It is well known that lipid metabolism is influenced by sex hormones in animals and humans(Reference Gevers Leuven1, Reference Shono, Kumagai and Sasaki2). Sex hormones, such as oestrogen, have a major impact on atherosclerotic processes, and studies in animal models have shown that oestrogen inhibits the development of atherosclerotic lesions(Reference Sullivan, Karas and Aronovitz3, Reference Chen, Li and Durand4). Oestrogen deficiency is associated with changes in blood lipid levels. It is now clear that oestrogen deficiency plays a key pathogenetic role in the development of CHD in women, as supported by several epidemiological findings(Reference Colditz, Willett and Stampfer5, Reference Rosano and Fini6). Oestrogen replacement therapy in postmenopausal women provides a protective effect against CHD. However, side effects such as breast cancer, resumption of menses and weight gain have consistently accompanied this treatment(Reference Judd, Meldrum and Deftos7, Reference Henderson, Ross and Pike8).
The amount and type of dietary protein influences blood cholesterol concentrations. In general, animal proteins are considered to be hypercholesterolaemic when compared with plant proteins(Reference Kritchevsky9). Fish consumption has been shown to be inversely associated with CHD(Reference Kris-Etherton, Harris and Appel10). In many clinical trials, the benefits of fish consumption have been attributed to the effects of fish oil; however, recent studies have reported hypocholesterolaemic effects of fish protein(Reference Zhang and Beynen11–Reference Spielmann, Kluge and Stangl14). It is also known that individual amino acids are involved in the hypocholesterolaemic effects of dietary protein(Reference Potter15, Reference Shukla, Brandsch and Bettzieche16). This concerns whether specific amino acid(s) of dietary proteins affect the metabolism of cholesterol either directly or indirectly after absorption. A potential explanation for the hypocholesterolaemic effect of soya protein when compared with casein lies in its amino acid profile, especially methionine, glycine, lysine and arginine. Sulphur-containing amino acids are recognised to be some of the most potent modulators of lipid metabolism among amino acids(Reference Oda17). Methionine (Met) was shown to increase serum cholesterol concentration in rats fed a cholesterol-free diet(Reference Sugiyama, Kanamori and Akechi18). Morita et al. (Reference Morita, Oh-hashi and Takei19) have reported that the hypocholesterolaemic effects of soya protein, potato protein and rice depended on their low Met content.
The water-insoluble fish protein (IFP) prepared by washing minced fish meat with water, a process that removes water-soluble proteins and fat, is a useful ingredient in processed sea foods, such as fish cakes (kamaboko), baked fish paste (chikuwa) and fish sausage in Japan. We reported that IFP from Alaska pollock (Theragra chalcogramma) meat had a hypocholesterolaemic effect in ovariectomised (OVX) rats(Reference Kato, Ogawa and Kishida20). As with soya protein, IFP from Alaska pollock meat has a lower content of Met and a higher content of arginine compared with casein. Therefore, in the present study, we investigated whether the hypocholesterolaemic effect of IFP from Alaska pollock in OVX rats was affected by Met addition.
Materials and methods
Materials
IFP prepared from Alaska pollock (T. chalcogramma) meat was donated by Nippon Suisan Kaisha Limited (Tokyo, Japan). IFP was prepared by washing minced Alaska pollock meat with water, a process that removes water-soluble proteins and fat. IFP was cut into small pieces, freeze-dried and powdered.
Acid-precipitated lactic casein (30 mesh) was purchased from New Zealand Dairy Board (Wellington, New Zealand).
The protein content of IFP and casein was determined by the Kjeldahl method, with an N-to-protein conversion factor of 6·25. The lipid content of IFP and casein was determined by the Soxhlet method. The protein content and lipid content of IFP and casein were 90·5 and 0·04 g/100 g, and 89·3 and 0·12 g/100 g, respectively.
Animals and diets
The present study was approved by the Laboratory Animal Care Committee of Ehime University. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University.
Female Wistar rats (6 months old) were housed individually in screen-bottomed, stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light–12 h dark cycle (light on 07.00–19.00 hours). Rats were acclimatised by feeding on a commercial solid diet (MF®; Oriental Yeast Company, Osaka, Japan) for 7 d. After acclimatisation, rats were anaesthetised by intraperitoneal injection of sodium pentobarbital (30 mg/kg body, Nembutal; Abbott Laboratories, Chicago, IL, USA), and bilaterally OVX. The rats were then fed a commercial solid diet (MF®; Oriental Yeast Company) during the 8 d recovery period, after which they were randomly divided into three groups (n 6), and allowed free access to one of the following diets for 28 d: casein, IFP or IFP+Met diet (Table 1)(Reference Reeves, Nielsen and Fahey21). In the present study, rats fed the casein and IFP+Met diets received 5·0 g Met/kg diet, which was a sufficient amount of Met recommended for rodents (4·9 g Met/kg diet) by the National Research Council(22). On the other hand, rats fed the IFP diet received 2·5 g Met/kg diet, which was half the amount of Met recommended for rodents by the National Research Council. For each rat, body weight and food intake were recorded daily in the morning before the food was replaced. The amino acid composition of the casein, IFP and IFP+Met diets was determined using an amino acid analyser (model JCL-555/V; Japan Electron Optics Laboratory Company Limited, Tokyo, Japan; Table 2)(Reference Hasegawa and Kuroda23).
Table 1 Composition of the experimental diets
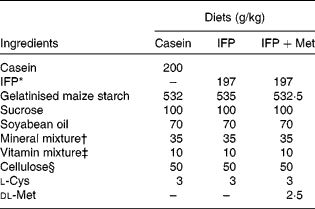
IFP, water-insoluble fish protein.
* SURIMI, prepared from Alaska pollock.
† Based on AIN-93G(Reference Reeves, Nielsen and Fahey21).
‡ The AIN-93 vitamin mixture used in the present study contained 20 g of choline bitartrate/100 g.
§ PC200 (Danisco Japan Limited, Tokyo, Japan).
Table 2 Amino acid composition of the experimental diets (n 3)*
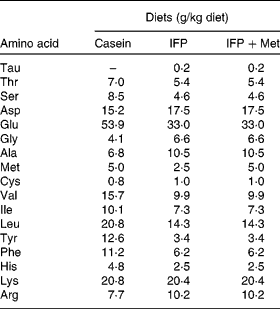
IFP, water-insoluble fish protein.
* Each value is the mean of three observations.
Sampling and analytical procedures
Before the rats were killed, faeces were collected from each rat over the final 3 d of the experimental period. The faeces were freeze-dried, weighed and milled. On the last day of the experimental period, a blood sample was collected at night (24.00–01.00 hours) from the neck of each rat into a blood collection tube (Vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA) containing heparin as an anticoagulant without fasting. The plasma was separated by centrifugation at 1400 g at 4°C for 15 min and stored at − 50°C until analysis. After blood collection, the liver was immediately perfused with ice-cold saline (9 g NaCl/l), removed, washed with cold saline, blotted dry on filter paper and weighed. After weighing, about 0·5 g of the liver was frozen immediately in liquid N2, and stored at − 80°C until RNA extraction. The remaining liver was stored at − 50°C until lipid analysis. After the liver had been removed, the small intestine was removed. The contents of the small intestine were transferred into a pre-weighed tube, freeze-dried and weighed. The middle 5 cm of the terminal 25 cm of the small intestine was flushed with ice-cold saline, frozen in liquid N2 and stored at − 80°C until RNA extraction.
Biochemical analysis
The concentration of total cholesterol and TAG in the plasma was determined enzymatically using commercial diagnostic kits (Cholesterol E-Test Wako and Triglyceride E-Test Wako; Wako Pure Chemical Industries, Osaka, Japan). The cholesterol and TAG concentrations of lipoprotein fractions were determined enzymatically using a commercial kit (Cholesterol E-Test Wako and Triglyceride E-Test Wako). The total lipid content in the liver was determined gravimetrically after extraction using the method of Folch et al. (Reference Folch, Lees and Sloane Stanley24). The liver cholesterol and TAG concentrations were determined enzymatically, as described previously(Reference Ebihara, Shiraishi and Okuma25).
The amount of bile acids in the small-intestinal contents and faeces was determined enzymatically using the 3α-dehydrogenase assay method of Sheltaway & Losowsky(Reference Sheltaway and Losowsky26), with taurocholic acid as a standard. Briefly, steroids were extracted from 50 mg of small-intestinal contents and faeces with 5 ml mixture of chloroform–methanol (1:1, v/v) at 70°C for 60 h(Reference Eneroth, Hellstrom and Sjovall27). After extraction, the volume of the steroids solution was adjusted to 5 ml with the same solution of chloroform–methanol (1:1, v/v). The extract (1·5 ml) was dried under a N2 stream, and the residue obtained was mixed with 1 ml methanol. This mixture (10 μl) was mixed with 260 μl of aqueous enzyme solution.
RNA extraction from the liver and RT-PCR analysis of gene expression
Total RNA was extracted from frozen livers according to the method described by Chomczynski & Sacchi(Reference Chomczynski and Sacchi28). RNA integrity was verified by agarose gel electrophoresis after purification of mRNA with Oligotex-dT30 (Takara Bio, Shiga, Japan). Then, 1 μg of mRNA was used for complementary DNA synthesis with 10 units of avian myeloblastosis virus (AMV) Reverse transcriptase (Takara Bio) and 2 μl of oligo(dT) primer (Novagen, Inc., Madison, WI, USA) according to the manufacturer's instructions. Expression of mRNA for acyl-CoA:cholesterol acyltransferase 1, acyl-CoA:cholesterol acyltransferase 2, cholesterol 27-hydroxylase, cholesterol 12α-hydroxylase, farnesoid X receptor, hydroxymethylglutaryl-CoA reductase, ileal bile acid transporter (IBAT), LDL-receptor, microsomal TAG transfer protein, sterol regulatory element-binding protein (SREBP)-1a, SREBP-1c, SREBP-2 and β-actin, as a housekeeping gene for normalisation, were determined by real-time monitoring of PCR using a Light Cycler instrument (Roche Diagnostics, Mannheim, Germany). Thereafter, 2 μl of complementary DNA were amplified in a total volume of 20 μl using the 2 × QuantiTect SYBR Green RT-PCR Master Mix (Qiagen, Hilden, Germany) and specific primers each at 0·5 m. After initial denaturation and activation of the polymerase at 95°C for 15 min, fifty cycles were performed with annealing temperatures for 25 s, synthesis at 72°C for 30 s and denaturation at 94°C for 15 s. Fluorescence was measured at the end of the elongation step at 72°C. The sequences of the gene-specific primers (Carl Roth, Karlsruhe, Germany) used in the present study are listed in the Supplementary table (the supplementary material for this article can be found at http://www.journals.cambridge.org/bjn). Relative gene expression was calculated using the crossing point of each target gene; the β-actin gene was used as a reference.
Statistical analyses
Data are expressed as means with their standard errors. Data were analysed by one-way ANOVA using Super ANOVA (Abacus Concepts, Berkeley, CA, USA), and the differences among groups were examined by Tukey's multiple range test using the Super ANOVA statistical software package (Abacus Concepts) when the F value was significant. A P value < 0·05 was considered significant.
Results
Body-weight gain, food intake, plasma lipids and liver lipids
Body-weight gain and food intake were not affected by the diet (Table 3). The concentration of plasma cholesterol in rats fed the IFP and IFP+Met diets was significantly lower compared with OVX rats fed the casein diet. The concentration of plasma TAG was not affected by the diet. Liver weight and the concentration of total lipids and TAG in the liver were not affected by the diet.
Table 3 Effects of water-insoluble fish protein (IFP) and IFP+methionine (Met) on body-weight, body-weight gain, food intake, plasma lipids, and liver lipids in overiectomised (OVX) rats*
(Mean values with their standard errors, n 6)
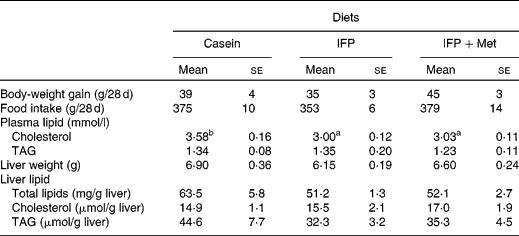
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* OVX rats were fed a test diet for 28 d.
Intestinal contents and faecal excretion
The dry weights of the small-intestinal contents and excreted faeces were not affected by the diet (Table 4). The amount of bile acids in the small-intestinal contents of OVX rats fed the IFP and IFP+Met diets was significantly higher compared with OVX rats fed the casein diet. The amount of bile acid excreted in the faeces was significantly higher in OVX rats fed the IFP diet compared with OVX rats fed the casein diet, but not in OVX rats fed the IFP+Met diet. The mRNA levels of IBAT were significantly lower in rats fed the IFP diet compared with rats fed the casein diet, but not in OVX rats fed the IFP+Met diet.
Table 4 Effects of water-insoluble fish protein (IFP) and IFP+methionine (Met) on dry weights and bile acids of the small intestinal contents, mRNA levels of ileal bile acid transporter (IBAT) and faecal bile acid excretion in overiectomised (OVX) rats*
(Mean values with their standard errors, n 6)
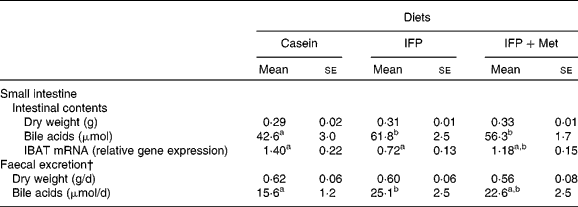
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* OVX rats were fed a test diet for 28 d.
† Faeces were collected on the final 3 d of the experimental period.
Hepatic gene expression
In the liver, the mRNA levels of cholesterol acyltransferase 1, cholesterol acyltransferase 2, cholesterol 7α-hydroxylase, cholesterol 27α-hydroxylase, farnesoid X receptor, hydroxymethylglutaryl-CoA reductase, LDL-receptor, liver X receptor, microsomal TAG transfer protein and SREBP-2 were not affected by the diet. When compared with OVX rats fed the casein diet, the mRNA levels of apo B and SREBP-1a were increased in OVX rats fed the IFP diet, but not in OVX rats fed the IFP+Met diet (Table 5). The mRNA levels of cholesterol 12α-hydroxylase tended to be increased in rats fed the IFP diet compared with rats fed the casein and IFP+Met diets (P = 0·0632). The mRNA levels of SREBP-1c were higher in rats fed the IFP and IFP+Met diets.
Table 5 Effects of water-insoluble fish protein (IFP) and IFP+methionine (Met) on hepatic mRNA levels in overiectomised (OVX) rats*
(Mean values with their standard errors, n 6)

ACAT, acyl-CoA:cholesterol acyltransferase; CYP7A1, cholesterol 7α-hydroxylase; CYP8B1, cholesterol 12α-hydroxylase; CYP27A1, cholesterol 27-hydroxylase; FXR, farnesoid X receptor; HMG-CoA, hydroxymethylglutaryl-CoA; LDL-R, LDL-receptor; LXR, liver X receptor; MTP, microsomal TAG transfer protein; SREBP, sterol regulatory element-binding protein.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* OVX rats were fed a test diet for 28 d.
† See the supplymentary table (the supplementary material for this article can be found at http://www.journals.cambridge.org/bjn).
Discussion
The concentration of plasma cholesterol was significantly lower in OVX rats fed the IFP and IFP+Met diets compared with OVX rats fed the casein diet. Because the diets did not contain cholesterol, it is clear that the hypocholesterolaemic effect of IFP in the OVX rats must be due to changes in endogenous sterol metabolism.
Although the mechanism of the hypocholesterolaemic effect of fish proteins is not yet fully understood, a potential explanation for the cholesterol-lowering effect may lie in their amino acid profiles(Reference Sugano, Ishiwaki and Nakashima29). Met was shown to elevate serum cholesterol concentration(Reference Sugiyama, Ohkawa and Muramatsu30). The hypocholesterolaemic effects of soya protein, potato protein and rice in rats depend on their low Met content(Reference Morita, Oh-hashi and Takei19). The Met content in the IFP diet was about half of that in the casein diet. However, in the present study, the hypocholesterolaemic effect of the IFP diet was not abolished by the addition of Met. Morita et al. (Reference Morita, Oh-hashi and Takei19) have reported that the higher ratio of Met:glycine in casein may be responsible for elevations in serum cholesterol. The ratios of Met:glycine (w/w) in the IFP and IFP+Met diets (0·38 and 0·76, respectively) were lower compared with the casein diet (1·22). Therefore, it seems that the amino acid profile, in part, linked to the hypocholesterolaemic effect of IFP.
Changes in amino acid metabolism after ovariectomy might affect cholesterol metabolism. After ovariectomy, serum alanine was significantly decreased in contrast to serum glycine and branched-chain amino acids; however, amino acid metabolism per se had not any significant link to cholesterol metabolism in OVX rats(Reference Frauscher and Lubec31).
An increased faecal excretion of bile acids is considered a factor in the hypocholesterolaemic effect of IFP. The primary bile acids produced in the liver are usually converted into either glycine- or taurine-conjugated bile acids before excretion into the bile. The active uptake of conjugated bile acids from the small intestine is mediated by IBAT located on the apical membrane of the ileal enterocyte(Reference Wong, Oelkers and Craddock32). The mRNA levels of IBAT were significantly lower in OVX rats fed the IFP diet compared with OVX rats fed the casein diet. In addition, the amount of bile acids in the small intestine and faecal excretion of bile acids were significantly higher in OVX rats fed the IFP diet compared with OVX rats fed the casein diet, which suggests that IFP might inhibit the ileal reabsorption of bile acids through decreased IBAT. The amount of bile acids in the small-intestinal content and the amount of bile acids excreted into faeces were significantly higher (P = 0·0491) in OVX rats fed the IFP+Met diet compared with OVX rats fed the casein diet; however, the mRNA levels of IBAT were not significantly different. The levels of hepatic cholesterol 7α-hydroxylase mRNA were not affected by the diet; however, there was a positive correlation between faecal excretion of bile acids and the levels of hepatic cholesterol 7α-hydroxylase mRNA (r 0·557, P = 0·0164). The undigested fraction of soya protein can bind bile acids, in particular conjugated bile salts, and increase faecal excretion of bile acids in rats(Reference Sugano, Goto and Yamada33). IFP may be able to bind bile acids. Therefore, an increased faecal excretion of bile acids by feeding the IFP diet might depend on decreased reabsorption due to bile acid binding of the undigested fraction of IFP rather than on decreased reabsorption of bile acids from the ileum by a decrease in IBAT.
Fatty acid synthase expression is increased by SREBP-1c activation(Reference Swinnen, Ulrix and Henys34, Reference Bennett, Lopez and Sanchez35). Fatty acids are the major components of TAG. The levels of SREBP-1c mRNA were significantly higher in OVX rats fed the IFP and IFP+Met diets compared with OVX rats fed the casein diet; however, total lipids in the liver of OVX rats fed the IFP and IFP+Met diets tended to be lower compared with OVX rats fed the casein diet (P = 0·0672). The concentration of TAG in the liver was not affected by the diet. It is not known why the TAG concentration in the liver was not affected by the diet.
In conclusion, plasma total cholesterol concentration decreased in OVX rats fed the IFP diet compared with OVX rats fed the casein diet, and the hypocholesterolaemic effect of the IFP diet was not abolished by Met addition to the IFP diet. The hypocholesterolaemic effect of IFP in OVX rats may be mediated by increased faecal excretion of bile acids coupled with decreased reabsorption of bile acids from the ileum through a decrease in IBAT and by the change in cholesterol metabolism linked to the amino acid profile. However, mRNA expression level is necessarily reflecting neither activity nor the amount of proteins. Therefore, there would be a limit in clarifying the mechanism of a hypocholesterolaemic effect of IFP by the change in the mRNA expression level of genes that relate to cholesterol metabolism. Further studies to clarify the hypocholesterolaemic effect of IFP in OVX rats are needed.
Acknowledgements
The present study was supported, in part, by the All-Japan Kamaboko Makers Association Grant Research. There are no conflicts of interest on the present study. The present study was supported by the grant of the All-Japan Kamaboko Makers Association. The contribution of each author to the manuscript is as follows: M. K. carried out the experimental plan, summarised the experimental results and discussed the experimental results with the other researchers. H. O. advised on all aspects of the experiment and discussed the experimental results. T. K. advised on all aspects of the experiment. K. E. designed the experiment and was involved in the manuscript preparation.







