Pregnancy and lactation are periods of maternal bone calcium mobilisation for fetal growth and breast milk production(Reference Kovacs1,Reference Olausson, Goldberg and Ann Laskey2) . The high calcium demand of lactation results in transient loss of bone mass in adult and adolescent women with a wide range of daily calcium intake (∼300 mg/d–1200 mg/d)(Reference Olausson, Goldberg and Ann Laskey2–Reference Diogenes, Bezerra and Donangelo6). These inferences are based on bone mineral density (BMD) measures at trabecular-rich bone sites (lumbar spine and hip), which is well accepted as the main determinant of fracture resistance(Reference Shuhart, Cheung and Gill7). It has been previously shown that hip geometry parameters are indeed useful information that reflects bone strength and susceptibility to femoral neck fractures, independent of BMD(Reference Nagaraj, Boudreau and Danielson8–Reference Kistler-Fischbacher, Yong and Weeks11).
Changes in bone geometric properties during adolescence in girls precede the peak bone mineral accrual in the total hip and femoral neck(Reference Wang, Chen and Cheng12) and appear susceptible to modulation by dietary intervention with low doses of vitamin D (200 μg/d)(Reference Al-Shaar, Nabulsi and Maalouf13). However, changes in bone geometry during lactation in adolescents are less understood. The only study conducted in adolescent mothers (∼ one month postpartum) reports cross-sectional peripheral measurements of bone geometry, with no differences observed when compared with non-pregnant, non-lactating adolescents(Reference Ward, Adams and Roberts14). In contrast, longitudinal transient changes in hip geometry have already been observed during ∼14 months postpartum in adult women(Reference Laskey, Price and Khoo15).
We have previously demonstrated that calcium plus vitamin D supplementation (600 mg Ca + 200 μg D) during adolescent pregnancy reduces the magnitude of bone mass loss in the femoral neck from the 5th to the 20th week postpartum(Reference Diogenes, Bezerra and Rezende16), although this effect was not sustained at one year postpartum(Reference Diogenes, Bezerra and Donangelo6). We raised the hypothesis that adolescent mothers would also develop longitudinal changes in hip geometry during postpartum and that calcium plus vitamin D supplementation could have an effect on these changes. Therefore, the present study is a secondary, post hoc analysis that builds upon our previous clinical trial (NCT01732328). Here we tested the effect of calcium plus vitamin D supplementation during pregnancy in hip geometry throughout one year postpartum in Brazilian adolescents with habitually low calcium intake (∼600 mg/d).
Methods and materials
Subjects and study design
Pregnant adolescents were recruited during prenatal care at the Maternity School, Federal University of Rio de Janeiro, from September 2009 to June 2011. Eligibility criteria were age between 13 and 19 years, pregnant for the first time, pregnancy with a single fetus, gestational age between 23 and 29 weeks, intending to exclusively or predominantly breastfeed, no chronic health problems, no pregnancy complications, nonsmokers and nonusers of nutritional supplements, except Fe and folic acid provided during prenatal care. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethical Committee of the Maternity School (CAAE 0002.0361.000-09). All adolescents and their parents or legal guardians provided written informed consent prior to participation. More details of the study protocol, sample size estimate and primary results of the clinical trial (ClinicalTrials.gov: NCT01732328) were previously published(Reference Diogenes, Bezerra and Donangelo6,Reference Diogenes, Bezerra and Rezende16) . Fifty-six pregnant adolescents were longitudinally followed from mid-pregnancy to 5 weeks postpartum. We considered data from a study on vitamin D supplementation in adolescents(Reference Al-Shaar, Nabulsi and Maalouf13) to estimate the minimum detectable differences between the placebo and calcium plus vitamin D groups at 5 weeks (n 56). Assuming a 95 % CI and 80 % statistical power, the estimated minimum detectable differences between groups in buckling ratio (BR) and outer diameter (OD) were 5·10 and 1·96 mm, respectively. To estimate the minimum detectable changes from the 5th to the 20th week (n 47), we referred to a study describing longitudinal changes in hip geometry in adult lactating women(Reference Laskey, Price and Khoo15). Using the same statistical assumptions, the estimated minimum detectable percent change from the 5th to the 20th week was –1·59 % in cross-sectional area (CSA).
The pregnant adolescents were randomised single-blind and assigned in a 1:1 ratio within permuted blocks of size 10 to receive a commercially available supplement (Rexall Sundown, Inc., NY) containing 600 mg CaCO3 plus 200 μg cholecalciferol or placebo (capsules of microcrystalline cellulose and corn starch; Quintessência) for daily use from 26 weeks of gestation until parturition. The dose of daily calcium was chosen to achieve recommendations for pregnant adolescents (1300 mg)(17) and provide the recommended adequate intake of vitamin D for adolescent pregnancy, at the time of study enrolment (200 μg)(18). Adherence to supplementation was obtained by counting the remaining capsules at each prenatal care visit.
Information about pregnancy and postpartum
Information on maternal age, gestational age, age at menarche, and pre-pregnancy BMI was collected during an interview at entering the study (∼26 weeks of pregnancy). Information on calcium intake was obtained by three 24-h dietary records applied between 26 weeks of pregnancy until parturition and one 24-h dietary record at each postpartum visit (5 weeks, 20 weeks and 56 weeks). Calcium intake was analysed on the basis of a Brazilian food database(19,20) with the use of the AVANUTRI software (Version Revolution 4.0; Avanutri & Nutrição Serviços e Informática Ltda ME) and WebDiet (Version 3.0; © 2023 WebDiet Health Manager). Label data were used for processed foods. At each postpartum visit, mothers were asked about breast-feeding practices and were classified as exclusive breast-feeding, predominant breast-feeding and complementary breast-feeding(21). Mothers who did not breastfeed their babies were classified as non-breast-feeding.
Laboratory analysis
Fasting blood samples were collected at 5 and 20 weeks postpartum, and serum aliquots were stored at −80°C until laboratory analysis. Serum 25(OH)D, intact parathyroid hormone (iPTH) and insulin-like growth factor I were measured by using a chemiluminescent enzyme-labelled immunometric assay (Liaison; Diasorin). Serum prolactin and oestradiol were analysed by using an immunoenzymetric assay (DiAsource ImmunoAssays). All samples were analysed in duplicate and reanalysed if duplicates were different by 10 %. More details were previously published(Reference Diogenes, Bezerra and Rezende16).
Anthropometric, lean mass and bone measurements
Height and body mass were measured by using a stadiometer (Seca®) and a calibrated electronic scale (Filizola®), respectively. Total lean mass and total femur BMD z-score and regions (femoral neck, wards and trochanter) were assessed using dual-energy X-ray absorptiometry (iDXA, enCore 2008 version 12.20, GE Healthcare, Madison, WI). BMD z-score values were obtained by comparison with a population matched for age, sex and race/colour using the manufacturer’s database as a reference. Hip geometry parameters were assessed using the manufacturer’s Advanced Hip Assessment software and included hip axis length, CSA, cross-sectional moment of inertia (CSMI), section modulus (SM), OD, neck shaft angle and BR. All anthropometric, lean mass and bone measurements were assessed at 5, 20 and 56 weeks postpartum. The performance of the equipment (dual-energy X-ray absorptiometry) was evaluated daily by the calibration block with a CV of 0·27 %.
Statistical analyses
The variables were analysed for normality using the Shapiro–Wilk test. Comparisons of general characteristics between intervention groups (placebo or calcium plus vitamin D) were performed by Student’s t test or Mann–Whitney U test. The effects of the intervention (placebo v. calcium plus vitamin D), time point (5 weeks, 20 weeks, and 56 weeks postpartum) and intervention by time point interaction in hip geometry measurements were examined unadjusted and adjusted for confounding variables using repeated-measures linear mixed models with Bonferroni post hoc tests. Potential covariates in the models were defined on the basis of the literature(Reference Laskey, Price and Khoo15,Reference Osborne, Weaver and McCabe22,Reference Al Rassy, Matta and Frenn23) and included total lean mass, height and time elapsed since menarche at each postpartum timepoint. Subsequently, the effect of calcium plus vitamin D and placebo group percent changes in hip geometry measurements from the 5th to the 20th week and from the 20th to the 56th week were examined by using ANCOVA and adjusted for the same covariates. For those variables for which time point had a significant effect in the repeated-measures linear mixed model (CSMI, CSA and SM), a comparison between groups for percent changes in hip geometry parameters throughout the study (from the 5th to the 20th week and to the 56th week) was also conducted by using ANCOVA and adjusted for the same covariates. Additionally, associations between serum iPTH, 25(OH)D, insulin-like growth factor I, prolactin and oestradiol concentrations at 5 and 20 weeks postpartum with hip geometry measurements were analysed by Spearman’s or Pearson’s correlation in the whole group. Statistical analyses were performed with SPSS 20.0 (SPSS, IBM Corp). Values at P < 0·05 were considered significant.
Results
Adolescent mothers’ characteristics
Of the 84 adolescents that were initially recruited when pregnant, 56 completed the longitudinal study at 5 weeks postpartum, 47 at 20 weeks and 30 at 56 weeks (Fig. 1). To assess possible selection bias, we compared characteristics of the adolescents who were in the study until the 5th week postpartum (n 56) v. those lost to follow-up (n 26; online Supplementary Table). The characteristics of adolescent mothers at baseline (∼26 weeks of pregnancy) and at each postpartum time point (5, 20 and 56 weeks) are shown in Table 1. At 26 weeks of pregnancy, the adolescents were, on average, 17 (sd 1·3) years old, with time elapsed since menarche 5·3 (sd 1·9) years and nutritional diagnosis of eutrophy (pre-pregnancy BMI: 21·5 (sd 3·9) kg/m2) (Table 1). At 26 weeks of pregnancy, daily calcium intake was higher in the placebo compared with the calcium plus vitamin D group (median: 602 v. 405 mg/d, respectively, P = 0·011) and did not differ between groups at 5, 20 or 56 weeks postpartum. There were no significant changes over time in calcium intake within each group (placebo, P = 0·139; calcium plus vitamin D, P = 0·744). Intervention adherence was above 80 % in both groups (Table 1). The weight of the adolescents did not differ between groups at 5th, 20th and 56th weeks postpartum (P > 0·05; Table 1), with significant reductions observed over time for both groups (time effect, P = 0·04). Serum 25(OH)D, iPTH, insulin-like growth factor I, prolactin and oestradiol concentrations showed no difference between groups at each postpartum time point. There were also no significant differences in the BMD z-scores for total femur and the femoral neck, wards and trochanter between groups over time (Table 1). A total of 95 % and 64 % of adolescent mothers breastfed their babies exclusively or predominantly at 5 and 20 weeks postpartum, respectively. At 56 weeks postpartum, 43 % of mothers were still breast-feeding their infants. No difference in breast-feeding practices between groups at each postpartum time point was observed.
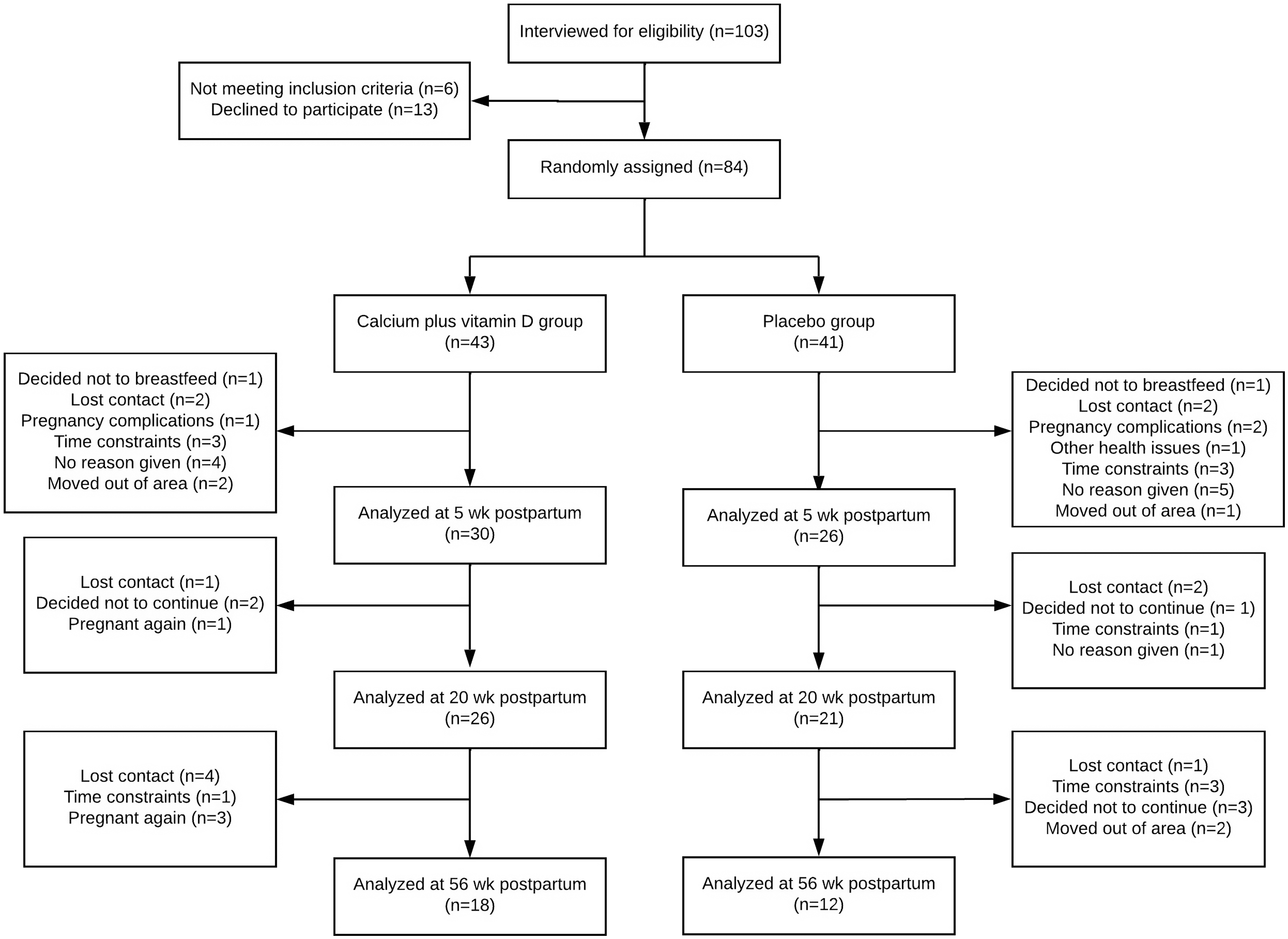
Fig. 1. Flow diagram of recruitment, random assignment, losses and follow-up of study participants (Diogenes et al. 2021).
Table 1. Characteristics of the adolescent mothers during the study (Mean values and sd; median values and 25–75 percentile)

* Values are means (sd) or median (percentile 25–percentile 75). Comparisons between groups were conducted by Student’s t test or Mann–Whitney U test.
IGF-I: insulin-like growth factor I
† Classification according to BMI-for-age (WHO, 2006).
‡ Average of three 24-hour dietary records collected between 26 weeks of pregnancy and parturition.
§ Information obtained through one 24-hour dietary record at each postpartum moment (5, 20 and 56 weeks postpartum).
Calcium plus vitamin D supplementation does not affect hip geometry
In the unadjusted model, no significant intervention effect nor intervention × time interaction was observed in hip geometry parameters (P > 0·05; Table 2). We observed time effects on CSA, CSMI and SM parameters with decreases from the 5th to the 20th week postpartum followed by recovery from the 20th to the 56th week (P < 0·05, unadjusted). Adjustments for confounding variables (lean mass, height and time elapsed since menarche) also resulted in no intervention effect or interaction and showed trend time point changes similar to the unadjusted results. No absolute changes in hip axis length were observed over time (P > 0·05). Calcium plus vitamin D supplementation did not affect hip geometry parameters but time effects in CSA, CSMI and SM were observed. Lean mass was a significant covariate for almost all parameters, except for BR. Height (for OD and hip axis length) and time elapsed since menarche (for CSMI and neck shaft angle) were also significant covariates in the analyses.
Table 2. Hip geometry parameters of adolescent mothers in both groups at 5, 20 and 56 weeks postpartum (Mean values with their sd)
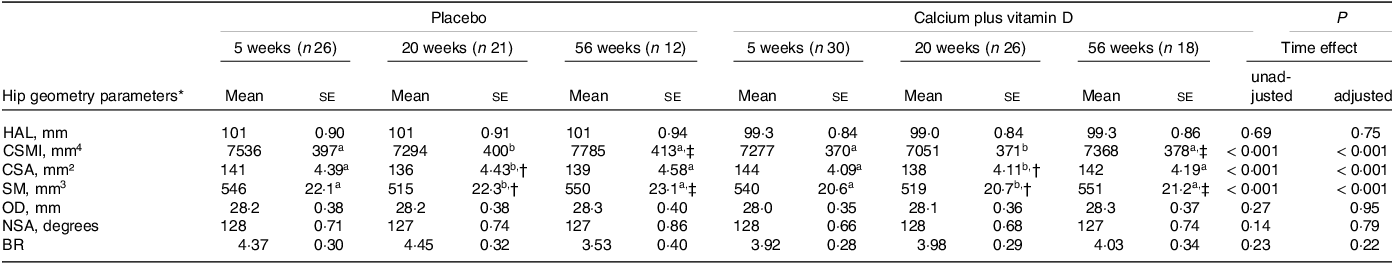
HAL, hip axis length; CSMI, cross-sectional moment of inertia; CSA, cross-sectional area; SM, section modulus; OD, outer diameter; NSA, neck shaft angle; BR, buckling ratio.
Adolescent mothers were given either placebo or calcium plus vitamin D during the last trimester of pregnancy.
a,b Labelled means (time effect) in a row without a common letter differ, P < 0·05.
* Values are unadjusted means (ses). P values refer to time postpartum in the repeated-measure linear mixed unadjusted model and in the model adjusted for lean mass, height and time elapsed since menarche as covariates.
† Significantly different from 5 weeks within the group, P < 0·05.
‡ Significantly different from 20 weeks within the group, P < 0·05.
Rate changes in hip geometry parameters did not differ between groups
Adjusted analyses were also conducted to verify differences between placebo and calcium plus vitamin D groups in the rate changes of hip geometry parameters from the 5th to the 20th week and from the 20th to the 56th week postpartum (Table 3). The rate changes in hip geometry parameters did not differ between groups (P > 0·05).
Table 3. Effects of calcium plus vitamin D supplementation during pregnancy on percent changes in hip geometry parameters of adolescent mothers from the 5th to the 20th week and from the 20th to the 56th week postpartum (Mean values with their sd)
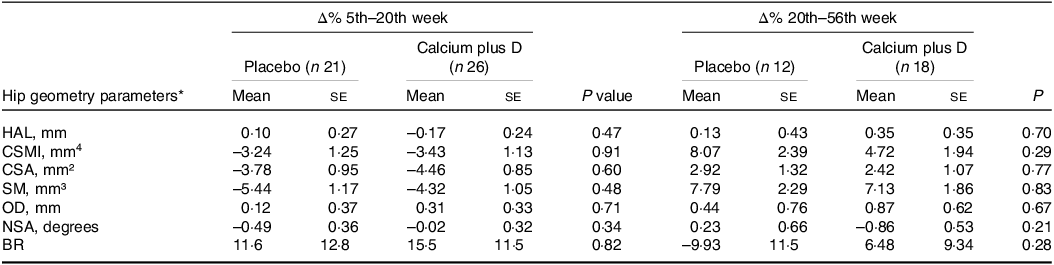
HAL, hip axis length; CSMI, cross-sectional moment of inertia; CSA, cross-sectional area; SM, section modulus; OD, outer diameter; NSA, neck shaft angle; BR, buckling ratio.
* Values are adjusted means (ses). P values refer to the comparison between calcium plus vitamin D and placebo groups by using ANCOVA and adjusted for lean mass, height and time elapsed since menarche, P < 0·05.
Decreases followed by recovery in CSA, CSMI and SM parameters
For those variables for which the time point had a significant effect on absolute values, we explored adjusted comparisons between groups for percent changes in hip geometry parameters throughout the study (Fig. 2). For both the placebo and calcium plus vitamin D groups, rate decreases were observed from the 5th to the 20th week (n 47) in CSMI (mean (s e) = −3·24 (se 1·25) % placebo v. −3·43 (se 1·13) % calcium plus vitamin D group; P = 0·914), CSA (–3·78 (se 0·95) % placebo v. −4·46 (se 0·85) % calcium plus vitamin D group; P = 0·602), and SM (–5·44 (se 1·17) % placebo v. −4·32 (se 1·05) % calcium plus vitamin D group; P = 0·483). From the 5th to the 56th week (n 30), a rate recovery was observed in CSMI (2·05 (se 2·74) % placebo v. 2·07 (se 2·23) % calcium plus vitamin D group; P = 0·996) and SM (0·21 (se 2·56) % placebo v. 2·74 (se 2·08) % calcium plus vitamin D group; P = 0·455) but not in CSA (–1·48 (se 1·52) % placebo v. −1·78 (se 1·23) % calcium plus vitamin D group; P = 0·880). Over time, we observed that in both groups a decrease was followed by a full recovery in percent changes in CSMI and SM while in CSA only a partial recovery was observed.
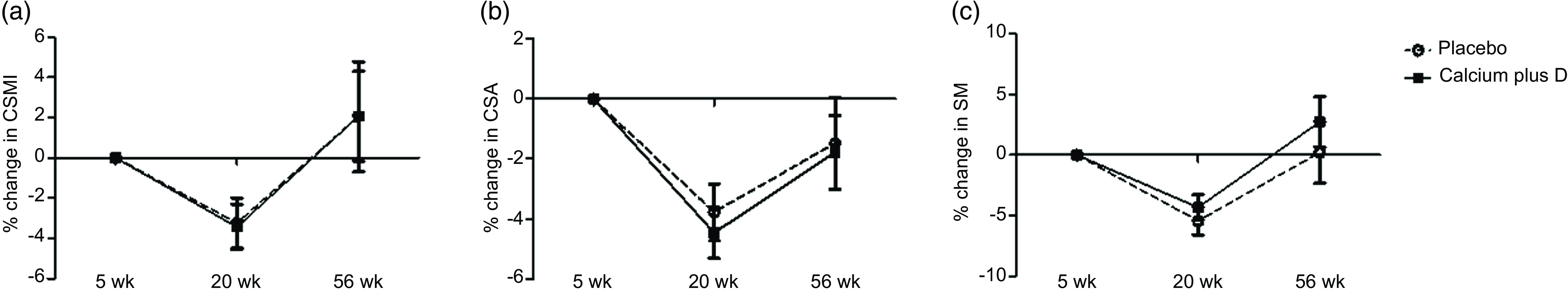
Fig. 2. (a)–(c) Percent changes in hip geometry parameters from the 5th to the 20th week and to the 56th week postpartum in the adolescent mothers given placebo or calcium plus vitamin D during the third trimester of pregnancy. The numbers of subjects at 5, 20 and 56 weeks were 26, 21 and 12 in the placebo group and 30, 26, 18 in the calcium plus vitamin D group. All values are adjusted means (ses). P values refer to comparisons between calcium plus vitamin D and placebo groups by using ANCOVA, with lean mass, height and time elapsed since menarche as covariates. There were no significant differences between placebo and calcium plus vitamin D groups over time (Δ% 5th–20th week: P = 0·914, P = 0·602 and P = 0·483 for CSMI, CSA and SM, respectively; Δ% 5th–56th week: P = 0·996, P = 0·880 and P = 0·455 for CSMI, CSA and SM, respectively). CSMI, cross-sectional moment of inertia; CSA, cross-sectional area; SM, section modulus.
Inverse correlations between serum hormones and hip geometry parameters
We explored potential correlations between serum iPTH, 25(OH)D, insulin-like growth factor I, prolactin and oestradiol concentrations with hip geometry parameters at 5 and 20 weeks postpartum. For all participants in this study, no correlations were observed between serum hormones at 5 weeks postpartum and hip geometry parameters. At 20 weeks postpartum, there were inverse correlations between serum iPTH and CSMI (Spearman’s rho = −0·371; P = 0·010), CSA (rho = –0·393; P = 0·006), and SM (rho = –0·418; P = 0·003) measurements, as well as serum prolactin and neck shaft angle (rho = −0·422; P = 0·003) (Fig. 3).
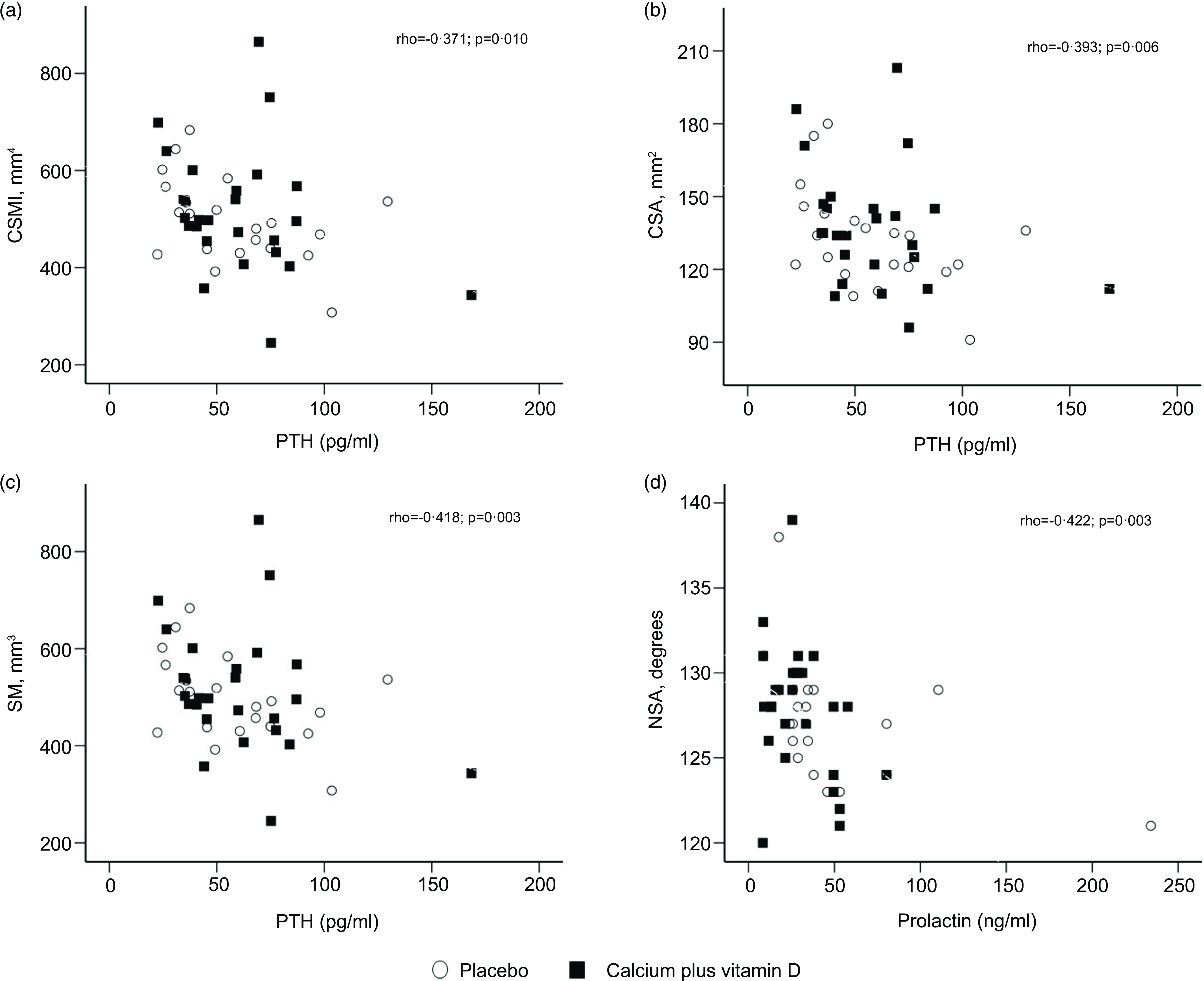
Fig. 3. (a)–(d) Correlations between hip geometry parameters and serum hormones at 20 weeks postpartum (rho for all participants, n 47). The numbers of subjects at 20 weeks were 21 in the placebo group and 26 in the calcium plus vitamin D group. CSA, cross-sectional area; CSMI, cross-sectional moment of inertia; NSA, neck shaft angle; SM, section modulus.
Discussion
In this study, we raised the hypothesis that at postpartum, adolescent mothers would develop changes in hip geometry and that calcium plus vitamin D supplementation could have an effect on these changes. Our results suggest that transient longitudinal changes do occur in the hip geometry of adolescent mothers with habitually low calcium intake. However, calcium plus vitamin D supplementation offered during pregnancy did not have any effect on reverting these hip geometry changes.
Information about changes in bone geometry during lactation is limited(Reference Ward, Adams and Roberts14,Reference Laskey, Price and Khoo15) . The only previous study evaluating adolescent mothers (∼1 month postpartum; from the UK) used a cross-sectional design and peripheral quantitative computed tomography to compare geometrical parameters at the radius and found no difference from non-pregnant or non-lactating adolescents(Reference Ward, Adams and Roberts14). Long-term transient changes in hip geometry (assessed by dual-energy X-ray absorptiometry) during lactation were previously described in well-nourished adult women from the UK(Reference Laskey, Price and Khoo15). In that study, decreases in CSA and SM and an increase in BR were observed from two weeks postpartum to peak lactation, which occurred ∼5 months postpartum. At the post-lactation period, defined as at least 3 months since weaning (∼14 months postpartum), hip geometry measurements were not significantly different from baseline (2 weeks postpartum), showing recovery of these parameters, except for CSA(Reference Laskey, Price and Khoo15). Similarly, in the present study, decreases were observed in the CSA, CSMI and SM measurements from the 5th to the 20th week postpartum, followed by full recovery in the CSMI and SM measurements and a partial recovery in the CSA from the 20th to the 56th week postpartum (Table 2 and Fig. 2). This finding is consistent with an age-independent physiological response to lactation. At 20 weeks postpartum, we also observed inverse correlations between serum iPTH concentrations and CSMI, CSA and SM measurements, as well as serum prolactin and neck shaft angle in all participants in this study (Fig. 3), suggesting that these hormones are mediating these changes during lactation.
The influence of dietary calcium and/or vitamin D on hip geometry has been explored but mainly in adult women(Reference Al-Shaar, Nabulsi and Maalouf13,Reference Nurzenski, Briffa and Price24–Reference Dhaliwal, Islam and Mikhail28) . No significant association was observed between different ranges of daily calcium intake (less than 400 mg/d, 400–799 mg/d, 800–1199 mg/d and 1200 mg/d or larger) and hip geometry parameters in women over 50 years(Reference Kim, Choi and Lim26). In postmenopausal women, most studies that investigated whether calcium and/or vitamin D supplementation improved bone strength observed a beneficial effect of supplementation on the geometric properties(Reference Nurzenski, Briffa and Price24,Reference Jackowski, Kontulainen and Cooper25,Reference Bislev, Langagergaard Rødbro and Rolighed27,Reference Dhaliwal, Islam and Mikhail28) . The only previous study during adolescence was conducted in non-pregnant non-lactating girls with vitamin D deficiency (median of serum 25(OH)D: 11·3 ng/ml) and shows that vitamin D supplementation, even in low doses (200 μg/d for 1 year), has long-term benefits on hip geometry (it improves OD and reduces BR)(Reference Al-Shaar, Nabulsi and Maalouf13). In our study, conducted in adolescent mothers who habitually consume (∼600 mg Ca/d) less than half the recommended intake of calcium (1300 mg Ca/d)(17), no benefits from calcium plus vitamin D supplementation during pregnancy were observed in the postpartum hip geometry parameters (Table 2 and Table 3). This is consistent with a previous study reporting a lack of association between dietary calcium intake and changes in hip geometry in lactating adult women(Reference Laskey, Price and Khoo15). Taken together, these results suggest that hip geometry physiologic adaptations during postpartum occur independently of calcium intake.
We have previously demonstrated that calcium plus vitamin D supplementation (600 mg Ca/d + 200 μg D/d) during pregnancy reduces the magnitude of bone mass loss in the femoral neck from the 5th to the 20th week postpartum, with no sustained effect on bone changes after one year postpartum and no effect on infant bone mass(Reference Diogenes, Bezerra and Donangelo6,Reference Diogenes, Bezerra and Rezende16,Reference Diogenes, Bezerra and Rezende29) . Strikingly, a study done with Gambian adult women with very low daily calcium intake (∼350 mg) and who received calcium supplementation (1500 mg Ca/d) during pregnancy shows that the practice disturbed their metabolic adaptation and resulted in greater reductions in bone mineral during lactation at the lumbar spine, hip, and distal radius(Reference Jarjou, Laskey and Sawo3). The result from the present study adds evidence to the knowledge that increasing calcium intake through supplementation during pregnancy brings no long-term benefits to the bone mass and/or strength in adolescent women accustomed to a low calcium diet. These results require attention from the scientific community when adopting reference dietary intake recommendations based on data collected in populations other than the one being considered.
It is important to highlight that previous studies have already reported that increasing calcium intake after delivery benefits the bone mass of adolescent mothers with adequate habitual intake in the first 6 months postpartum(Reference Chan, McMurry and Westover30,Reference Malpeli, Mansur and Santiago31) . Therefore, we cannot rule out the possibility that this effect extends to bone geometry parameters if supplementation is continued after birth. Additionally, we do not know if offering different amounts of calcium and/or vitamin D could yield different results. This is especially relevant for vitamin D, as its recommendations were revised shortly after the time of study enrolment (from an adequate intake of 200 μg to a recommended dietary allowance of 600 μg)(17,18) .
One potential limitation of our study is that a substantial number of participants were lost to follow-up over time since randomisation, limiting the statistical power of our study to detect differences between groups. Larger dropouts occurred at longer intervals between measurements, highlighting the difficulty of following adolescent mothers throughout one year, especially after they lose connection with the health unit where they received prenatal care and gave birth, which occurs during the second month postpartum. Additionally, in adolescents, bone mineralisation is incomplete which can lead to an underestimation of the results once the software assumes that the bone is fully mineralised(Reference Beck32). This study’s strength was the longitudinal design in a less-explored group. To our knowledge, this is the first study to test the effect of calcium plus vitamin D supplementation during pregnancy on long-term postpartum hip geometry measurements of adolescents with low daily calcium intake. Further research is required to confirm these findings in adolescent mothers with a larger number of participants.
Conclusions
In the present study, we tested the effects of calcium plus vitamin D supplementation during pregnancy on hip geometry parameters throughout one year postpartum in Brazilian adolescents with habitually low calcium intake (∼600 mg/d). Our study indicates that postpartum period is associated with transient changes in the hip geometry of lactating adolescent mothers, regardless of the low calcium intake and the supplementation offered during pregnancy, suggesting that a physiological adaptation of these adolescents to the low calcium intake in the postpartum period is at play.
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; F.F.B., grant number 422290/2021) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; V.R., grant number E-26/201.518/2021), Brazil. V.R. Formal analysis, investigation and writing – original draft. M.E.L.D. Conceptualisation, methodology, investigation, writing – review & editing and supervision. M.C.: Conceptualisation, methodology and investigation. C.M.D.: Methodology, writing – review & editing and funding acquisition. F.F.B.: Conceptualisation, methodology, writing – review & editing, supervision, project administration and funding acquisition. The authors declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452400165X









