Most previous studies on the consumption of sugar-sweetened beverages (SSB) have focused on its effect on total energy intake (TEI) and body weight. In fact, Malik et al. concluded in their recent review that greater consumption of sugar-sweetened drinks is associated with weight gain and obesity in both children and adults(Reference Malik, Schulze and Hu1). SSB are usually energy-dense, but have a relatively low micronutrient content(Reference Kant2). Therefore, SSB consumption might have a diluting effect on micronutrient intake and total diet quality if SSB substitute nutrient-dense foods such as milk. Decreasing diet quality scores were found to increase disease risk and mortality rates in adulthood(Reference Waijers, Feskens and Ocke3). As eating and drinking habits are known to track between childhood and adulthood(Reference Kelder, Perry and Klepp4), the potential nutritional consequences of SSB consumption should already be considered in childhood and adolescence.
To date, the effect of SSB consumption on diet quality has predominantly been evaluated indirectly. SSB consumption has been associated with decreasing intakes of milk in children and adults(Reference Vartanian, Schwartz and Brownell5). Furthermore, Duffey & Popkin observed a positive correlation between an unhealthy beverage pattern, characterised by a high consumption of sweetened drinks, and an unhealthy food pattern in adults(Reference Duffey and Popkin6). In German children and adolescents, SSB were found to be one of the main contributors to added sugar intake(Reference Alexy, Sichert-Hellert and Kersting7). An increasing intake of added sugars has been linked with decreasing intakes of micronutrients in several studies(Reference Alexy, Sichert-Hellert and Kersting7–Reference Forshee and Storey10). Additionally, a recent meta-analysis that was predominantly based on US-American studies observed a diluting effect of SSB consumption on Ca and riboflavin intake(Reference Vartanian, Schwartz and Brownell5). Due to differences in beverage and food patterns between children in the USA and European countries, results from one country cannot necessarily be transferred to another. Furthermore, most previous studies focused on the association between SSB consumption and the intake of single nutrients, but did not evaluate the effect on total diet quality.
Therefore, the primary objective of this examination was to analyse the relationship of SSB consumption with intake of single nutrients and also total diet quality in a group of German adolescents from the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study. The total diet quality was estimated using the nutrient-based nutritional quality score (NQI). The secondary objective was to investigate possible sex and age interactions between SSB consumption and diet quality in order to identify subgroups at particular risk of dilution of total diet quality.
Study design and methods
Study design
The DONALD Study is an ongoing longitudinal, open cohort study that was established in 1985 at the Research Institute of Child Nutrition (Dortmund, Germany). The main aim of the study is the collection of information on nutrition, development, metabolism and health status of subjects between infancy and early adulthood. The regular assessments include 3 d weighed dietary records, anthropometry, urine sampling, as well as interviews on lifestyle and medical assessments. The regular visits begin at 3 months of age and take place annually from the age of 2 years until the age of 18 years. To date, over 1200 subjects have been enrolled in the DONALD Study; about forty subjects are joining each year. Further details of the DONALD Study are provided elsewhere(Reference Kroke, Manz and Kersting11).
The DONALD Study is approved by the ethical committee of the Rheinische Friedrich-Wilhelms-Universität Bonn. All examinations and assessments are performed with parental and, later on, with the children's written consent.
Subjects
Dietary records (n 7740) of subjects aged between 2 and 19 years have been collected in the DONALD Study since 1985. Of those, we considered dietary records with a ratio of reported TEI and predicted individual BMR(Reference Schofield12) above the age- and sex-specific cut-off values, i.e. 0·97 for boys and girls aged 1–5 years; boys 1·04 and girls 1·01 aged 6–13 years; boys 1·07 and girls 0·97 aged 14–18 years(Reference Sichert-Hellert, Kersting and Schoch13). As a consequence, 506 records had to be excluded due to implausible dietary recording. Also, dietary records covering less than 3 d were excluded from evaluation. This selection resulted in a total of 7145 three-day dietary records from 1069 subjects (524 boys). The individual number of 3 d records ranged from one record (n 163) to seventeen records (n 18); mean number was 4·8 records per subject. Mean age was 8·0 (sd 4·5) years in boys and 8·0 (sd 4·4) years in girls.
Dietary survey
All foods and beverages before consumption as well as leftovers were weighed to the nearest g and recorded by the children's parents, or by the older subjects themselves, on three consecutive days using electronic food scales (3 d weighed dietary records). If weighing was not possible, semi-quantitative recording (for example, numbers of glasses, cups) was allowed. Weekdays (69·8 %) and weekend days (30·2 %) were proportionally distributed in the evaluated study sample.
Energy and nutrient intakes were calculated using our continuously updated in-house nutrient database LEBTAB, which contains detailed data on the energy and nutrient contents of all recorded food items(Reference Sichert-Hellert, Kersting and Chahda14). The nutrient content of basic food items was taken from standard nutrient tables; the content of commercial food items was derived either from their product labels or from simulating recipes from the ingredients listed on the labels including fortified nutrients.
For distinction from low-energy beverages, SSB were defined as carbonated and uncarbonated beverages providing more than 40 kJ/100 ml, the energy arising predominantly from added sugars (mono- and disaccharides). Since no universal definition existed to distinguish between low-energy and regular SSB, we chose this cut-off based on information from our food database LEBTAB. Unfortunately, LEBTAB does not have information on added sugar for all SSB. However, carbohydrates in total provided more than 90 % of total energy in more than 90 % of all regular SSB. Therefore, SSB include lemonades, iced tea and fruit drinks (diluted and sugar-sweetened fruit juices). Juices made from 100 % fruit and alcoholic beverages were not classified as SSB.
Nutrient intake and diet quality
For each 3 d record individual mean daily intakes of energy from added sugars and macronutrients (carbohydrates, fat, protein) and mean daily intake of vitamins and minerals (including micronutrients from fortified SSB) in three recorded days considering vitamin losses due to food preparation were calculated. Mean values of energy from macronutrients and added sugars were related to TEI. Added sugars were defined as all refined sugars (for example, sucrose, glucose, fructose, maltose), but also honey, syrup and sugar substitutes such as mannitol, eaten separately or as an ingredient in processed or prepared foods. For the particular vitamins and minerals, the intake quality score (IQS)(Reference Gedrich and Karg15, Reference Gedrich, Sichert-Hellert and Kersting16) was calculated as percentage of the latest age- and sex-specific dietary reference values for nutrient intakes of the German Nutrition Society(17):
where i denotes the particular nutrient.
We found two different definitions for nutrient adequacy in the literature, i.e. 77 %(Reference Forshee and Storey10) and > two-thirds(Reference Farris, Nicklas and Myers9) of the respective reference value. We decided to choose the more conservative cut-off value to determine nutrient inadequacy. Therefore, inadequate mean intake was defined as IQS < two-thirds of the particular reference value(Reference Farris, Nicklas and Myers9). IQS values of the following seventeen vitamins and minerals were considered separately:
(1) Vitamin A, vitamin E, vitamin K, thiamin, riboflavin, vitamin B6, vitamin B12, vitamin C, niacin, pantothenic acid and folate;
(2) Ca, Mg, Fe, P, K and Zn.
Since vitamin D values were not reliable for all foods in LEBTAB, we decided not to include vitamin D in our analysis. Some other nutrients (for example, Mn) were not included, because recommendations for these nutrients are only estimates.
The nutrient-based NQI was used to estimate the individual total diet quality(Reference Gedrich and Karg15, Reference Gedrich, Sichert-Hellert and Kersting16). NQI was calculated as the individual harmonic mean of each subject's IQS values of the seventeen micronutrients:
To avoid mathematical compensation of deficient intakes of one nutrient by exceeding intakes of another nutrient, IQS values were truncated at 100 if the intake of a nutrient exceeded the reference value. NQI theoretically ranges from 0 to 100, where 100 represents a high diet quality meeting or exceeding the reference values in all considered micronutrients within three recorded days.
Statistical analysis
SAS procedures (version 8.02, 2001; SAS Institute, Inc., Cary, NC, USA) were applied for data analysis. Descriptive data are given as mean values and standard deviations, unless indicated otherwise.
The association between SSB consumption and diet quality was tested using a repeated-measures regression model (PROC MIXED; SAS Institute, Inc.). To concurrently regard absolute intake of SSB and differences in energy density of SSB, energy (MJ) deriving from SSB was chosen as the parameter for SSB consumption and included as an independent variable. The percentage of energy from macronutrients, IQS values of single vitamins and minerals, and NQI were separately used as dependent variables. Model 1 was controlled for time since onset of the study and age of subjects. As TEI was not adjusted in model 1, this model analyses the association between SSB consumption and total nutrient intake. The coefficient β in model 1 represents the predicted change in total nutrient intake (% reference values or % of TEI respectively) for each additional 1 MJ of SSB consumption. In model 2, energy from SSB was adjusted for TEI using the residual method separately in particular age groups. Additionally, TEI was included as a covariable in model 2. In this case, the association between SSB consumption and nutrient intake was estimated independently from TEI, i.e. model 2 analyses if a change in SSB consumption at a constant TEI level affects micronutrient intake. Since total nutrient intake depends on micronutrient density of a diet and TEI, the adjustment for TEI in model 2 is leading to prediction of the association between SSB consumption and micronutrient density.
Due to a significant sex interaction between SSB consumption and NQI in model 1 and model 2, we analysed boys and girls separately. We further tested for possible age interactions using two age groups (2–10 years and 11–19 years) to identify subgroups at particular risk of dilution of total diet quality. In all models, an exponential variance structure was assumed, which supposes a stronger correlation of assessments of one subject in two consecutive years than for longer time intervals. A separate model of repeated-measures regression analyses with time and age as sole independent variables was used for the description of time-adjusted age trends in energy and nutrient intake respectively for boys and girls (Tables 1 and 2). Subsequently, sex was included as an additional independent variable (Table 2). Positive coefficient values for sex suggest a higher nutrient intake in boys. In all statistical tests a P value < 0·05 was considered as significant.
Table 1 Mean daily nutrient intakes and diet quality in 2- to 19-year-old male subjects of the Dortmund Nutritional and Anthropometric Longitudinally Designed Study
(Mean values and standard deviations)

TEI, total energy intake; SSB, sugar-sweetened beverages; NQI, Nutritional Quality Index.
* P < 0·05, ** P < 0·01, *** P < 0·0001.
† Results from repeated-measures regression analyses on the time-adjusted effect of age (years) on the intake of single nutrients.
Table 2 Mean daily nutrient intakes and diet quality in 2- to 19-year-old female subjects of the Dortmund Nutritional and Anthropometric Longitudinally Designed Study
(Mean values and standard deviations)

TEI, total energy intake; SSB, sugar-sweetened beverages; NQI, Nutritional Quality Index.
* P < 0·05, ** P < 0·01, *** P < 0·0001.
† Results from repeated-measures regression analyses on the time-adjusted effect of age (years) on the intake of single nutrients.
‡ Results from repeated-measures regression analyses on the age- and time-adjusted effect of sex on the intake of single nutrients; positive values indicate a higher nutrient intake in boys.
Results
Sugar-sweetened beverage consumption and nutrient intakes
Mean values of daily SSB consumption and nutrient intake in boys and girls are shown in Tables 1 and 2. Mean intakes of most micronutrients met or even exceeded the reference values in both sexes. Inadequate mean intakes (IQS < two-thirds) were observed for folate and Fe in several age strata in girls and intake of Ca was borderline in older girls. In boys, intake of folate was predominantly deficient in younger age groups.
Sex differences and age trends in sugar-sweetened beverage consumption and nutrient intake
TEI (MJ) and energy deriving from SSB (MJ) were higher in boys in comparison with girls (Table 2). Additionally, boys had higher IQS values of most vitamins and all minerals resulting in a higher NQI.
TEI and energy deriving from SSB increased with age in both sexes. However, TEI and energy from SSB increased faster with age in boys, resulting in the mentioned sex differences. The composition of macronutrients did not change substantially as boys grew older, whereas girls substituted to a moderate degree fat by carbohydrates. At the same time, the intake of added sugars increased with age in both sexes. While NQI increased with age in boys because of increasing IQS values in critical nutrients such as folate and Fe, NQI slightly decreased in girls mainly due to decreasing mean IQS values of various minerals.
Association between sugar-sweetened beverage consumption and nutrient intake
Results of the repeated-measures regression analysis of the association between SSB consumption and macronutrient intake are shown in Table 3. SSB consumption was positively associated with energy from carbohydrates in both sexes in model 1. However, the increase in energy from added sugars was higher than for total carbohydrates, i.e. energy from non-sugar carbohydrates decreased with increasing SSB consumption. Concomitant to the increase in energy from total carbohydrates the intake of protein and especially fat decreased as SSB consumption increased. Results were similar when SSB consumption was adjusted for TEI (model 2).
Table 3 Results of the mixed linear regression model of the association between sugar-sweetened beverage (SSB) consumption and intake of macronutrients in a sample of the Dortmund Nutritional and Anthropometric Longitudinally Designed Study
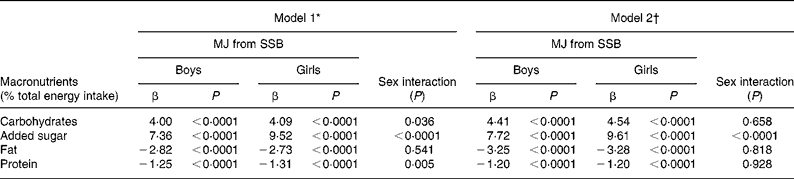
* Adjusted for time and age.
† Adjusted for time, age and total energy intake.
Results for micronutrients are shown in Table 4. In contrast to macronutrients, the associations between SSB consumption and total micronutrient intakes were different between boys and girls (model 1). In boys, the intake of riboflavin, pantothenic acid, Ca, Fe, P and K significantly decreased by 6, 5, 6, 6, 5 and 3 % respectively in relation to reference values for each additional 1 MJ of SSB consumption. The total intake of vitamin E, niacin and vitamin C, which are commonly fortified in SSB (Table 5), was positively associated with SSB consumption. In boys, NQI decreased only by 1·4 points for each 1 MJ of SSB consumption. In girls, the intake of vitamin A, vitamin K, riboflavin, folate, Ca, Mg, P and K significantly decreased by 9, 37, 8, 5, 9, 10, 6, and 9 % respectively for each additional 1 MJ of SSB consumption in model 1. These lower nutrient intakes resulted in a decrease of 2·6 points in NQI for each MJ of SSB consumed. In model 1, the decrease in NQI for each 1 MJ of SSB consumption was higher in girls. In model 2, SSB consumption was negatively associated with the nutrient density of most vitamins and all minerals in both sexes. Also, energy-adjusted NQI decreased in boys and girls. However, in line with model 1, this decrease was larger in girls.
Table 4 Results of the mixed linear regression model of the association between sugar-sweetened beverage (SSB) consumption and intake of micronutrients in a sample of the Dortmund Nutritional and Anthropometric Longitudinally Designed Study

IQS, Intake Quality Score; NQI, Nutritional Quality Index.
* Adjusted for time and age.
† Adjusted for time, age and total energy intake.
Table 5 Fortification of micronutrients in sugar-sweetened beverages (SSB)
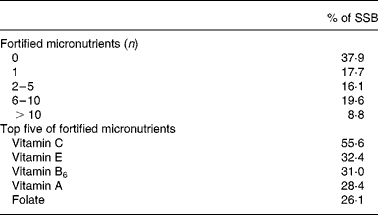
The association between SSB consumption and NQI in model 1 significantly differed between younger and older boys (P for age interaction = 0·006). In boys aged 2–10 years, NQI decreased by 2·28 points for each 1 MJ of SSB consumed (P ≤ 0·001), while the NQI did not significantly decrease in boys aged 11–19 years (β = − 0·77; P = 0·149). In girls, there was no significant difference in the association between SSB consumption and NQI in model 1 between younger and older girls (P for age interaction = 0·86).
Discussion
In the present examination a negative association between SSB consumption and the total intake of various nutrients was observed which resulted in a lower total diet quality (model 1). The diluting effect of SSB consumption on diet quality was larger in girls than in boys. In addition, SSB consumption at a constant level of TEI was adversely associated with the intake of many micronutrients and diet quality in both sexes in the energy-adjusted model 2. Thus, the supposed diluting effect of SSB on diet quality was confirmed in a sample of German children and adolescents.
In girls SSB consumption was negatively associated with the total intake of various nutrients in model 1. Therefore, SSB apparently displaced more nutritious foods or were associated with a high consumption of other nutrient-poor foods. Most remarkable was the negative effect on folate and Ca due to inadequate mean intakes of these nutrients in various age groups in girls and to their supposed effects in the prevention of CVD(Reference Spence, Bang and Chambless18, Reference McCully19) and on bone health(Reference Cashman20). Our findings in girls on folate and Ca intake are in line with results from previous studies. Especially in the case of Ca, most studies in children and adolescents found a significantly lower intake in high consumers of SSB(Reference Kant2, Reference Harnack, Stang and Story21–Reference Marshall, Eichenberger Gilmore and Broffitt27), while one study found no association(Reference Forshee, Anderson and Storey28) and another a positive association(Reference Farris, Nicklas and Myers9). In a recent meta-analysis, the authors conclude that there is a moderate negative effect of SSB consumption on Ca intake in children(Reference Vartanian, Schwartz and Brownell5). In terms of folate intake, two studies also found a negative association with SSB consumption(Reference Harnack, Stang and Story21, Reference Frary, Johnson and Wang26), whereas one study did not find any association(Reference Mrdjenovic and Levitsky22). Beside these two nutrients, we found negative associations with intakes of some other micronutrients such as vitamin K, Mg and riboflavin. A meta-analysis supports this finding for riboflavin(Reference Vartanian, Schwartz and Brownell5). However, the mean intake of riboflavin as well as vitamin K exceeded the reference values in girls of the present study sample and should not get critical even in high consumers of SSB. As IQS values were truncated at 100, the negative association with riboflavin and vitamin K intake had no effect on NQI values as an indicator for total diet quality. Regarding all micronutrients, the present examination suggests that absolute diet quality decreased with increasing SSB consumption in girls and to smaller degree in boys. So far, the few, mostly cross-sectional, studies on the effect of SSB consumption on total diet quality support the supposed negative effect of SSB consumption on total diet quality. Two studies found a negative association with total diet quality in younger children aged between 1 and 7 years using the food-based healthy eating index(Reference Rodriguez-Artalejo, Garcia and Gorgojo23) or the nutrient-based mean adequacy ratio (MAR)(Reference Marshall, Eichenberger Gilmore and Broffitt27). A significant lower diet quality measured by the healthy eating index was also observed in high soda consumers between 6 and 11 years of age(Reference LaRowe, Moeller and Adams29). In contrast, a weak positive effect of SSB consumption on the healthy eating index was found in a cross-sectional analysis in a population ranging from childhood to adulthood(Reference Forshee and Storey30). LaRowe et al. concluded that diet quality of all children could benefit from decreasing soda and sweetened beverage consumption(Reference LaRowe, Moeller and Adams29). Our findings suggest that this conclusion could be transferred to adolescence at least in girls.
In boys, SSB consumption was negatively associated with the intake of riboflavin, pantothenic acid, Ca, Fe, P and K. These negative associations resulted in a decrease in absolute NQI. Due to positive associations with the intake of vitamin E, niacin and vitamin C, which are commonly fortified in SSB, in this examination the decrease in NQI was smaller in boys in comparison with girls. Here, the diluting effect of SSB consumption on total diet quality was reduced by fortification. Earlier results from the DONALD Study demonstrated that fortification had a masking effect on the nutrient dilution of added sugars(Reference Alexy, Sichert-Hellert and Kersting31). Since total diet quality was just slightly impaired in boys, SSB probably did not widely displace other foods in diets, but was drunken additionally. This resulted in nearly unaffected total nutrient intake (model 1), but decreasing nutrient density (model 2) due to higher TEI. Actually, a previous analysis of the DONALD Study found no adequate compensation for energy from SSB by restriction of other energetic foods resulting in higher TEI(Reference Libuda, Alexy and Sichert-Hellert32). Although the effect of SSB consumption on absolute diet quality is small in boys, the diluting effect on nutrient density, the concomitant increase in TEI and the consequences for energy balance are unfavourable.
In girls the diluting effect of SSB consumption was larger in comparison with boys. In contrast to boys, the negative association was still significant as girls grew older. Since mean NQI decreased with age in girls, high SSB consumption in adolescent girls in particular might be cause for concern.
For evaluation of total diet quality, we used the nutrient-based NQI. Another common nutrient-based score is the MAR(Reference Madden and Yoder33, Reference Guthrie and Scheer34), which is also based on single nutrient intake levels given as a percentage of reference values (i.e. IQS). As values of single nutrients were truncated at 100, NQI and MAR are unaffected by nutrients which exceeded the reference values. In contrast to NQI, which is the harmonic mean of the IQS values, MAR is calculated as the arithmetic mean. One advantage of the harmonic mean is that it is more sensitive to imbalances in nutrient intake than the arithmetic mean. As a consequence, a low intake of one nutrient is not as easily compensated for by a high intake of another nutrient(Reference Gedrich and Karg15). Gedrich et al. concluded that the NQI is likely to be a more sensitive instrument to classify a deficient food quality(Reference Gedrich, Sichert-Hellert and Kersting16). However, when using MAR instead of NQI as the dependent variable in our sample, the consumption of SSB similarly affected diet quality in girls (results not shown). As intake levels of only a few micronutrients were affected in boys, there was no significant relationship between SSB consumption and MAR in model 1. Therefore, NQI is more appropriate for evaluation of the effect of SSB consumption on diet quality due to higher sensitivity for deficiency nutrients.
In terms of macronutrients, the present results (Table 3) confirm findings of various studies that observed higher carbohydrate and lower protein intakes in relation to TEI in high consumers of SSB(Reference Kant2, Reference Vartanian, Schwartz and Brownell5, Reference Farris, Nicklas and Myers9, Reference Harnack, Stang and Story21, Reference Mrdjenovic and Levitsky22, Reference Davy, Harrell and Stewart35). In the present examination, the relative increase in added sugars was higher than the increase of all carbohydrates, i.e. relative intake of non-sugar carbohydrates was lower in those who consumed more SSB. A meta-analysis also primarily attributed the observed increase in carbohydrate intake in SSB consumers to the greater consumption of added sugar(Reference Vartanian, Schwartz and Brownell5). Findings on the relationship with fat intake were less consistent(Reference Kant2, Reference Vartanian, Schwartz and Brownell5, Reference Farris, Nicklas and Myers9, Reference Harnack, Stang and Story21, Reference Davy, Harrell and Stewart35).
The DONALD Study has various strengths, but also limitations that need to be mentioned. Information on dietary habits and food intake are self-reported and might be influenced by recording errors and under-reporting. However, we excluded obviously implausible records. Most other studies on beverage consumption and nutrient intake used either single 24 h recalls or FFQ, both of which are prone to misjudge portion size and therefore total nutrient intake in children and adolescents in contrast to weighed dietary records. The high standard of the dietary records in the DONALD Study has recently been shown in an analysis comparing dietary iodine intake from weighed amounts of different food groups with concurrent 24 h urinary iodine excretion(Reference Remer, Fonteyn and Alexy36). The elaborate design of the DONALD Study results in a relative small study sample, which is not representative with respect to socio-economic status. Nevertheless, a recent nationwide survey observed similar micronutrient intakes in German children and adolescents(Reference Mensink, Heseker and Richter37). Additionally, an earlier nationwide German dietary survey from 1987–8 observed no or only minor differences in dietary habits in comparison with the DONALD sample(Reference Kersting, Sichert-Hellert and Alexy38, Reference Alexy, Kersting and Sichert-Hellert39). Therefore, our selected sample appears to reflect common patterns of nutrient intakes in German children and adolescents.
Conclusion
In a sample of German children and adolescents, the consumption of SSB was negatively associated with total diet quality in boys and girls. These findings suggest that SSB consumption might decrease mean diet quality despite an adequate mean intake of most micronutrients. However, diet quality of girls suffered more from SSB consumption. Most remarkable were the negative effects on the already low folate and Ca intake. As food and beverage patterns might track from adolescence to adulthood, the present results suggest that SSB consumption should preferably be limited in childhood in order to prevent lasting dilution effects on diet quality.
Acknowledgements
The present study was supported and funded by the German Federal Ministry of Food, Agriculture and Consumer Protection. The DONALD Study is further supported by the Ministry of Education, Science and Research North Rhine-Westphalia, Germany.
L. L. conducted the statistical analyses and wrote the manuscript. M. K., L. L. and U. A. conceived of the research project. W. S.-H. provided technical support and statistical expertise. P. S. and M. K. supervised the study. All authors contributed to the interpretation of the data and revision of the manuscript. M. K. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had any conflict of interest.
We are very grateful to the staff of the Research Institute of Child Nutrition for carrying out the anthropometric measurements and for collecting and coding the dietary records.







