We have previously confirmed that higher levels of phospholipid hydroperoxides (PLOOH), the primary oxidation products of phospholipids (PL)(Reference Miyazawa, Yasuda and Fujimoto1, Reference Miyazawa, Suzuki and Fujimoto2), accumulate abnormally in the erythrocytes of dementia patients(Reference Miyazawa, Suzuki, Yasuda, Yagi, Kondo, Niki and Yoshikawa3). Such erythrocytes with high levels of lipid hydroperoxides have been postulated to have a decreased ability to transport oxygen to the brain, which may impair blood rheology, thus facilitating dementia(Reference Bosman, Bartholomeus and de Man4–Reference Mohanty, Eckley and Williamson8). Recently, we have developed an HPLC method to determine erythrocyte carotenoid content(Reference Nakagawa, Kiko and Hatade9). Using this method, we gathered evidence that accumulation of polar oxygenated carotenoids (xanthophylls) occurs predominantly in human erythrocytes(Reference Nakagawa, Kiko and Hatade9), and that a decrease in xanthophylls and an increase in PLOOH levels in erythrocytes correlate with the severity of dementia(Reference Nakagawa, Miyazawa and Kiko10). These findings led to the hypothesis that xanthophyll supplementation may minimise the accumulation of erythrocyte PLOOH, and that xanthophylls could be used therapeutically as drugs or functional foods to prevent the disease. Although there is still scarce information on whether orally administered xanthophylls are distributed to human erythrocytes and actually inhibit erythrocyte PLOOH formation, our recent human study has revealed antioxidant properties of the xanthophyll lutein towards erythrocyte PLOOH formation(Reference Nakagawa, Kiko and Hatade11). Animal studies have also supported this hypothesis(Reference Nakagawa, Fujimoto and Miyazawa12, Reference Asai, Nakagawa and Miyazawa13).
Among xanthophylls, astaxanthin has recently received attention for its potent antioxidant activity(Reference Higuera-Ciapara, Félix-Valenzuela and Goycoolea14, Reference Hussein, Sankawa and Goto15). Astaxanthin is naturally synthesised by plants and algae, and is now commercially available as a food supplement from Haematococcus alga(Reference Guerin, Huntley and Olaizola16). The recommended daily intake is estimated to be 1–12 mg/d; however, there is not much information regarding the bioavailability of astaxanthin in humans. To the best of our knowledge, the occurrence and antioxidant roles of astaxanthin in human erythrocytes have not been reported.
In this investigation of whether administered astaxanthin is distributed to erythrocytes and inhibits erythrocyte PLOOH formation, we conducted a randomised, double-blind, placebo-controlled human trial. The efficacy of 12-week astaxanthin supplementation (6 or 12 mg) on both astaxanthin and PLOOH levels in the erythrocytes of thirty middle-aged and senior subjects was investigated. For erythrocyte astaxanthin analysis, a newly developed HPLC coupled with tandem MS (MS/MS) method was applied. Our findings (the inhibitory effect of astaxanthin on erythrocyte PLOOH) would provide new insights into the possible application of astaxanthin as an anti-dementia agent.
Subjects and methods
Subjects and materials
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committee of Anti-Aging Science (Tokyo, Japan; ethics no. I030807). All subjects were recruited from the Anti-Aging Science volunteer database, and written informed consent was obtained from all subjects. Exclusion criteria included pregnancy, lactation and severe medical illness.
Two doses of the test materials were prepared by filling soft capsules with astaxanthin-rich Haematococcus pluvialis oil (Puresta®; Yamaha Motor Company, Limited, Shizuoka, Japan)(Reference Satoh, Ishikura, Murakami, Bagchi, Lau and Ghosh17). Compositions of the test materials were 75 mg Puresta oil 80 and 145 mg olive oil/capsule for the low dose (equivalent to 6 mg astaxanthin dialcohol); and 150 mg Puresta oil 80 and 70 mg olive oil/capsule for the high dose (equivalent to 12 mg astaxanthin dialcohol). To prepare placebo capsules, 150 mg maize oil was used instead of 150 mg Puresta oil 80. Placebo capsules were coloured to appear identical to test capsules.
Supplementation trial
A 12-week randomised, double-blind, placebo-controlled trial was conducted. A total of thirty healthy subjects (fifteen men and fifteen women), between 50 and 69 years of age (mean 56·3 (sd 5·3) years), with a BMI of 27·5 (sd 2·1) kg/m2 were recruited, and randomly received 0 (placebo), 6 or 12 mg astaxanthin. During the 12-week trial, subjects ingested one of the three astaxanthin-dosed (0, 6 or 12 mg) capsules with an appropriate amount of water once daily after breakfast. Before and after the supplementation period (weeks 0 and 12, respectively), anthropometric data (e.g. height, body weight and blood pressure) and blood samples were collected from the subjects after they had fasted overnight, adverse effects were assessed by interviews and self-reports, and compliance was checked by self-reports and returned capsule counts. Throughout the study period, subjects were instructed to maintain their usual lifestyle (avoid excessive eating and drinking, intense exercise and lack of sleep). Dietary intake, alcohol consumption and physical activity (pedometer count) during the 3 d before each blood collection (weeks 0 and 12) were also assessed by self-reports.
Measurement of erythrocyte astaxanthin and other carotenoids
Blood samples were subjected to centrifugation at 1000 g for 10 min at 4°C. After the plasma and buffy coat were removed, erythrocytes were washed three times with PBS (pH 7·4) to prepare packed cells. For the determination of erythrocyte carotenoids (including astaxanthin), packed cells (2·5 ml) were diluted with 2·5 ml of water and were mixed with 5 ml of 80 mm-ethanolic pyrogallol, 1·0 ml of 1·8 m-aqueous KOH and 40 μl of 1 μm-ethanolic echinenone (internal standard)(Reference Nakagawa, Kiko and Hatade9). After the addition of 1·25 ml of 0·1 m-aqueous SDS, the sample was mixed with 15 ml of hexane–dichloromethane (5:1) to extract erythrocyte carotenoids. The extract was purified by a Sep-Pak silica cartridge (Waters, Milford, MA, USA), and then was subjected to HPLC-MS/MS for the determination of astaxanthin. The HPLC-MS/MS apparatus consisted of a liquid chromatograph (Shimadzu, Kyoto, Japan) and a 4000 QTRAP MS/MS instrument (Applied Biosystems, Foster City, CA, USA). The MS/MS parameters (e.g. collision energy) were optimised with an astaxanthin standard under positive atmospheric pressure chemical ionisation. The standard and erythrocyte extracts were separated with a C30 carotenoid column (4·6 × 250 mm, 5 μm; YMC, Kyoto, Japan). The column was eluted using a binary gradient consisting of the following HPLC solvents: A, methanol–methyl tert-butyl ether–water (83:15:2; containing 3·9 m-ammonium acetate); B, methanol–methyl tert-butyl ether–water (8:90:2; containing 2·6 m-ammonium acetate). The gradient profile was as follows: 0–12 min, 10–45 % B linear; 12–24 min, 45–100 % B linear; 24–38 min, 100 % B. The flow rate was adjusted to 1 ml/min, and the column temperature was maintained at 25°C. Astaxanthin was detected in the post-column by MS/MS with multiple reaction monitoring (MRM) for the transition of the parent ion to the product ion. The concentration of erythrocyte astaxanthin was calculated using the standard curve of astaxanthin and was adjusted by the percentage recovery of the added echinenone (internal standard). For the determination of other carotenoids, erythrocyte extracts were analysed by HPLC coupled with UV diode array detection and atmospheric pressure chemical ionisation MS(Reference Nakagawa, Kiko and Hatade9).
Measurement of erythrocyte phospholipid hydroperoxides
For the determination of erythrocyte PLOOH(Reference Miyazawa, Yasuda and Fujimoto1–Reference Miyazawa, Suzuki, Yasuda, Yagi, Kondo, Niki and Yoshikawa3), total lipids were extracted from packed cells with a mixture of 2-propanol and chloroform containing butylated hydroxytoluene. PLOOH (i.e. phosphatidylcholine hydroperoxide (PCOOH) and phosphatidylethanolamine hydroperoxide (PEOOH)) in the total lipids were measured by HPLC with chemiluminescence (CL) detection. The column was a 4·6 × 250 mm, 5 μm Finepak SIL NH2-5 (Japan Spectroscopic Company, Tokyo, Japan), the eluent was 2-propanol–methanol–water (135:45:20), and the flow rate was 1 ml/min. Post-column CL detection was carried out using a CLD-100 detector (Tohoku Electronic Industries Company, Sendai, Japan). A mixture of luminol and cytochrome c in 50 mm-borate buffer (pH 10·0) was used as a hydroperoxide-specific post-column CL reagent. Calibration was carried out using PCOOH or PEOOH standards.
Other biochemical measurements
For plasma samples, astaxanthin, other carotenoids and PLOOH were determined by HPLC-MS/MS, HPLC-UV(Reference Nakagawa, Kiko and Hatade9) and HPLC-CL(Reference Miyazawa, Yasuda and Fujimoto1–Reference Miyazawa, Suzuki, Yasuda, Yagi, Kondo, Niki and Yoshikawa3), respectively. Tocopherols in erythrocytes and plasma were measured by HPLC with fluorescence detection(Reference Ikeda, Tohyama and Yoshimura18). Also, blood chemistries such as haematological and blood biochemical parameters were analysed using standardised methods.
Statistical analysis
Data are expressed as means and standard deviations. One-way ANOVA was used to compare means among the three groups. If a statistically significant difference (P < 0·05) was detected, Dunnett's test was performed for comparison between the control group and one of the two astaxanthin groups. For comparison of baseline (week 0) and post-dosing (week 12) values in each treatment group, a paired t test was used. These statistical analyses were done using SPSS for Windows (SPSS Inc., Chicago, IL, USA).
Results
As mentioned in the introduction, the distribution of astaxanthin in erythrocytes has not been reported, mainly due to the lack of an analytical method. We, therefore, developed an HPLC-MS/MS method to analyse for erythrocyte astaxanthin before we conducted the 12-week astaxanthin supplementation study. In brief, an astaxanthin standard was analysed by MS/MS with flow injection, and astaxanthin showed an intense molecular ion at m/z 597 (M+H)+. Product ion scanning was conducted for the ion and astaxanthin-specific fragment ions (e.g. m/z 147) were identified. These ions (m/z 597 and 147) allowed us to quantitatively determine erythrocyte astaxanthin concentrations using HPLC-MS/MS with MRM (Fig. 1(a)).
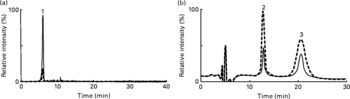
Fig. 1 Typical multiple reaction monitoring (MRM) and chemiluminescence (CL) chromatograms of (a) astaxanthin and (b) phospholipid hydroperoxides (PLOOH) in erythrocytes taken (![]() ) before and (
) before and (![]() ) after 12-week supplementation of astaxanthin (12 mg/d). Erythrocyte astaxanthin and PLOOH were determined by HPLC-MS/MS with MRM and HPLC-CL, respectively. Peak identifications are as follows: 1, astaxanthin; 2, phosphatidylcholine hydroperoxide; 3, phosphatidylethanolamine hydroperoxide.
) after 12-week supplementation of astaxanthin (12 mg/d). Erythrocyte astaxanthin and PLOOH were determined by HPLC-MS/MS with MRM and HPLC-CL, respectively. Peak identifications are as follows: 1, astaxanthin; 2, phosphatidylcholine hydroperoxide; 3, phosphatidylethanolamine hydroperoxide.
In the human trial, before administration (baseline), there were no significant differences in anthropometric parameters among the three groups (Table 1). The trial was completed without any of the subjects withdrawing. The mean compliance to the prescribed dose for the 6 mg astaxanthin, 12 mg astaxanthin and placebo groups was 99·5 (sd 3·7), 98·1 (sd 1·9) and 98·7 (sd 2·4) %, respectively. The average energy intake during the 12-week trial, as calculated from the self-reports, did not statistically differ among the groups. Furthermore, no significant differences were observed among the groups in the intake of each type of nutrient (carbohydrate, protein, fat, cholesterol and fibre), alcohol consumption and pedometer counts. Data from all thirty subjects were, therefore, included in the statistical analysis.
Table 1 Baseline characteristics of the study subjects in the 0 (placebo), 6 and 12 mg astaxanthin groups
(Mean values and standard deviations)
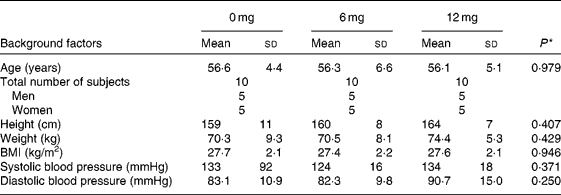
* One-way ANOVA test among groups.
The results of the physical, haematological and blood biochemical measurements before and after 12 weeks of dosing are shown in Tables 2 and 3. Some parameters (i.e. Hb, haematocrit, mean corpuscular volume, mean corpuscular Hb, uric acid, total cholesterol, LDL, fasting glucose and HbA1c) showed changes from baseline in the astaxanthin groups and/or the placebo group. However, these changes were small and were observed to be within the normal range irrespective of the test materials administered. Moreover, no differences were noted among the three groups in any parameters at baseline or post-dosing. Therefore, the physical and metabolic states of the subjects were considered to be randomised homogeneously throughout the trial.
Table 2 Changes in physical and haematological parameters before and after the 12-week administration of 0, 6 or 12 mg astaxanthin
(Mean values and standard deviations)

bpm, Beats/min.
Mean values were significantly different in the paired t test between before and after astaxanthin administration: *P < 0·05, **P < 0·01.
† One-way ANOVA test among groups.
Table 3 Changes in blood biochemical parameters before and after the 12-week administration of 0, 6 or 12 mg astaxanthin
(Mean values and standard deviations)

GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; ALP, alkaline phosphatase; γ-GTP, γ-glutamic transpeptidase.
Mean values were significantly different in the paired t test between before and after astaxanthin administration: *P < 0·05, **P < 0·01.
† One-way ANOVA test among groups.
In the typical MRM chromatogram of the erythrocyte extract taken before and 12 weeks after supplementation, astaxanthin was clearly detected (Fig. 1(a)). After supplementation, the erythrocyte astaxanthin concentration significantly increased and was higher than that of the placebo group (Table 4). On the other hand, in a typical CL chromatogram of erythrocyte total lipids, PCOOH and PEOOH were identified as the predominant PLOOH forms (Fig. 1(b)). After supplementation, erythrocyte PLOOH concentration decreased and was lower than that of the placebo group (Table 4). In plasma, the only detectable PLOOH was PCOOH, and a somewhat lower PCOOH level was found after astaxanthin supplementation (Table 5). In both erythrocytes and plasma, astaxanthin supplementation did not affect the levels of other carotenoids (except for small changes in lycopene and lutein) and tocopherols. These results suggest that upon ingestion of astaxanthin, it is absorbed, distributed and accumulated in erythrocytes, where it acts as an antioxidant molecule, thereby reducing PLOOH, an index of oxidative stress.
Table 4 Changes in carotenoids, phospholipid hydroperoxides (PLOOH) and tocopherol contents in erythrocytes taken before and after the 12-week administration of 0, 6 or 12 mg astaxanthin
(Mean values and standard deviations)

PCOOH, phosphatidylcholine hydroperoxide; PEOOH, phosphatidylethanolamine hydroperoxide.
Mean values were significantly different in the paired t test between before and after astaxanthin administration: *P < 0·05, **P < 0·01.
Mean values were significantly different in Dunnett's test between the control group and one of the two astaxanthin groups: †P < 0·05, ††P < 0·01.
‡ One-way ANOVA test among groups.
§ Xanthophylls are the sum of astaxanthin, lutein, zeaxanthin and β-cryptoxanthin.
∥ Non-polar carotenoids are sum of α-carotene, β-carotene and lycopene.
¶ PLOOH are the sum of PCOOH and PEOOH.
Table 5 Changes in carotenoids, phospholipid hydroperoxides (PLOOH) and tocopherols contents in plasma before and after the 12-week administration of 0, 6 or 12 mg astaxanthin
(Mean values and standard deviations)

PCOOH, phosphatidylcholine hydroperoxide; PEOOH, phosphatidylethanolamine hydroperoxide.
Mean values were significantly different in the paired t test between before and after astaxanthin administration: *P < 0·05, **P < 0·01.
Mean values were significantly different in Dunnett's test between the control group and one of the two astaxanthin groups: †P < 0·05, ††P < 0·01.
‡ One-way ANOVA test among groups.
§ Xanthophylls are the sum of astaxanthin, lutein, zeaxanthin and β-cryptoxanthin.
∥ Non-polar carotenoids are sum of α-carotene, β-carotene and lycopene.
Discussion
In recent years, medical and nutritional experts have seriously considered the antioxidant properties of food constituents, since the reactive oxygen species-mediated peroxidation of biological molecules (e.g. lipids) has been postulated to induce a variety of pathological events such as atherogenesis, ageing and dementia. Although many in vitro studies on the antioxidant properties of food constituents have been reported, little is known about the biological functions of dietary antioxidants in vivo (especially in humans), except for a few major antioxidants (e.g. tocopherols and ascorbic acid). Since the bioavailability of food constituents is limited by their digestibility and metabolic fate, oral administration trials are favoured in evaluating their biological functions.
The present randomised, double-blind, placebo-controlled human trial shows that when human subjects ingest astaxanthin, it is absorbed, distributed and accumulated in erythrocytes, where it exhibits antioxidative effects (inhibition of erythrocyte PLOOH). It is interesting to note that the antioxidative effect observed in the present study was produced by a relatively short-term supplementation with astaxanthin (12 weeks).
In the present study, since the distribution of astaxanthin in erythrocytes had not previously been reported, we developed an HPLC-MS/MS method to analyse the erythrocyte astaxanthin content before we conducted the astaxanthin supplementation study. Using MS/MS, we found that protonated astaxanthin tended to generate product ions (e.g. m/z 147). The product ion indicated that MRM could be adapted to the HPLC-MS/MS analysis of astaxanthin. Under optimised conditions, the detection limit of the HPLC-MS/MS with the MRM method was very sensitive at 0·02 pmol astaxanthin/injection. The characteristics and advantages of our HPLC-MS/MS method are as follows. The method was selective and sensitive enough to measure astaxanthin in erythrocytes (Fig. 1(a)) as well as in the plasma. Also, the method was sufficiently simple and convenient to be applicable to a large number of samples. The method, therefore, would be a powerful tool for studying the metabolic fate of astaxanthin as well as its bioavailability.
Until now, there are few reports concerning human erythrocyte carotenoids. Some studies have successfully detected erythrocyte carotenoids (mainly β-carotene)(Reference Fotouhi, Meydani and Santos19), while other studies have been unable to detect these species(Reference Norkus, Bhagavan and Nair20). Incorporation of a carotenoid (β-carotene) into erythrocytes after oral supplementation has been described in some reports(Reference Murata, Tamai and Morinobu21). However, there has been no study evaluating whether administered carotenoids other than β-carotene are distributed to erythrocytes, except for our recent human study of the xanthophyll lutein(Reference Nakagawa, Kiko and Hatade11). In the present study, using the newly developed HPLC-MS/MS analysis method, incorporation of astaxanthin into erythrocytes after oral supplementation was established (Fig. 1(a)). Because both the erythrocyte and plasma astaxanthin concentrations increased (Tables 4 and 5), it seems likely that astaxanthin in plasma lipoprotein particles is partly transferred into erythrocytes. By this hypothesis, astaxanthin would be located on the outer region of plasma lipoproteins, which would facilitate its transfer to erythrocytes. On the other hand, the concentrations of endogenous antioxidants (i.e. carotenoids and tocopherols) showed virtually no change before and after astaxanthin supplementation (Tables 4 and 5). This is advantageous for elucidating the antioxidant contribution of astaxanthin. By the way, it was known that blood carotenoid concentration in females is somewhat higher than that in males. In the present study, we compared sex difference in blood carotenoids, but no statistical differences were observed between males and females in each carotenoid at baseline or post-dosing.
In the present study, to evaluate peroxidisability, we measured the PLOOH content. Because PLOOH are the primary oxidation products of PL, an increase in PLOOH directly reflects in vivo oxidative stress(Reference Miyazawa, Yasuda and Fujimoto1–Reference Miyazawa, Suzuki, Yasuda, Yagi, Kondo, Niki and Yoshikawa3, Reference Miyazawa22, Reference Moriya, Nakagawa and Santa23). As has been observed, astaxanthin supplementation clearly reduced the erythrocyte PLOOH concentration (Fig. 1(b)), indicating that astaxanthin incorporation into erythrocytes attenuated PL peroxidation of erythrocyte membranes. On the other hand, the antioxidant effect of astaxanthin seemed to be more apparent on the erythrocyte membrane, as compared with the plasma (Tables 4 and 5). Erythrocytes are rich in PUFA in their PL bilayer, and contain high concentrations of molecular oxygen and ferrous ions as constituents of oxyhaemoglobin. The oxidation of Hb is accompanied by the formation of superoxides, a source of reactive oxygen species. Therefore, erythrocyte membrane PL would be more susceptible to peroxidation than other organelle membranes, even though they are protected by several antioxidative systems such as superoxide dismutase, catalase and glutathione peroxidase. For plasma PCOOH, unexpectedly, at baseline (week 0), groups taken 6 or 12 mg astaxanthin showed significantly less PCOOH than the group taken 0 mg astaxanthin. Because no differences were observed among the three groups in other parameters (e.g. haematological and blood biochemical values), it might be other factor(s) affecting plasma PCOOH before the start of the study. This possibility needs further investigation.
In the present study, when comparing erythrocyte PCOOH between the placebo and astaxanthin groups, PCOOH levels after astaxanthin supplementation (5·2 and 6·6 pmol/ml packed cells for 6 and 12 mg astaxanthin groups, respectively) were somewhat lower but not statistically significant as compared with those of the placebo group (9·1 pmol/ml packed cells; P = 0·122; Table 4). In contrast, PEOOH levels after astaxanthin supplementation (2·8 and 3·0 pmol/ml packed cells for 6 and 12 mg astaxanthin groups, respectively) were significantly lower (P = 0·011) than those of the placebo group (5·8 pmol/ml packed cells). PLOOH (sum of PCOOH and PEOOH) is, therefore, considered to show significant changes (P = 0·031) between the placebo (14·9 pmol/ml packed cells) and astaxanthin groups (8·0 and 9·7 pmol/ml packed cells for 6 and 12 mg astaxanthin groups, respectively). Considering these, the antioxidant effect of astaxanthin appears likely to be through the reduction of erythrocyte PEOOH rather than PCOOH. This possibility needs to be clarified in future studies. On the other hand, for the efficacy of astaxanthin, inhibitory effects of the 6 mg astaxanthin group on PCOOH and PEOOH were as good as or even better than those of the 12 mg astaxanthin group (Tables 4 and 5). Concentrations of erythrocytes and plasma astaxanthin were not different between the 6 and 12 mg astaxanthin groups, suggesting that 6 mg astaxanthin is effective enough to show antioxidative benefit in vivo. Thus, to estimate the optimal dose of astaxanthin, we are now conducting a human study by administering 3–6 mg astaxanthin to volunteers.
Among the carotenoids (xanthophylls), astaxanthin has recently received increased scientific interest due to its potent antioxidant activity and hence possible anti-metabolic syndrome, anti-brain ageing and anti-atopic dermatitis effects(Reference Uchiyama and Okada24–Reference Satoh, Kawamura and Horibe26). We have previously found that there was a higher accumulation of PLOOH in the erythrocytes of dementia patients(Reference Miyazawa, Suzuki, Yasuda, Yagi, Kondo, Niki and Yoshikawa3). Erythrocytes high in lipid hydroperoxides have been suggested to have a decreased ability to transport oxygen to the brain and may impair blood rheology, thus facilitating dementia(Reference Bosman, Bartholomeus and de Man4–Reference Mohanty, Eckley and Williamson8). In the present study, orally administered astaxanthin was incorporated into erythrocytes, and erythrocyte PLOOH levels decreased. On the basis of these points, it seems that similar to lutein(Reference Nakagawa, Kiko and Hatade11), astaxanthin has the potential to act as an important antioxidant in erythrocytes, and thereby may contribute to the prevention of dementia. This possibility warrants the testing of astaxanthin in other models of dementia with a realistic prospect of its use as a human therapy.
Acknowledgements
The present study was supported in part by Grants-in-Aid for Scientific Research (KAKENHI; 20228002) of the Japanese Society for the Promotion of Science (JSPS; Tokyo, Japan). K. N. was involved in data collection, data analysis, data interpretation, literature search and manuscript preparation. T. K., T. M., G. C. B., F. K. and A. S. were involved in data collection, data analysis and data interpretation. A. S. and T. M. were involved in the study design, data interpretation and review of the manuscript. None of the authors has conflicts of interest with respect to the present study.








