Introduction
Acute revascularization treatments, including endovascular treatment (EVT) and intravenous thrombolysis, are the standard treatment for acute ischemic stroke patients with large vessel occlusions (LVO).Reference Powers, Rabinstein and Ackerson1 Achieving a successful reperfusion and shorter time to treatment metrics are among the most robust predictors of functional outcome in patients with LVO.Reference Saver, Goyal and van der Lugt2 The current organization of most stroke networks is based on primary stroke centers capable of providing intravenous thrombolysis, and comprehensive stroke centers which are also able to provide EVT. It became clear early after the completion of the successful randomized controlled trials which demonstrated the benefit of EVT, that the optimization of stroke networks at different levels (spatial distribution, transport organization, hospital resources and logistics, and personnel) would play a decisive role in improving the care of patients with acute ischemic stroke.Reference Mocco, Fargen and Goyal3 The organization of stroke networks varies greatly from region to region, and is highly dependent on population density, geographical extension, installed health-care facilities and availability of neurointerventional expertise. Because comprehensive stroke centers are scarce resources, many acute stroke patients are typically admitted in nearby primary stroke centers, where intravenous thrombolysis may be initiated and the indication to interhospital transfer for EVT may be decided. This “drip-and-ship” model contrasts with the “mothership” model, where patients with clinical suspicion of acute stroke and LVO are directly transferred to comprehensive stroke centers, bypassing nearby primary stroke centers.Reference Campbell, Donnan and Davis4 A recent meta-analysis of 19 studies reporting outcomes in almost 1500 stroke patients who underwent EVT showed that patients admitted primarily in EVT-capable centers presented better 3-month functional outcomes when compared to patients who were admitted primarily in primary stroke centers.Reference Zhao, Ma, Chen and Yue5 In addition to that, interhospital transfer also reduces eligibility for EVT.Reference Nikoubashman, Pauli and Schurmann6 However, the recently published RACECAT trial showed no benefit of actively bypassing primary stroke centers in patients with suspected LVO.Reference de la Ossa, Abilleira and Jovin7 The “mothership” model relies on correctly identifying patients with suspicion of acute ischemic stroke and LVO in the prehospital setting. Several clinical scales were developed for this purpose, but present relevant limitations, namely low sensitivity (<70%) for detection of LVO.Reference Duvekot, Venema and Rozeman8 One of the limitations of RACECAT trial is exactly the use of a prehospital clinical scale (RACE) which presented a positive predictive value of 67% for the identification of LVO among patients with confirmed ischemic stroke or transient ischemic attack.Reference de la Ossa, Abilleira and Jovin7
We conducted a proof-of-concept study where we used point-of-care ultrasound (POCUS) of the common carotid arteries (CCA) as a non-invasive and simple method to screen for LVO in patients with suspected acute ischemic stroke.
Methods
For this proof-of-concept observational study we included 15 acute ischemic stroke patients with LVO, and bedside emergent CCA duplex ultrasonography (DUS) during the acute diagnostic work-up (before EVT was performed) by one of the investigators. All of these 15 patients had a LVO of the anterior circulation, which was confirmed in the diagnostic digital subtraction angiography before mechanical thrombectomy. For the control group we selected 15 patients with no evidence of LVO in computed tomography angiography (CTA), who were admitted to our comprehensive stroke unit and underwent carotid DUS in the first 24 hours after admission. The study inclusion period was between December 2017 and April 2018, patients were included by convenience. We collected clinical and imaging data from our local stroke register or from the electronical patient record when needed. The following stroke scales were retrospectively scored based on patients records and detailed baseline neurological examination: 3ISS,Reference Singer, Dvorak, de Rochemont, Lanfermann, Sitzer and Neumann-Haefelin9 LAMS,Reference Llanes, Kidwell, Starkman, Leary, Eckstein and Saver10 RACE,Reference de la Ossa Pérez, Carrera and Gorchs11 CPSSS.Reference Katz, McMullan, Sucharew, Adeoye and Broderick12 This study complies with the guidelines for Good Clinical Practice and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The local institutional ethics committee of the Medical Faculty, RWTH Aachen University, Aachen, Germany (EK 335/15) approved the study protocol. All participants signed informed consent to use the data for research and to publish their data anonymously.
Data Analysis
The flow profiles in both CCAs for each patient were independently analyzed by one experienced neurologist with accreditation in neurovascular ultrasound and one junior neurologist, both of whom were blinded to imaging findings of CT and CTA. The images were displayed by the same machine Siemens Acuson X300 using 2,5–10 MHz probe, with B-mode baseline and color Doppler ultrasound profile, and they consisted of still-frame records of DUS exams (span of 5 seconds) of both CCA that were shown next to each other for 30 seconds to each observer individually and in randomized order. Raters were provided with clinical information describing the major neurological deficits and the side of the neurological deficits, but no information concerning stroke scale scores was provided. CCA flow profiles compatible with distal LVO were defined as presence of increased pulsatility with systolic spikes and significantly decreased or absent diastolic flow, as well as marked asymmetry by side comparison, as exemplified in Figure 1a. With this definition we aimed to simplify the emergency POCUS assessment, so that non-expert users of sonographic techniques could also be able to visually recognize the flow pattern. No peak systolic velocities, end-diastolic velocities, pulsatility indexes or resistance indexes were presented to the observers.

Figure 1: Duplex ultrasonography images of the common carotid arteries in a patient with acute right-sided carotid T-occlusion, showing increased pulsatility and absent end-diastolic flow in the right common carotid artery (a). False positive case, in a patient incorrectly judged to have an intracranial large vessel occlusion on the right side (b). False negative case, in a patient with a large vessel occlusion on the right side (c).
Statistical Analyses
The characteristics of the groups of patients with LVO and without LVO were summarized. Baseline characteristics, time metrics and DUS parameters of patients with and without LVO were compared using chi-square tests and Mann-Whitney U tests. Univariable logistic regression analyses of DUS parameters and profile using presence of LVO as the dependent variable were calculated. Variables found to be associated with LVO with p < 0.10 in the univariable analysis were included in the multivariable logistic regression analysis using the presence of LVO as the dependent variable. Interrater agreement for the presence of CCA flow profile compatible with LVO was assessed using Cohen´s Kappa. Diagnostic statistics were carried out for the evaluation of CCA flow profiles by observers with and without accompanying clinical information, and for the following stroke scales in isolation: NIHSS, 3ISS, LAMS, RACE, CPSSS. The reported optimal cut-off values for identifying LVO for each scale were used.Reference Singer, Dvorak, de Rochemont, Lanfermann, Sitzer and Neumann-Haefelin9,Reference de la Ossa Pérez, Carrera and Gorchs11–Reference Noorian, Sanossian and Shkirkova14 Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), c-statistic and respective 95% confidence intervals (95%CI) were calculated. The threshold for significance was set at an alpha value of 0.05. Statistical analyses were performed in SPSS® 25 and MedCalc® Version 20.022.
Results
Patient Characteristics
Table 1 summarizes baseline demographic and clinical characteristics of the groups of patients with and without LVO. Among patients with LVO, six patients presented with an occlusion of the carotid-T and nine patients presented with an M1-occlusion. Among patients with no LVO, seven had an acute ischemic stroke, three had an intracerebral hemorrhage, two had a transient ischemic attack, two had a stroke mimic, and one had asubarachnoidal hemorrhage. Both groups presented a similar time interval between symptom onset and hospital admission (p = 0.189), but LVO patients underwent DUS after admission faster than patients without LVO (p < 0.001). Patients with LVO presented lower end diastolic velocities (p < 0.001), lower mean flow velocities (p = 0.020), higher pulsatility index (p < 0.001), and higher resistance index (p < 0.001) in the CCA contralateral to neurological deficits, when compared to patients with no LVO. These DUS parameters and CCA flow profile compatible with LVO were associated with presence of LVO detected in CTA in the univariable logistic regression analysis, but none of these variables were independently associated with presence of LVO in the multivariable logistic regression analysis (Supplementary Table 1). Individual patient data concerning presence of LVO, DUS parameters and presence of CCA flow profile compatible with LVO is presented in Supplementary Table 2. No significant differences were found among any of the analysed DUS parameters between patients with M1-occlusion or occlusion of the carotid-T (Supplementary Table 3).
Table 1: Baseline characteristics of the groups of patients with and without large vessel occlusion
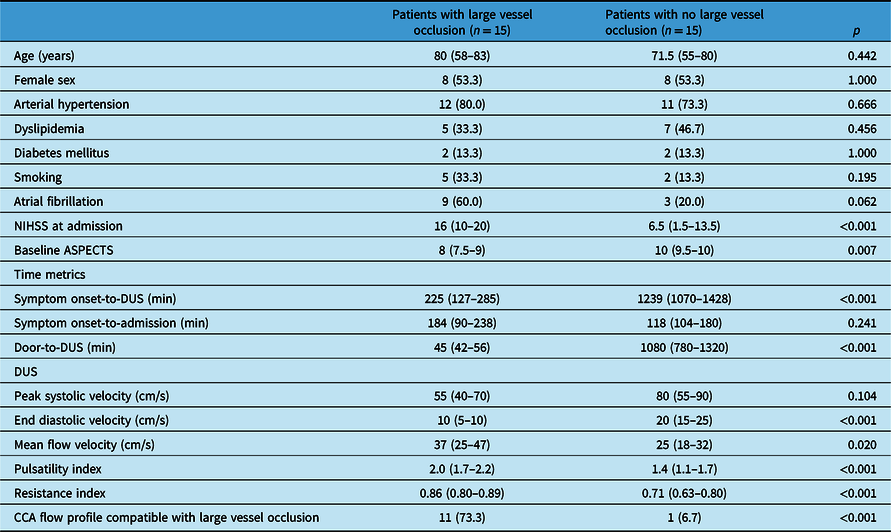
Values are presented as n (%) or median (interquartile range).
NIHSS: National Institutes of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT Score); CT: computed tomography; DUS: duplex ultrasonography.
Diagnostic Performance of DUS for Detection of LVO
Interobserver agreement for identification of a CAA flow profile compatible with LVO between the experienced neurologist and the junior neurologist was excellent (Kappa 0.813, p < 0.001). Table 2 provides sensitivity, specificity, PPV, NPV and c-statistics of CCA flow profile compatible with LVO and of stroke scales for the presence of LVO. The identification of a CCA flow profile compatible with LVO by the experienced neurologist revealed a sensitivity of 73%, a specificity of 93%, a PPV of 92%, and a NPP of 78%. The identification of a CCA flow profile compatible with LVO by the junior neurologist revealed a sensitivity 73%, a specificity of 100%, a PPV of 100% and a NPP of 79%. The higher sensitivity for identifying the presence of LVO was found for NIHSS ≥ 7 (100%), followed by CPSSS (87%), DUS by an experienced neurologist and DUS by a junior neurologist. The best trade-off between sensitivity and specificity was found for DUS by a junior neurologist (c-statistics = 0.87, 95%CI = 0.69–0.94) followed by DUS by an experienced neurologist (c-statistics = 0.83, 95%CI = 0.65–0.94), and 3ISS ≥ 4 (c-statistics = 0.82, 95%CI = 0.64–0.94).
Table 2: Diagnostic performance of duplex ultrasonography of the common carotid arteries and of stroke scales for the presence of large vessel occlusion

DUS: duplex ultrasonography; PPV: positive predictive value; NPP: negative predictive value.
Discussion
The main conclusion of our proof-of-principle study is that POCUS is a feasible method for screening the presence of LVO in patients with suspected acute ischemic stroke, and its preliminary favourable diagnostic performance is a suggestion that it could be used to select patients for primary admission in EVT-capable centers. Our definition of CCA flow profile compatible with LVO, which was evaluated visually by raters blinded to the presence of LVO, was shown to reliably reflect higher pulsatility and resistance indexes and lower end diastolic velocities. These characteristics are known sonographic hallmarks for the presence of an occlusion or severe stenosis distal to the examined segment of the artery.Reference Alexandrov15 Similar to some of the objective DUS parameters (end diastolic velocity, mean velocity, resistance index, and pulsatility index), CCA flow profile compatible with LVO was associated with presence of LVO in CTA in the univariable analysis. However, none of the parameters independently predicted the presence of LVO in the multivariable analysis, which is probably related to the small sample size and collinearity. Although our study focused on qualitative DUS analysis for prediction of LVO, objective DUS parameters may also be used to define high-risk profiles and provide the opportunity to develop automated machine-learning based methods to identify LVO, which are independent from user interpretation.
Currently, many stroke networks operate using the drip-and-ship model, where LVO is identified in primary stroke centers using CTA. The reliable identification of LVO before stroke patients reach the primary stroke center, i.e., during the prehospital phase, would allow these patients to be directly transferred to EVT-capable centers, hence shortening the time between symptom onset and groin puncture. Time delays in achieving reperfusion with EVT are associated with a significant negative impact on the patient´s functional outcome,Reference Kunz, Hunink and Almekhlafi16 and the eligibility for EVT reduces with the passing of time since symptom onset.Reference Nikoubashman, Pauli and Schurmann6 The benefit of the “mothership” strategy was recently put to test in the RACECAT trial, which showed similar results for both “mothership” and “drip-and-ship” strategies.Reference de la Ossa, Abilleira and Jovin7 However, the study presents several limitations, including that the results do not apply to other stroke networks with different geographical characteristics and with different organization of the health care resources,Reference Holodinsky, Williamson and Demchuk17 and that the RACE scale did not present an optimal accuracy for identification of LVO.
The accurate identification of acute ischemic stroke patients with LVO in a prehospital setting is of major importance for optimizing strategies to implement the “mothership” model in stroke networks. The method used in the identification of stroke patients with LVO in a prehospital setting should, in our view, have the following characteristics: 1) have an excellent sensitivity, in order not to miss patients with LVO; 2) have a good positive predictive value, in order not to overload comprehensive stroke centers with patients who otherwise could benefit more from a rapid direct admission in primary stroke centers; 3) be readily available in the prehospital setting without causing delays in patient transfer; 4) be noninvasive; 5) have a simple and rapid application; and 6) have a good reliability. DUS of the CCA appears to fulfill most of these criteria, and the main obstacle being its reliability in the setting of prehospital application. Additional advantages of POCUS as a screening method for LVO in the prehospital setting include the fact that it is a handheld portable device easy to implement in ambulances, the fact that it does not require an extensive training and does not involve radiation, the relatively low cost of these devices, and the possible use of the device for other medical emergencies.
The use of clinical stroke scales for the identification of LVO presents several limitations, mainly related to the relatively low sensitivityReference Duvekot, Venema and Rozeman8 and need for training prehospital personnel in the recognition of specific neurological signs, which is sometimes challenging. The performance of the RACE scale as a screening method in the RACECAT trial, namely the proportion of patients with no LVO despite high RACE scores (which represented 54% of the study population and 33% of patients with confirmed ischemic stroke or transient ischemic attack), probably influenced the results of the trial.Reference de la Ossa, Abilleira and Jovin7 The implication is that a relevant proportion of stroke patients actively deviated to an EVT-capable center will ultimately not benefit from such strategy because they do not present LVO and may receive intravenous thrombolysis at a later time point. At the same time, a relevant proportion of patients with LVO despite low RACE scores (up to 1/3 of patients with LVO),Reference de la Ossa Pérez, Carrera and Gorchs11 who were not included in RACECAT, could potentially benefit from the “mothership” strategy.
An alternative strategy is the use of mobile stroke units with capability to perform CT and CTA, initiate intravenous thrombolysis in the ambulance, and directly transfer patients to comprehensive stroke centers in the presence of LVO.Reference Harris18 This strategy presents the major limitation of being highly resource demanding. The accuracy of other promising strategies for detection of LVO, such as the volumetric impedance phase shift spectroscopy device,Reference Kellner, Sauvageau and Snyder19 and portable devices using electroencephalography and somatosensory-evoke potentials,Reference Sergot, Maza and Derrick20 still needs to be established in a prehospital setting. Transcranial doppler was also shown to reliably predict ischemic stroke due to occlusion of the internal carotid or middle cerebral arteries,Reference Thorpe, Thibeault, Canac, Wilk, Devlin and Hamilton21–Reference Herzberg, Boy and Holscher23 but it is technically more demanding, requires more expertise than DUS of CCA, and is limited by absent acoustic temporal bone window in 20–30% of all stroke patients,Reference Gahn, Gerber and Hallmeyer24 which may limit its use in the prehospital setting by non-expert users.
The main limitation of our study is that it is a small-sized proof-of-concept study that needs to be confirmed in unselected patients with suspected acute ischemic stroke. The group comparisons and logistic regressions we conducted are, therefore, to be interpreted with caution. The noninclusion of patients with occlusions of smaller arteries of the anterior circulation (A1, M2, M3) and occlusions of arteries in the vertebrobasilary territory may have influenced our results and may represent a major limitation for the use of POCUS with this purpose. We could not demonstrate objective differences in DUS parameters between M1 and carotid-T occlusions, but our study population size is too small to allow conclusions in this respect. Another potential limitation of POCUS for identification of LVO is its feasibility in the hyperacute setting in the emergency department or in the ambulance, which we did not analyze in our study. Patients with LVO received DUS significantly earlier after symptom begin than patients with no LVO, which is explained by the study design. This may induce bias and influence the pattern of blood flow seen in DUS, even though raters were unaware of the time point at which DUS was performed. However, no patient with no LVO developed new neurological symptoms between CTA and DUS (<24 hours in all patients), therefore the likelihood of a new nondiagnosed LVO in these group of patients is very low. DUS pitfalls which could explain false positive and false negative results must also be recognized, and it may be difficult for observers to discern them in the hyperacute phase of ischemic stroke.Reference Alexandrov15 High cardiovascular flow states and severe aortic stenosis may cause Doppler waveforms that resemble CCA flow profile compatible with LVO. On the contrary, more distal occlusions may not cause a significant change in Doppler waveforms, and occlusions which last for several hours may allow compensation and ongoing collateralization, which could cause partial normalization of Doppler waveforms. Other possible pitfalls that could influence CCA flow profiles include heart arrhythmia and significant intracranial and extracranial atherosclerotic vessel changes with increased arterial stiffness.
Nevertheless, these results are a basis for future research, and larger studies both in the emergency department and the prehospital setting are needed to confirm the results found in the current study. Follow-up studies with larger patient populations, which standardize the time interval between symptom onset and performance of POCUS, and which examine both cervical and intracranial arteries are needed to explore the questions raised by the current study. Despite the lack of benefit of “mothership” when compared to “drip-and-ship” in the RACECAT trial, the authors suggest that the employment of a “mothership” strategy using more accurate and novel technological triage tools warrants additional investigation.Reference de la Ossa, Abilleira and Jovin7
In conclusion, DUS of the CCA in the acute stroke setting is feasible and may serve as a complementary tool for the detection of LVO in the prehospital setting.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.275.
Data Availability Statement
Anonymized data will be shared by the corresponding author upon reasonable request and in compliance with the local ethic guidelines.
Author Contributions
Conceptualization: PH, ID, AR, ON. Methodology: PH, ID, JP, KS, PH, ON. Investigation/experiments: PH, ID, KS. Validation: ID, KS, MW, AR. Formal analysis: PH, ID, KS, AR, MW, JP, JBP, JSB, ON. Visualization: ID, KS, PH. Resources: AR, ON. Original draft preparation: PH, ID, JP. Review and editing: PH, ID, KS, AR, MW, JPB, JP, JBS, ON. Supervision: AR, ON. All authors have read and approved the manuscript.
Conflict of Interest Statement
The authors have no conflict of interests to declare.





