INTRODUCTION
Parvovirus B19 (B19V) is a small, non-enveloped DNA virus that belongs to the genus Erythrovirus of the family Parvoviridae [Reference Heegaard and Brown1–Reference Brown3]. B19V is best known as the causative agent of erythema infectiosum, a generally mild febrile rash illness that mainly affects children [Reference Anderson4]. However, the spectrum of clinical signs of B19V infection can range from asymptomatic to chronic or recurrent illnesses, including arthritis and arthropathy [Reference Heegaard and Brown1–Reference Brown3]. Due to the efficient replication of B19V in the erythroid progenitor cells [Reference Ozawa and Young5], the infection can also lead to life-threatening aplastic crisis in patients with underlying haemoglobinopathies, as well as to chronic anaemia in immunocompromised patients [Reference Kurtzman6, Reference Plentz7]. B19V is usually spread through respiratory droplets [Reference Anderson8], but it can also be transmitted via contaminated blood products [Reference Norja, Lassila and Makris9].
Importantly, B19V can also be transmitted vertically from mother to fetus where it can cause severe fetal anaemia, miscarriage, fetal death or hydrops fetalis [Reference Brown10–Reference Puccetti14]. The risk of vertical transmission of B19V is up to about one third of acutely infected pregnant women [15] and the excess fetal death rate after maternal infection during the first 20 weeks of gestation was estimated to be 5·6% [Reference Enders16]. Notably, the probability of fetal death is highest after B19V infection in early gestation [Reference Enders16–Reference Yaegashi19]. The incidence of fetal anemia and hydops fetalis is particularly high during the second trimester when the erythrocyte mass expands rapidly, combined with the short lifespan of fetal erythrocytes [Reference Enders16, Reference Yaegashi19]. Timely transfusion of packed erythrocytes of fetuses is the treatment of choice in severe fetal anaemia and hydrops resulting in a significant reduction of fetal mortality [Reference Enders16, Reference Fairley20, Reference Rodis21]. The risk of acquiring B19V infection during pregnancy is about 1–2% in endemic periods [Reference Mossong22, Reference van Gessel23], but it may rise to >10% during epidemic periods [Reference Valeur-Jensen24]. The reported seroprevalences of B19V in pregnant women differ between countries ranging between ~35% in Spain [Reference Gratacós25] and 81% in Sweden [Reference Skjöldebrand-Sparre26].
In many developed countries, the epidemiology and trends of B19V infection in women of childbearing age are well known [Reference Vyse27, Reference Röhrer28]. However, the epidemiological data on B19V infection are generally lacking in many African countries including Sudan. Therefore this study aimed to provide preliminary information about the seroprevalence of B19V infection in Sudan through investigating pregnant women who attended antenatal clinics in Khartoum state, Sudan.
METHODS
Study area
Khartoum state, the national capital of Sudan, covers an area of 22 000 km2. The state is geographically divided into three regions; Khartoum, Khartoum North, and Omdurman, and is administratively divided into seven localities. In addition, it is the most populated Sudanese state with an estimated 5·3 millions residents, with 68% living in urban areas, 21% in rural areas, and 11% internally displaced people as reported by the Sudan Central Bureau of Statistics [29]. Furthermore, the state is a centre of several medical facilities where 94·8% of its pregnant women receive antenatal care at least once during their pregnancy and 89·0% of them are seen by skilled personnel as detailed in the Sudan Household Health Survey, 2006 [30].
Study settings
This cross-sectional study was conducted between November 2008 and March 2009, at the antenatal clinics of seven main hospitals located at the different localities of Khartoum state. The study included 500 healthy pregnant women, who came for routine follow-up at any gestational age and who agreed to participate in the study. Three millilitres of blood sample was collected in plain containers from each woman. Serum samples were separated by centrifugation and stored at −20°C until tested. A questionnaire including the demographic and obstetrical characteristics of the study subjects was administered by the research team.
Ethics
This study was approved by the Health Research Ethics Committee, Ministry of Health, Sudan. All subjects were informed about the study and consented before enrolment.
Serology testing
All serum specimens were screened for B19V IgG and IgM antibodies by the Parvovirus B19-IgG-ELISA PKS® and Parvovirus B19-IgM-ELA Test PKS® (μ-capture) assays, respectively, based on baculovirus-expressed B19V capsid proteins VP1 and VP2 as antigen (Medac, Germany). All samples testing equivocal for B19V IgG or equivocal or positive for B19V IgM by the Medac assays were retested by Parvovirus B19 IgG EIA® and Parvovirus B19 IgM EIA® (μ-capture) microtitre plate assays, respectively, based on baculovirus-produced VP2 antigen (Biotrin, Ireland, distributed by DiaSorin, Germany). All assays were performed and interpreted according to the manufacturers' instructions.
Real-time polymerase chain reaction (PCR)
All serum samples testing equivocal or positive for B19V IgM antibodies by the Medac assay were analysed for B19V DNA by real-time PCR. Nucleic acid was prepared by the QIAamp DNA Blood Mini kit (Qiagen, Germany; 200 μl input volume), and PCR was performed according to the protocol for an in-house assay described by Baylis et al. [Reference Baylis, Shah and Minor31] except for labelling the probe with BHQ1 instead of TAMRA. Cycling was conducted on a LightCycler® 480 II instrument (Roche, Germany). The PCR assay is able to detect all three genotypes of B19V.
Statistical analysis
The statistical analysis was performed using SPSS software, version 12 (SPSS Inc., USA). A P value of <0·05 was considered statistically significant by χ 2 test.
RESULTS
Characteristics of the study population
The demographic and obstetrical characteristics of the 500 pregnant women enrolled in this study are shown in Table 1. The median age (25th–75th percentiles) of the women was 26 years (22–30) ranging from 16 to 47 years. In terms of their education, 37·6% of the women had a primary-school education, 48·6% had secondary-school or university education while 13·8% were illiterate. The obstetrical characteristics revealed that the majority (66·4%) of the women were at third trimester of pregnancy, and 74·6% were multigravidae with a number of past pregnancies ranging between one and 14. Of these multigravid women, the mean number of living children was 2·5 (range 0–8). Moreover, 149 (29·8%) women had experienced pregnancy loss.
Table 1. Demographic and obstetric characteristics of the Sudanese pregnant women (n = 500) in the study

Prevalence of B19V IgG antibodies
The initial testing of the enrolled pregnant women for B19V IgG antibodies identified 287 women as positive, 190 as negative and 23 as equivocal. These 23 equivocal samples were retested by a second B19V IgG ELISA where 20 tested low positive, while three samples remained equivocal. Classifying these 20 low-positive samples as being B19V IgG positive, the overall prevalence of B19V IgG was 61·4% (307/500, Table 2).
Table 2. Results of parvovirus B19V IgG and IgM antibody testing of 500 Sudanese pregnant women

* Re-testing by a second enzyme immunoassay of the 23 serum samples whose results were initially equivocal for IgG antibodies and re-testing of the 16 and 10 serum samples whose results were initially positive and equivocal, respectively, for IgM antibodies.
Prevalence of B19V IgM antibodies
The initial examination of the subjects investigated for B19V IgM antibodies identified 474 women as negative, 16 as positive, and 10 as equivocal. Re-examination of these 16 positive subjects by the alternate B19V IgM assay revealed only one subject who tested positive, 13 who tested negative and two who were equivocal. In addition, all the 10 initially equivocal samples were negative for B19V IgM when re-examined by the second assay. Accordingly, the overall prevalence of B19V IgM was calculated as 0·2% (1/500, Table 2). The single sample that was concordantly positive for B19V IgM was also positive for B19V IgG antibodies.
B19V PCR analysis
Next, sera of subjects testing positive or equivocal for B19V IgM by the first assay were examined for B19V DNA. None of these 26 subjects were positive for B19V DNA.
Risk factors associated with B19V seroprevalence
In this study, the statistical analysis showed insignificant association between the prevalence of B19V IgG antibodies and the demographic and obstetrical factors except for the gravidity factor, i.e. B19V IgG seroprevalence was significantly higher in multigravid women than in primigravidae (χ 2 = 3·999, P = 0·046) (Table 3).
Table 3. Association between the seroprevalence of B19V IgG antibodies and various socioeconomic factors in Sudanese pregnant women (n = 497; the three individuals testing equivocal by both IgG assays were omitted from analysis)
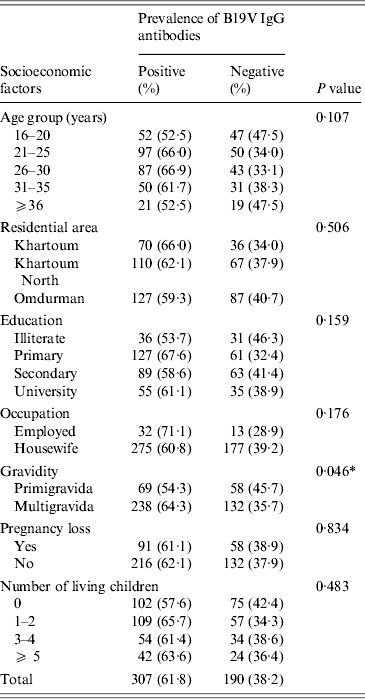
* Statistically significant: P < 0·05.
DISCUSSION
Data on B19V epidemiology in Sudan are very limited. To our knowledge, this study is the first of its kind about the seroprevalence of B19V in Sudanese adults. In this study, 61% of the participating women were positive for B19V IgG antibodies indicating a considerable circulation of B19V in Sudan. Furthermore, our results revealed only one woman (0·2%) who tested positive for B19V IgM antibodies. This lack of IgM antibodies in our study population increases the likelihood that B19V infection of these women was acquired earlier in life.
To date, only one study is known about B19V infection in Sudan [Reference Khider32]. In that PhD thesis (unpublished), serum specimens from 90 patients with haemoglobinopathies and from 90 controls aged 1 month to 18 years were examined for B19V IgG and IgM antibodies using ELISA assays. The overall prevalence of B19V IgG in the control group was 26·7%, with an increase from 19·2% in children aged <5 years to 30·0% in the 15–18 years age group. Obviously, the B19V prevalence generated in our study is higher than in the former study. This difference between the two studies is due to the older age of our study cohort (median age 26 years) and may, in part, be due to the assays applied.
Interestingly, the B19V IgG prevalence rate we calculated in Sudanese pregnant women was comparable to that of several other countries regionally and worldwide. In Libya, a neighbouring country of Sudan, a seroprevalence of 61·0% in pregnant women was reported [Reference Elnifro33]. Moreover, our B19V seroprevalence was similar to those reported in women of childbearing age in developed countries mostly ranging between about 55% and 70% [Reference Plentz and Modrow12]. By contrast, the seroprevalence rate we determined in Sudan differed from that observed in some other African countries such as the 82·0% seroprevalence detected in Eritrea, also neighbouring Sudan, and the 27·5% detected in Nigeria, a country located in West Africa [Reference Tolfvenstam34, Reference Emiasegen35]. However, fewer numbers of pregnant women were recruited in the Eritrean (n = 112) and Nigerian (n = 273) studies. Data on the prevalence of B19V IgM antibodies in pregnant women were available from the studies in Libya [Reference Elnifro33] and Nigeria [Reference Emiasegen35], both reporting higher rates than we observed. In Libya, the percentage of pregnant women testing positive for B19V IgM was 5·3% (8/150) and in Nigeria it was 13·2% (36/273).
The seroprevalence of B19V in the pregnant women population may be influenced by various demographic and obstetrical variables. Here in this study, we noticed a significant association between B19V seroprevalence and the number of pregnancies (P = 0·046), as the seroprevalence was higher in multigravidae (64·3%) compared to primigravid (54·3%) women. We speculate that such difference might be due to the higher age of multigravidae in our study (P< 0·001, data not shown) or the exposure of the multigravid women to their children. However, we were unable to unambiguously assign the difference in B19V IgG seroprevalence to the maternal age or the mothers' number of children, probably due to the relatively small size of our cohort. From studies in industrialized countries it is known that infection occurs throughout adult life increasing the seroprevalence rate from ~60% at age 18 years to >80% in geriatric populations [Reference Röhrer28, Reference Cohen and Buckley36, Reference Kelly37]. Moreover, multigravid B19V-susceptible women are more liable to acquire B19V infection from their living children [Reference Yaegashi19, Reference Röhrer28, Reference Enders, Weidner and Enders38]. In a large Danish study, including 30 946 pregnant women, the seroprevalence of B19V in mothers significantly increased with the number of their living children, particularly, children aged 5–7 years [Reference Valeur-Jensen24].
In practice, serology is the most used method for the diagnosis of B19V infection in pregnant women. In addition, confirmatory testing for B19V DNA or IgG quality is crucial for the accuracy of maternal diagnosis in cases of unreliable IgM results [Reference Enders39]. In the present study, 16 specimens were initially reactive for B19V IgM antibodies. Re-testing by an alternate commercial ELISA did not reproduce these results. Only one of these serum samples tested concordantly B19V IgM positive and two of these sera re-tested as equivocal. PCR analysis revealed negative results for all 16 samples. Furthermore, B19V avidity testing of the 16 samples by a line immunoassay [recomLine Parvovirus B19 IgG (Avidität), Mikrogen, Germany; data not shown] showed high-avidity IgG antibodies, also arguing against recent B19V infection, except for two specimens. These two did not contain the B19V IgG antibodies which are relevant for avidity testing. Unfortunately, the specimen that tested concordantly positive by the two IgM enzyme immunoassays belonged to this category. The two sera with an equivocal second enzyme immunoassay result were included in the group of high-avidity B19V IgG antibodies. Thus, finally, we classified 15 of these initial results as ‘not confirmed’ and as B19V IgM false positive. Likewise, ten sera with initial equivocal IgM results gave negative results by employing the second ELISA and tested negative for B19V DNA. Our concern about the initially reactive IgM results was underpinned by the finding that six and three specimens that tested IgM positive and equivocal, respectively, did not contain B19V IgG antibodies. Because the samples were also devoid of B19V DNA, the possibility of a very early stage of acute B19V infection was excluded. These results underline the necessity of performing additional testing for accurate diagnosis, especially in case of pregnancy. By contrast to IgM testing, 80 sera, initially testing B19V IgG positive, were also positive by the alternate ELISA and, vice versa, 69 samples, initally negative for B19V IgG, were concordantly negative when re-tested.
Currently, several viral infections are known to cause febrile rashes that are similar to B19V infection. Therefore, laboratory testing is important to differentiate between those infections, especially in the context of measles and rubella surveillance [40]. During 2009, the Sudanese National Laboratory for Measles and Rubella identified a large proportion (47·5%) of cases negative for both measles and rubella IgM antibodies in patients presenting with rash and fever (n = 655) according to the database of the Measles and Rubella Initiative [41]. Thus, we suggest the implementation of B19V testing of the febrile rashes that were previously detected in Sudan, in accordance with our present results. This suggestion is consistent with the findings of different studies that confirmed B19V infection in similar patients in Belarus [Reference Yermalovich42] and Argentina [Reference Pedranti43]. Furthermore, we recommend screening of patients for B19V not only as means of differential diagnosis of febrile rash illness but also to provide more information about the epidemiology, the pattern of circulation, and the burden of B19V in Sudan.
In conclusion, this study is the first report about the seroepidemiology of B19V in Sudanese adults. We documented a 61% seroprevalence rate of B19V in Sudanese pregnant women. We recommend conducting further studies in pregnant Sudanese women, mainly in those with complications and adverse outcomes of pregnancy, as well as in other high-risk groups including patients with haemoglobinopathies and immunological disorders.
ACKNOWLEDGEMENTS
We are grateful to all women who participated in the study and to the doctors and technicians who helped during sample collection in different hospitals.
DECLARATION OF INTEREST
None.






