, Best, Bourbonnais-Boyce, Clement, Cogswell, MacDonald, MacEachern, MacIsaac, MacNab, Paon, Reid, Roach, Shea, Themelis and Kenchington2009, Reference Kenchington, Benjamin, Best, Cogswell, Cook, DeVaney, Lirette, MacDonald, MacIsaac, Mallam, McIntyre, McMillan, Moors-Murphy, Morton, Paon, Roach, Shea, Themelis and Kenchington2014b). Three were in late summer (August–September 2007 to 2009) and one in early spring (March 2010). Each followed a depth-stratified, fixed-station, replicated design. Three principal stations (denoted ‘Head’, ‘Main’ and ‘Deep’) were established along the canyon thalweg (Figure 2). The depth strata were defined as 0–250, 250–750, 750–1250 and 1250–1750 m, though only the first two were available on the Head Station, while only the Deep Station could be fished to 1750 m. By design, each survey usually made two (sometimes one or three) replicate standard IYGPT sets in each available stratum at each station in each of daylight and darkness. Depth control utilized a headline-mounted acoustic sensor while recording pressure sensors provided more accurate post hoc measurements of the maximum depth of each set. Limitations on ship time prevented the design from being fully achieved after 2007, though some additional trawling (not considered in the analyses presented here) was done at other stations and other depths.
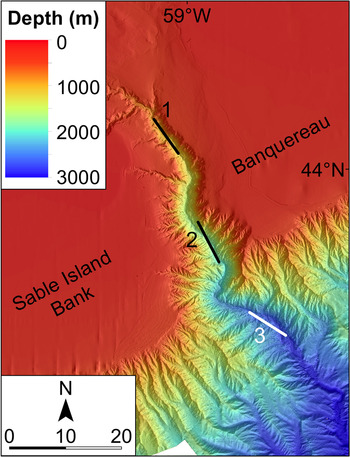
Fig. 2. Bathymetry of The Gully and the locations of the principal trawling stations: 1: Head Station, 2: Main Station, 3: Deep Station. Scale bar in kilometres. (High resolution bathymetric data are lacking for a small area near the southern limit of this map.)
The IYGPT is an open net, of 60 m2 mouth area, and necessarily fishes from the surface to the maximum depth of the set. In the Gully surveys, sets which fished below 250 m followed double-oblique (‘V’) profiles. After the 2007 survey, shooting and hauling were controlled to ensure that the net fished for 1 h in each stratum (except 30 min above 250 m) down to the intended maximum depth. The catch taken by a particular set in its nominal stratum (its ‘stratum catch’) can thus be crudely estimated by subtracting the mean catch taken by sets which fished the next-shallower stratum (in the same survey, at the same station and during the same diel phase) from the actual catch taken by the set in question. Such estimates cannot be precise but, with the fixed-station design of the Gully surveys and the high volumes of water filtered by the IYGPT, variability amongst the catches of replicate sets was generally low and such estimated stratum catches have proven informative (e.g. MacIsaac et al., Reference MacIsaac, Kenchington, Kenchington and Best2014). In 2007, the net was dropped to and recovered from its nominal stratum as quickly as possible, somewhat reducing fishing time in shallower strata and hence depressing both the actual catches and the estimated stratum catches of sets that fished below 250 m. No corrections for that difference were attempted. The sets confined to the 0–250 m stratum involved extra complications but took so few Cyclothone spp. that they are here only used qualitatively. Instead, the two shallowest strata are collapsed into a 0–750 m stratum, sampled by the sets that fished to 750 m depth.
The 2007 and 2008 surveys used standard IYGPT nets, which were then replaced by one constructed of knotless mesh. From 2008, the trawls were fitted with a rigid ‘aquarium’ codend. The combination of those advances resulted in less damage to the catch and hence a higher proportion of Cyclothone spp. specimens with their internal organs in place.
Being inadequately sampled by the IYGPT and very often badly damaged during capture, Cyclothone spp. were not an intended focus of the surveys. At sea, they were only sorted to genus and the catch weight recorded. Most were then discarded but a few catches from the early surveys were fixed in formalin and returned to shore. The latter became standard practice in 2010.
Analyses of spatio-temporal distribution
Despite more than 100 standard sets being made on the three principal stations, replication remained too low to allow statistical analysis of the full four-factor survey design. Both the actual catches and the estimated stratum catches taken by each set were, therefore, averaged within each survey/station/stratum/diel-period cell of the design (using arithmetic means) and then across those factors that showed no distributional pattern, the resulting matrices being examined for spatio-temporal patterns.
Within the constraints of available data, the statistical significances of three such observed patterns were checked by factorial ANOVA of log-transformed catch weights, with post hoc testing using Tukey's HSD, all calculated using JMP v.6.0.3 software (SAS Institute Inc., Cary, NC). A two-way test, with Station and Survey as factors, was applied to the actual catches taken by night sets that fished to 750 m on the Head Station, to 1250 m on the Main Station and to 1750 m on the Deep Station (representing depth-integrated biomasses across the full surveyed portion of the water column at each station) during the 2008–2010 surveys. A second test was applied to actual catches taken by 0–750 m sets on the Head Station and 0–1250 m sets on the Main Station and on each of the four surveys, with Diel Phase, Station and Survey as the factors. Lastly, a three-way test of Station, Stratum and Survey was applied to the estimated stratum catches taken by night sets in the 0–750, 750–1250 and 1250–1750 m strata, on the Head, Main and Deep stations, during the 2008–2010 surveys. Throughout, the use of log transformations reduced non-normality and heteroscedasticity but residual data deficiencies and, especially, the testing only of hypotheses that appeared supported by the data will have tended to depress calculated probabilities. The limited amount of data will have had the reverse effect, while unequal numbers of replicates will have further distorted the results. Hence, the hypothesis tests may be indicative but were not definitive.
Laboratory methods
The Cyclothone spp. catches from 41 sets were returned to shore. Eighteen of those were selected for examination, in part haphazardly but also so as to include examples from various stations, depths, diel periods and both the 2007 and 2010 surveys. Samples of specimens were taken from each selected catch. The sampling fraction was chosen, in the form of a ratio 1:x, to produce a sample of at least 20 fish. Specimens were then extracted haphazardly from the preserved material, every x th individual being processed.
The specimens taken for processing were identified to species, to the extent possible, following Badcock's (Reference Badcock, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984) key, and their standard lengths were measured to the nearest millimetre. If the stomach was present, it was opened and the lumen examined, the content being recorded as either empty or containing prey – the latter being extracted and retained for further examination. If gonad tissue was present, the sex of the individual was determined, as was, for adult females only, the developmental stage of the ovaries. Both sexing and staging followed Badcock & Merrett (Reference Badcock and Merrett1976), in so far as their scheme could be applied without histological preparations.
The retained stomach contents were initially screened, removing those which contained only unidentifiable material. The remainder were examined (by J.M. Spry, SpryTech Biological Services Inc., Elmsdale, Nova Scotia) and identified in as much detail as their condition allowed.
RESULTS
Spatio-temporal distribution
Cyclothone spp. were found by each survey, on every station, and in the catches of sets made in each nominal stratum, though not those of every set that fished exclusively above 250 m depth. The catches barely differed between daylight and night. There were gaps in the data from the Deep Station but the depth distribution of catches taken on the Main Station gave no indication of diel migrations (Table 1).
Table 1. Arithmetic mean estimated stratum catch of Cyclothone spp. (in grams) at each of the three stations along the canyon thalweg, with confidence intervals of one standard deviation.

(For the sub-surface strata, each estimated stratum catch is the actual catch taken by a set, less the average of the catches taken by sets nominally made in the next-shallower stratum during the same survey, the same diel period and on the same station).
The depth-integrated biomass of Cyclothone spp., excluding any fish below the deepest stratum at each station, showed only a weak trend along the canyon. The mean catch by sets which reached 1750 m depth on the Deep Station was 273 g, compared with 232 g taken by sets made to 1250 m on the Main Station and 238 g by 750 m sets on the Head Station. To avoid empty cells, formal testing of that trend was confined to night sets during the latter three surveys. Two-way ANOVA of those catches showed a non-significant Station × Survey interaction term and, after its elimination, a significant Station effect (F (2,9) = 11.566, P = 0.003). Post hoc tests found no significant difference between the Head and Main stations but the Deep Station stood out for its higher catches.
Full arrays of daylight and night catch data from all four surveys, suited to statistical examination of inter-annual trends, are only available for 0–750 m sets on the Head and Main stations and 0–1250 m sets on the Main Station alone. For the Deep Station, near-complete coverage of both 0–750 and 0–1250 m sets is available. Four of those five station/depth-range series show 2007 catches averaging higher than those taken in 2008 or 2009 (Table 2) – the reverse trend to that expected from the inter-annual differences in field survey methods. The spring 2010 catches were generally lower than those taken during the summer. The inter-annual differences on the Head and Main stations were, however, only marginally significant (F (3,22) = 3.422, P = 0.035), after removal of non-significant interaction terms, while post hoc testing indicated that the only significant difference was between the 2007 and the 2010 catches. If a Bonferroni correction were applied, in response to the selection of hypotheses to test, based on apparent trends, that result would be judged non-significant.
Table 2. Arithmetic mean catches of Cyclothone spp. (in grams), with confidence intervals of one standard deviation, for each survey and each of the station/depth-range combinations for which data are available for near-complete coverage of the survey design.
The depth distribution of Cyclothone spp. catches did vary along the canyon. On the Deep Station, the fish were primarily bathypelagic but substantial catches were taken above 750 m depth on the Head Station (Table 1). The estimated stratum catches taken by night sets during the latter three surveys showed a significant Station × Stratum interaction (F (1,15) = 13.808, P = 0.002), indicative of the shallower distribution of the fish further up the canyon. That trend might be less pronounced than it appears if other Cyclothone spp. were concentrated between the seabed and the deepest stratum on the Main and Head stations but the very limited trawling undertaken in those near-bottom layers did not take large amounts of Cyclothone spp.
Of 43 sets that did not pass 250 m depth, 15 had no recorded catch of Cyclothone spp., while the average catch by such shallow sets on the Main and Deep stations was 5 g, suggesting minor contamination carried over from previous, deeper trawling. However, the 12 shallow sets made on the Head Station took an average of 22.8 g, one catching 108 g. While that set was immediately preceded by a large catch taken at greater depth, one each on the Head and Deep stations took 26 and 25 g of Cyclothone spp. respectively, despite each following some hours with no trawling. The measured maximum headline depths of those sets were 261 and 273 m, while their footropes reached 4–6 m deeper (Kenchington et al., Reference Kenchington, Best, Bourbonnais-Boyce, Clement, Cogswell, MacDonald, MacEachern, MacIsaac, MacNab, Paon, Reid, Roach, Shea, Themelis and Kenchington2009). Moreover, the stomachs of four individual Cyclothone microdon extracted from the catches of 250 m night sets (two on the Head Station and one on the Main: maximum headline depth 264 m) were found to contain incompletely digested prey material. Thus, in the canyon and especially near its head, the depth range of the genus extends very nearly to, and probably above, 250 m depth.
Specimens examined
A total of 736 Cyclothone individuals taken by 18 sets were examined, the entire catches of those sets totalling more than 6100 fish of the genus. 354 of the examined individuals were identified as Cyclothone microdon and four as Cyclothone pseudopallida, while 125 were one or the other of those species. The remaining specimens were too damaged for specific identification but none had any observable characteristics diagnostic of other species.
Sex could be determined for 310 of the specimens, of which 259 were female (the great majority being in Stage VI, with ripe eggs), while the remainder included males, immatures and hermaphrodites – C. microdon being a protandrous hermaphrodite (Badcock, Reference Badcock1986). Examination of the specimens indicated that the preponderance of specimens recorded as ripe females resulted primarily from that sex and stage being more readily recognized amongst the often-damaged material.
Standard length (after fixation) could be measured for 662 specimens. Following expansion by the inverse of the sampling fractions for each set, those measurements showed a length frequency (Figure 3) with modes at 32 and 53 mm (average 42.0 mm). The larger mode was primarily composed of females, which also comprised nearly a third of the 32 mm mode, most of which was male. The lengths of the four confirmed C. pseudopallida averaged slightly less than the rest of the collection, at 27, 28, 33 and 46 mm respectively. The available length data were mostly from fish taken in 2010. The summer 2007 length frequency tended towards smaller fish, with a mode at 38 mm and few individuals larger than 50 mm.
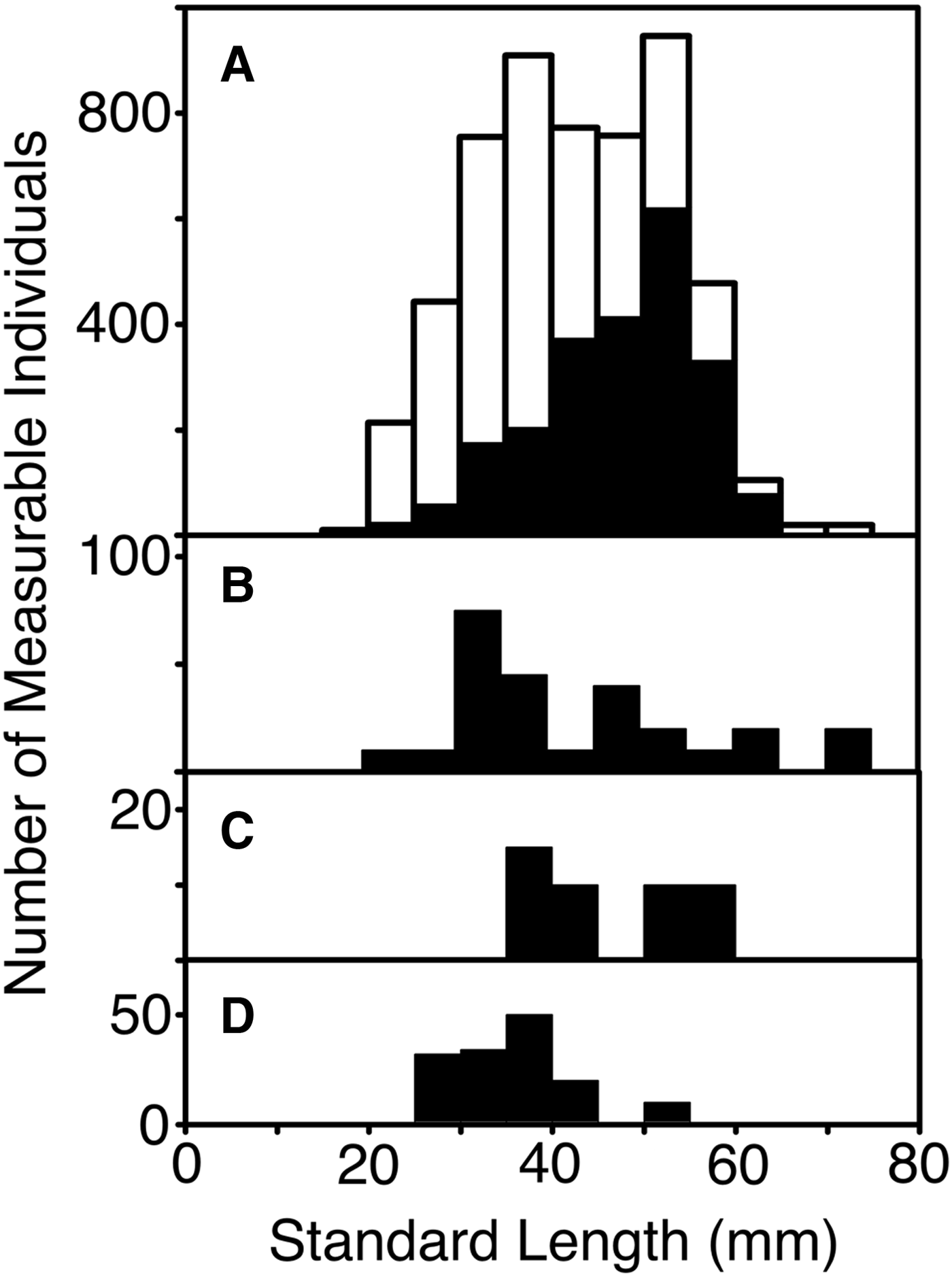
Fig. 3. Length frequency of measurable Cyclothone spp. caught by the sets selected for examination of samples (standard lengths in millimetres, grouped in 5 mm increments). Numbers of fish are expanded by inverse of sampling ratio applied to the catch from each set. (A) All specimens (white) and confirmed females (black), (B) confirmed males, (C) confirmed hermaphrodites, (D) confirmed immature specimens.
Stomach contents
Of the examined specimens, 490 had surviving stomachs, 82% of which were empty. No diel cycle was apparent in that proportion. The 89 non-empty stomachs included at least one specimen from each of 16 of the selected sets. Nine sets each yielded more than 20% non-empty stomachs. Those nine were spread across all three stations, both daylight and night fishing and both the 2007 and 2010 surveys. In the catches taken by sets made to 750 m depth, the proportion of non-empty stomachs declined up the canyon, from 36% on the Deep Station through 25% on the Main Station to 17% on the Head Station. Sets that did not fish below 250 m depth had non-empty proportions (mean: 20.3%, maximum: 33.3%) almost identical to those of sets that reached 750 m (mean: 23.1%, maximum 34.5%). In contrast, the sets which reached 1750 m depth on the Deep Station produced a lower non-empty proportion (mean: 11.6%, maximum 21.1%), despite fishing through shallower depths. If both the catches and the proportions of individuals with non-empty stomachs that were taken by those 1750 m sets while they fished above 750 m depth were assumed equal to the catches and non-empty proportions taken by sets that went no deeper than 750 m, then the proportion of non-empty stomachs in individuals taken between 750 and 1750 m could be crudely estimated. For the Cyclothone spp. on the Deep Station in 2010, that estimate is 8.3%.
The 89 fish with non-empty stomachs included 38 confirmed females (some with ripe eggs and one spent), one hermaphrodite, two immatures and eight males. The proportion of males was higher, and that of females lower, than amongst the individuals with empty stomachs but not significantly so. Amongst the females, there was no apparent difference in the proportions of the various maturity stages between those with empty or non-empty stomachs. The standard lengths of the 89 individuals in the latter group lay in the range 24–59 mm, with a mean of 41.2 mm. Two of the four identified C. pseudopallida were among the individuals with non-empty stomachs, though one contained no identifiable prey material. Another 63 individuals with non-empty stomachs were confirmed as C. microdon, 32 of which had identifiable prey items amongst their stomach contents.
Those were among the 42 specimens which contained identifiable prey. The numbers of non-empty stomachs with and without identifiable prey were generally approximately equal across depths and stations. Every identifiable prey item in the 42 stomachs was derived from a small crustacean (Table 3). Three stomachs contained parts of at least four amphipods. Eleven ostracods of the subfamily Conchoeciinae were found in 10 stomachs (one being that of a C. pseudopallida). Otherwise, all prey identifiable below subphylum were copepods and all identifiable to order were calanoids. Those included members of six families and seven genera. No particular genus was especially prominent, five being represented by four to six prey items each. Only a few of the copepod pieces could be identified to species. Those that could be comprised individuals of Metridia lucens Boeck, 1865, Paraeuchaeta norvegica (Boeck 1872) and Pleuromamma gracilis Claus, 1863. One prey item was probably Calanus finmarchicus (Gunnerus 1770), while both of the recorded Euchirella spp. were probably Euchirella rostrata (Claus 1866).
Table 3. Prey taxa found in stomachs of Cyclothone spp., showing the number of stomachs that contained each taxon and the minimum number of ingested individuals necessary to explain the observed pieces of prey.
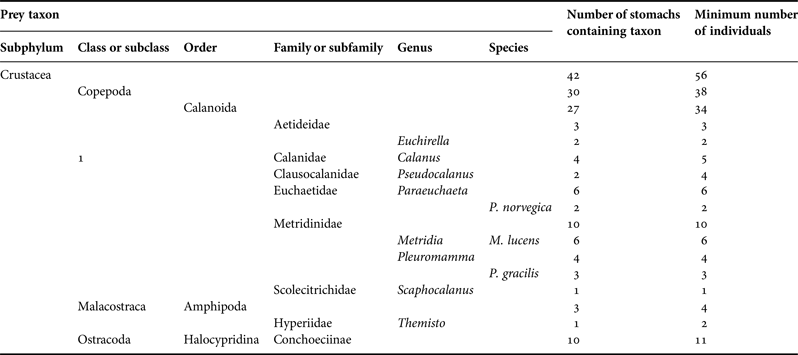
Counts presented for higher taxa include those shown for lower constituent taxa. Prey items are here listed under the lowest taxon to which they were identified, even if that identification was uncertain. However, every taxon named here was definitively identified from at least one piece extracted from a stomach.
The observed stomach contents of the 42 Cyclothone spp. may have comprised as few as 56 prey individuals. One small (25 mm) mature female, taken above 250 m depth on the Main Station, had two Calanus spp., three probable Pseudocalanus spp., one Paraeuchaeta sp., four unidentifiable digested copepod fragments and parts of two Themisto spp. in her stomach. Three other fish each contained the remains of one copepod and one or two ostracods. Two fish had each eaten two copepods. The remaining 36 Cyclothone spp. individuals may have ingested only a single prey item each.
DISCUSSION
Few Cyclothone spp. from the continental margin off Nova Scotia have been identified to species but it was clear before the Gully surveys that Cyclothone microdon is the principal member of the genus there (McKelvie & Haedrich, Reference McKelvie and Haedrich1985; Scott & Scott, Reference Scott and Scott1988). Small numbers of Cyclothone pseudopallida have also been reported (e.g. Scott & Scott, Reference Scott and Scott1988). A range of sizes and both sexes of C. microdon are present in the canyon, some females being in spawning condition. In the eastern North Atlantic, sex reversal seems universal in C. microdon, length ranges of juveniles and adult females showing little overlap, while the largest individuals are all female (Badcock & Merrett, Reference Badcock and Merrett1976; Badcock, Reference Badcock1986; McKelvie, Reference McKelvie1989). Hence, the difference in modal sizes of the sexes in The Gully, along with the presence of some hermaphrodites, were expected. However, in the present study female and juvenile length ranges overlapped broadly, while the largest individual examined was male. No less remarkable was the change in size composition between summer 2007 and spring 2010, suggestive of a recruitment event followed by growth of the abundant year-class.
On the Deep Station, Cyclothone spp. were mostly distributed across the deeper-mesopelagic and upper bathypelagic zones, as expected for C. microdon (Badcock, Reference Badcock, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984), but they were found at lesser depths further up the canyon, such that they were mesopelagic fish on the Head Station, with some caught near or above 250 m. That is within the expected depth range for C. pseudopallida but remarkably shallow for C. microdon – and three individuals caught by shallow sets were confirmed as that species. Elevation of biological and physical features towards a canyon head is not unusual but the extent of this trend in the vertical distribution of Cyclothone spp. was exceptional. Being small and weak-bodied, Cyclothone spp. appear as much planktonic as nektonic. They are also oceanic and perhaps poorly adapted to life in a canyon. Certainly, their distribution in The Gully is consistent with their being passively carried up-canyon and then upwards by the known water flow (Greenan et al., Reference Greenan, Petrie and Cardoso2014; Shan et al., Reference Shan, Sheng and Greenan2014), until increasing light levels discourage further ascent, while their abundance is thinned by predation.
The greater biomass observed in summer 2007, relative to that in the following summers, was at best marginally significant but a parallel trend has been noted in the highly abundant krill, Meganyctiphanes norvegica Holt & Tattersall, 1905, taken by the same surveys (MacIsaac et al., Reference MacIsaac, Kenchington, Kenchington and Best2014). Both may have been consequences of the unusual oceanographic conditions that year (Kenchington et al., Reference Kenchington, Best, Bourbonnais-Boyce, Clement, Cogswell, MacDonald, MacEachern, MacIsaac, MacNab, Paon, Reid, Roach, Shea, Themelis and Kenchington2009). The relative depression in the depth distribution of Cyclothone on the Deep Station during the 2009 survey was probably related to the presence of Warm Slope Water: the shelf/slope front which delimits that water mass usually lies much further south but cut across the Station during that one survey (Kenchington et al., Reference Kenchington, Cochrane, Gjerdrum, Greenan, Lirette, Moors-Murphy and Thompson2014c).
With their weak bodies, lack of diel migratory behaviour and an absence of the bioluminescent lures seen in many bathypelagic fishes, Cyclothone spp. appear adapted for minimizing energy use and relying on chance encounters with prey, which necessarily limits their consumption rates and energy intake (Maynard, Reference Maynard1982). The 82% empty stomachs observed in The Gully, along with the very low numbers of stomachs containing more than a single prey item, are typical for the genus (e.g. Collard, Reference Collard1970; De Witt & Cailliett, Reference De Witt and Cailliett1972; Gorelova & Tseytlin, Reference Gorelova and Tseytlin1979; Gorelova, Reference Gorelova1980, Maynard, Reference Maynard1982; Mauchline & Gordon, Reference Mauchline and Gordon1983; Roe & Badcock, Reference Roe and Badcock1984; Gordon et al., Reference Gordon, Nishida and Nemoto1985; Palma, Reference Palma1990; Burghart et al., Reference Burghart, Hopkins and Torres2010; McClain-Counts, Reference McClain-Counts2010). Cyclothone microdon above 750 m depth on the Deep Station do seem to have enjoyed better feeding opportunities, with only 64% of stomachs empty. That figure rose to 75% inside the canyon mouth and 83% at the Head Station, at least in March 2010, suggesting lowered prey availability further up The Gully.
Use of stomach contents as an indicator of diets, especially in a species that has a low proportion of stomachs containing identifiable prey, necessarily requires an assumption that the fish do not consume substantial amounts of soft-bodied animals which leave little trace in their stomachs. Within that limitation, previous studies of the diets of Cyclothone spp. have found them to be dominated by small crustaceans, especially calanoid copepods (De Witt & Cailliett, Reference De Witt and Cailliett1972; Gorelova, Reference Gorelova1980; Maynard, Reference Maynard1982; Mauchline & Gordon, Reference Mauchline and Gordon1983; Roe & Badcock, Reference Roe and Badcock1984; Gordon et al., Reference Gordon, Nishida and Nemoto1985; Palma, Reference Palma1990; Yoon et al., Reference Yoon, Nival, Choe, Picheral and Gorsky2007), as was seen in The Gully. Off Hawaii, Maynard (Reference Maynard1982) found some evidence of selection of preferred prey types but it seems that differences in recorded Cyclothone spp. diets owe more to variations in prey availability than anything else. Thus, the relatively high proportion of continental-shelf species in the stomachs of fish in The Gully is unsurprising. Pleuromamma spp., diel migrants to mesopelagic depths and hence species of the continental margins and open ocean, have frequently been reported as principal constituents of the diets of Cyclothone spp. (e.g. Gorelova, Reference Gorelova1980; Maynard, Reference Maynard1982; Mauchline & Gordon, Reference Mauchline and Gordon1983; Roe & Badcock, Reference Roe and Badcock1984; Gordon et al., Reference Gordon, Nishida and Nemoto1985; Palma, Reference Palma1990; Hopkins et al., Reference Hopkins, Sutton and Lancraft1996; Burghart, Reference Burghart2006; Yoon et al., Reference Yoon, Nival, Choe, Picheral and Gorsky2007; McClain-Counts, Reference McClain-Counts2010). They were only recorded in four of the stomachs from The Gully.
The depth distributions of copepods in The Gully may be as atypical for their respective species as is that of C. microdon and, if so, must remain unknown in the absence of site-specific data. However, the expected distributions of each of the identified prey types eaten by Cyclothone spp. in the canyon would have made them available to the fish between depths of 250 and 750 m, where most of the Cyclothone spp. with non-empty stomachs appear to have been caught. The depth distributions of the copepods are summarized in Table 4. The amphipods in stomachs from The Gully could not be identified to species but the Themisto spp. taken by the IYGPT were primarily Themisto compressa Goës 1865, with a small admixture of Themisto libellula (Lichtenstein in Mandt 1822). Themisto abyssorum Boeck 1870 may also have been present but not retained by the large meshes of the trawl (MacIsaac, Reference MacIsaac2011). Themisto compressa is primarily epipelagic but has been taken along the North American continental margin at bathypelagic depths (to a maximum of more than 1400 m: Bowman et al., Reference Bowman, Cohen and McManus McGuiness1982). The depth ranges of the Conchoeciinae have been little studied. Bashmanov & Chavtur (Reference Bashmanov and Chavtur2008) have recently summarized the available information on Boroecia borealis (Sars 1866) in the North Atlantic, finding that species most abundant around 500 m depth in waters north of 45°N, while the known depth range along the North American continental margin is 75–1500 m. In the north-east Atlantic, Angel (Reference Angel1977, Reference Angel1984) found a variety of species in the subfamily to be most abundant at depths of some hundreds of metres.
Table 4. Depth distributions of copepod taxa found in the stomachs of Cyclothone spp.

In summary, the diet of C. microdon in The Gully is consistent with Mauchline & Gordon's (Reference Mauchline and Gordon1983) observations of the species in the Rockall Trough. Both differ only in detail from the diets previously described for the mesopelagic species in the genus. The bulk of their prey comprises calanoid copepods which have themselves fed in the epipelagic layer and then migrated down, either seasonally or at dawn. In essence, instead of swimming up to feed on the herbivores near the surface, as the myctophids do, these most abundant of fishes remain at depth and wait for the copepods to descend – though the deep diel migrants are only a small fraction of all surface-feeding calanoids, limiting the prey supply for non-migratory fish.
ACKNOWLEDGEMENTS
This study could not have been undertaken without the exceptional efforts of the captains, crews and scientific staffs of the four survey cruises made in The Gully. It was no less dependent on the expertise and identifications of Jacquelyn Spry, SpryTech Biological Services Inc. We are further indebted to Kevin MacIsaac and Megan Best who, in addition to their work at sea, provided essential support in the laboratory ashore, to Cam Lirette for figure drafting and especially to Ellen Kenchington, who coordinated our efforts, obtained necessary funding, performed the ANOVAs and provided valuable comment on earlier drafts.
FINANCIAL SUPPORT
The authors undertook the work reported here as unfunded volunteers at the Bedford Institute of Oceanography. The overall program of research into the pelagic ecosystems of The Gully, including the four field surveys, was undertaken by the Department of Fisheries and Oceans, with support from the Government of Canada's ‘Health of the Oceans’ initiative.









