Implications
Sex-sorting of semen has entered a new era with advanced biochemical techniques and high-throughput machinery. This has made the technology easily accessible with the performance of the product improving continually. Coupled with the genomic selection, the use of sex-sorted semen has the potential to rapidly accelerate genetic gain in all farmed species. It reduces animal wastage and allows the farmer choices on how to best improve the productive characteristics of their herds.
Introduction
It is quite clear that global agriculture and the associated animal industries will face a crunch in the next few decades. As population pressures mount, the competition for land by both man and animals will intensify. Estimates suggest that in the period between 1961 and 2014, the available agricultural land area measured as Ha/Capita decreased from 0.371 Ha to 0.195 Ha (FAO, 2014). At the same time, the total number of livestock (cattle, buffaloes, pigs, chickens, sheep and goats) estimated by the FAO had risen from 6.72 billion head in 1965 to 12.9 billion head by 2010 and rising (FAO, 2009). Of these numbers, cattle and buffaloes contributed to the largest increase from 1.03 billion head in 1961 to about 4.6 billion in 2010, a 4.5-factor increase in this period. In all these animals, pregnancy and parturition is an essential step to continue producing meat and milk, products that are valued by the growing human population. The outcome of this pregnancy is of significant consequence and can result in animal wastage because of the wrong sex of the calf. Sex ratio of the resulting progeny through natural mating or through an artificial breeding programme is genetically controlled, but the one disadvantage is the fixed probability of 51 : 49 in favour of male calves. This is one of the few genetic traits that cannot be controlled or manipulated efficiently by breeding programmes (Seidel, Reference Seidel2003). In the animal industries, the future characteristics of the newborn are dependent on its phenotypic sex; the choices are, in the case of a dairy calf, the animal could either become a valued herd replacement or an unprofitable male sold as a bobby calf for veal or as a steer. This issue of animal wastage is something the animal industries do need to take cognizance of particularly with dwindling land and feed resources. Hence, using every available uterus for productive animals that will join the herd and contribute efficiently should be the goal of the farmer. The use of sex-sorted semen affects a breeding programme both at the farm level as well as the stud. To use all available pregnancies to generate productive animals (females) and also targeted sex skew to produce high-value bulls or heifers from a certain sire/dam combination will maximize the use of these animal resources for genetic gain as well as production.
A brief history: the early years of sex-sorting semen
It is now almost three decades since Dr Larry Johnson of the US Department of Agriculture (USDA) published the landmark paper on semen sexing (Johnson et al., Reference Johnson1989). A culmination of a series of investigations that started in the mid-1970s, this publication signalled a breakthrough for animal breeding where the sex outcome of a pregnancy could be reliably skewed in either direction. The difficult task of an in situ probe that provided a fluorescent signal and also maintained the integrity of the sperm cell during the separation process was the real breakthrough and this followed on with demonstration of live births of rabbits using this technology (Johnson et al., Reference Johnson1989). What is quite remarkable is that the Hoechst 33342 dye easily permeates cell membranes, selectively binds to the DNA and quantitatively distinguishes the X- and Y-sperm without any apparent cellular toxicity or impaired sperm function (Penfold et al., Reference Penfold, Holt, Holt, Welch, Cran and Johnson1998; Garner and Seidel, Reference Garner, Evans and Seidel2008; Garner, Reference Garner and Seidel2009). Advances in flow cytometry continued and soon high-purity sorting was possible with input rates of greater than 40 000× and Y-sperm cells per second which could then be sorted at a high purity of either sex at over 8000 cells/s (Sharpe and Evans, Reference Sharpe and Evans2009).
Dr George Seidel and a team from Colorado State University are credited with validating the reduction to practice and field application of this technology. Extensive studies were conducted using low-dose inseminations and fresh semen (Garner and Seidel, Reference Garner, Evans and Seidel2008). In many ways, it was these experiments that led to large-scale field trials with sex-sorted semen in cattle (Seidel, Reference Seidel and Garner2012) and other species (de Graaf et al., Reference de Graaf, Leahy and Vishwanath2014). There are some very elegant reviews on the history of sex-sorted semen and the progress made over time. We point the reader to the following reviews that mostly focus on the pre-commercial phase of this technology and the science that contributed to its development (Seidel and Garner, Reference Seidel2002; Seidel, Reference Seidel and Garner2012; de Graaf et al., Reference de Graaf, Leahy and Vishwanath2014). Today, this technology of sexing mammalian sperm using a flow cytometer and measuring DNA content of sperm through the fluorescence of the DNA bound Hoechst 33342 remains the only, commercially viable method to sex-sort mammalian sperm and obtain pregnancies. In the last 10 years, the process has undergone several improvements both in sperm handling and preparation for sorting. Also, significant enhancements in sorter technology such as advanced digital processing, multiple heads and automation has made this process more efficient and it now compares favourably with conventional semen (Sharpe and Evans, Reference Sharpe and Evans2009; Evans, Reference Evans2010; Vishwanath, Reference Vishwanath, Sedoglavich, Evans, Morad and Sharpe2014; Vishwanath et al., Reference Vishwanath, Nebel, McMillan, Pitt and Macmillan2014). This paper describes the history and the developments that have occurred in the recent past and puts into perspective the current performance of sex-sorted semen. With the exception of poultry, this technology has applications in a wide variety of animal industries (dairy, beef, pigs, sheep, goats, deer and horses, de Graaf et al., Reference de Graaf, Leahy and Vishwanath2014 and references therein) as well as in exotic and conservation efforts (dolphins, rhinos, whales, brown bears, O’Brien et al., Reference O’Brien, Crichton, Evans, Schenk, Stojanov, Evans, Maxwell and Loskutoff2002 and Reference O’Brien, Steinman and Robeck2009).
Overview of the semen sexing process
Essentially, the difference in DNA content between X- and Y-sperm remains the primary and only discriminating feature to separate them (Garner, Reference Garner and Seidel2009). Alternatives to this have been investigated but they mostly fail in accuracy, repeatability and practical application (Seidel, Reference Seidel and Garner2012 and references therein). In the farmed species, the difference in DNA content range between 3.6% in swine to about 4.4% in deer. On average, the difference in size between X- and Y-sperm is about 4% in cattle with some subtle differences between breeds (Garner et al., Reference Garner2013). The process relies on the Hoechst 33342 dye diffusing through an intact cell membrane and selectively binding to the A/T base pairs within the minor groove. The absorption and fluorescence emission spectra of H33342 are about 350/460 nm and this shift makes it a very convenient marker to determine the precise amount of DNA in the sperm cell (Seidel and Garner, Reference Seidel2002; Garner, Reference Garner and Seidel2009). The flow cytometric process then quantifies the DNA difference between the two types of sperm using two fluorescence detectors that measure the intensity of the signal from the H33342 bound to the DNA when excited by a laser. The jet in air flow cytometer allows the sperm to flow through in a single file and creates a terminal droplet and a differentiating droplet charge to separate the two populations of sperm. The presence of charged plates at the discharge point allows for the two separated populations to be deflected into opposite streams for collection. The sorted populations are distinguished by fluorescence histograms on the flow cytometer and the software also allows for gating out the dead and moribund sperm. The relative gating of individual populations enables the collection of highly enriched populations of X- and/or Y-sperm (Figure 1).

Figure 1 Plot 1 is forward 0° (FAF) and side 90° (SAF) fluorescence images. Plot 1 is used to identify live/dead sperm populations and to gate only the cells within the oriented region to plot 2 and plot 3. This removes all dead sperm from the sorting process. These resulting flow cytometry histograms are used to analyze and sort on the relative fluorescence of X- and Y-sperm populations. The plot 2 allows for the gating of the required sex (X or Y or both), while plot 3 is monitoring resolution by means of peak to valley ratio (PVR). Sort speeds of >9000 cells/s can be achieved of each sex. Parallelism can triple productivity through the development of multiple head sorters such as Genesis III.
Differences in semen processing conventional v. sex-sorted sperm
A high-level overview allows one to appreciate the significant differences in the processing of conventional and sex-sorted semen. In contrast to conventional semen processing which has minimal intervention points (about three or four depending on the processing method), the processing of sex-sorted semen involves in excess of 20 steps before it is subjected to cryopreservation (Vishwanath, Reference Vishwanath, Sedoglavich, Evans, Morad and Sharpe2014). The process has undergone refinement since the initial publications by Seidel and Garner (Reference Seidel2002), Garner and Seidel (Reference Garner, Evans and Seidel2008) and Johnson (Reference Johnson, Flook and Hawk2000). It primarily involves the extension of the sperm sample to between 60 and 400×106 sperm/ml before the cells are loaded with the H33342 stain (Seidel and Garner, Reference Seidel2002), followed by the process of sorting as outlined above. Sorted sperm are collected into tubes containing appropriate buffers to protect cells during the sorting and cooling processes. After sorting, tubes are slowly cooled to 5°C, additional extenders containing cryoprotectants are added, and tubes are centrifuged to obtain concentrated sperm pellets. The number of recovered sperm is determined and extenders added to obtain the desired concentration. After a period of equilibration, semen is loaded into straws and frozen in a programmable freezer (Johnson, Reference Johnson, Flook and Hawk2000; Seidel and Garner, Reference Seidel2002). Post-thaw quality control usually involves evaluation of sperm motility and acrosome integrity after 3 h of incubation at 35°C and analysis of purity using an analytical sorter where the histogram differentiates the relative populations of X and Y. Typically the purities are around 90% of the desired sex (Sharpe and Evans, Reference Sharpe and Evans2009). Each of the processing steps is physically and bio-chemically challenging and the logical conclusion is that the function of the sperm cell may be compromised as a consequence. The challenge in many ways has been to minimize the effect of the multiple steps that sperm have to go through during the sorting process before they are finally frozen and stored for artificial insemination (AI). The XY technology as described in previous publications (Johnson and Welch, Reference Johnson and Welch1999; Schenk et al., Reference Schenk, Suh, Cran and Seidel1999; Seidel et al., Reference Seidel1999) has been modified and now changed into the new SexedULTRA™ (Navasota, TX, USA) technology. Basically, this has been a complete revamp of all the media used for the process from the initial holding and preparation of sperm for staining, the sheath fluid and the subsequent collection and freezing of sperm. The SexedULTRA™ process has been designed to be more benign to sperm during the various stages particularly in buffer conditions, pH and managing oxidative load during the sorting process (Gonzalez-Marin et al., Reference Gonzalez-Marin, Lenz, Gilligan, Evans, Gongora, Moreno and Vishwanath2016; Lenz et al., Reference Lenz, Gonzalez-Marin, Gilligan, DeJarnette, Utt, Helser, Hasenpusch, Evans, Moreno and Vishwanath2016).
SexedULTRA™: raising the fertility bar of sex-sorted semen
Over the years, those working in semen production centres have used a rather blunt tool to combat sub-fertility in cattle. The principal method has been simply to increase sperm concentration per insemination dose in order to accommodate the potential adverse effects of compromised sperm on fertility. In some cases this strategy has worked but in the main, increasing sperm numbers has only increased fertility up to the asymptotic maximum for the given bull (Pace et al., Reference Pace, Sullivan, Elliott, Graham and Coulter1981; den Daas et al., Reference den Daas, de Jong, Lansbergen and van Wagtendonk-de Leeuw1998). The field fertility of sex-sorted sperm has been discussed in numerous reviews and the reader is referred to those and the references there in for more detail (Seidel and Garner, Reference Seidel2002; Seidel, Reference Seidel and Garner2012; de Graaf et al., Reference de Graaf, Leahy and Vishwanath2014). In all of these reviews, the biggest challenge highlighted was the relative fertility of sex-sorted sperm compared with unsorted sperm. The fertility of sex-sorted semen in cattle has always lagged behind that of conventional semen and dealing with compensable elements that normally would lift the fertility of a sub-fertile sire, such as higher sperm numbers or a higher proportion of sperm with better morphological features, did not yield better results with sexed sperm (DeJarnette et al., Reference DeJarnette, Leach, Nebel, Marshall, Cleary and Moreno2011). The relative fertility of sex-sorted semen at concentrations of 2.1 million and 10 million sperm was around 70% of that of conventional semen (DeJarnette et al., Reference DeJarnette, Leach, Nebel, Marshall, Cleary and Moreno2011; Vishwanath, Reference Vishwanath, Sedoglavich, Evans, Morad and Sharpe2014). The prevailing opinion was that the compounded effect of the multiple processes that sperm were subjected to during the sorting process, led to uncompensable changes to sorted sperm with a reduction in fertility. This reduction in fertility has, in the past, been the principle reason why this technology had not been more widely adopted (Seidel, Reference Seidel, Schenk, Herickhoff, Doyle, Brink and Green2014). The difference in fertility between conventional semen and sex-sorted semen, in the order of 10 percentage points was not bridged by increasing the number of sex-sorted sperm per inseminate (DeJarnette et al., Reference DeJarnette, Cleary, Leach, Moreno, Nebel and Marshall2010 and Reference DeJarnette, Leach, Nebel, Marshall, Cleary and Moreno2011). The causes of the lower fertility of sex-sorted semen have been attributed to the varied biochemical changes that sperm undergo during the process of sex-sorting. As described earlier, there are in excess of 20 different sub-processes involved in the sex-sorting procedure including an extended holding time before staining, exposure to a laser beam to induce fluorescence, separation into X- and Y-sperm and finally exposure to an electrical field for drafting as a relatively pure population into an appropriate vessel, all of which may contribute to the reduction in fertility (Seidel and Garner, Reference Seidel2002). The challenge therefore, for those involved with the development of the technology, has been to seek imaginative ways to improving the sex-sorting process through the use of new hardware, software as well as new semen processing techniques during both the pre- and post-sorting phases.
The sex-sorting protocol involves multiple steps and quite drastic changes in the environment the sperm cell transitions through and collectively, these steps contribute to additional stress on the sperm cells. There is good reason for this as the physiology of the sperm needs to be altered in a manner that facilitates the entry of the H33342 stain and also retains it within the cell to finally fluoresce and allow discrimination (Johnson, Reference Johnson, Flook and Hawk2000; Seidel, Reference Seidel and Garner2012; de Graaf et al., Reference de Graaf, Leahy and Vishwanath2014). The cryopreservation step is an added burden on a sperm cell that had already been subjected to some significant stress. The SexedULTRA™ process in many ways was devised as a system to simplify and optimize the media used for the sex-sorting protocol so that these stressors are removed and the sperm are retained in a medium that is far more benign. The main changes included a modified protocol to pre-treat sperm before the staining step and also a new staining medium that balanced the pH and kept it stable across an extended period. The sheath fluid and the freezing medium were also modified to take into account the low-dose freezing required for sex-sorted semen. All these changes were reflected in the initial laboratory evaluations and in vitro semen quality tests where semen processed using the SexedULTRA™ media had improved sperm motility as well as acrosome integrity compared with the XY Legacy technology at the same sperm concentrations (Figure 2, Gonzalez-Marin et al., Reference Gonzalez-Marin, Lenz, Gilligan, Evans, Gongora, Moreno and Vishwanath2016). In addition, SexedULTRA™ semen used in in-vitro fertilization (IVF) trials resulted in greater number of freezable embryos compared with the XY method (13.2% and 9.2%, respectively, Gonzalez-Marin et al., Reference Gonzalez-Marin, Lenz, Gilligan, Evans, Gongora, Moreno and Vishwanath2016). The first small-scale field trials with SexedULTRA™ involved industry partners and there was an improvement of 7.4 percentage points (15.6% relative) in heifer conception rates compared with the XY Legacy technology (Table 1). This was followed by significantly larger field trial in collaboration with Select Sires Inc. Semen from eight Holstein bulls was sorted by either the SexedULTRA™ or XY processing methods and used to inseminate 6930 Holstein heifers across 41 commercial herds in the USA. The SexedULTRA™ method resulted in a 4.5 percentage points (10.8% relative) improvement (P<0.001) in conception rate compared with the XY method (46.1% v. 41.6%, respectively, Table 1, Vishwanath, Reference Vishwanath, Sedoglavich, Evans, Morad and Sharpe2014; Lenz et al., Reference Lenz, Gonzalez-Marin, Gilligan, DeJarnette, Utt, Helser, Hasenpusch, Evans, Moreno and Vishwanath2016). A pertinent observation from all these trials was that the deleterious effect of the XY Legacy style of semen processing was partly alleviated through the use of the SexedULTRA™ technology, and the next logical step was to determine whether the corresponding sperm fertility was compensable through increasing dose rates. In the past, increasing sperm dosage did not compensate for lower conception rates with sex-sorted semen and the relative differences between sex-sorted and conventional remained (DeJarnette et al., Reference DeJarnette, Cleary, Leach, Moreno, Nebel and Marshall2010). A study conducted in collaboration with German Genetics International wherein split ejaculates from five bulls were processed in four ways into XY Legacy at 2.1 million sperm, SexedULTRA™ method with 2.1, 3 and 4 million sperm per dose, and this was compared with conventional semen from contemporaneous ejaculates from the same bulls at 15 million sperm per dose. The data are shown in Table 2 (Lenz et al., Reference Lenz, Gonzalez-Marin, Gilligan, DeJarnette, Utt, Helser, Hasenpusch, Evans, Moreno and Vishwanath2016). Fifty-six-day non-return rates (NRR) were calculated from a total of 7855 inseminations in heifers with sex-sorted semen and 62 398 inseminations with conventional semen. Overall, the 2.1 million XY Legacy resulted in lower NRRs compared with all the SexedULTRA™ treatments and conventional. The 2.1 and 3 million SexedULTRA™ treatments were similar and lower than conventional but increasing the dose rate to 4 million resulted in NRR comparable to conventional (Table 2; Lenz et al., Reference Lenz, Gonzalez-Marin, Gilligan, DeJarnette, Utt, Helser, Hasenpusch, Evans, Moreno and Vishwanath2016). Two important observations were noted from this trial: first, this was the first time a dose response was demonstrated using sex-sorted semen and the second was that fertility rates between conventional semen and sex-sorted semen approached equivalence.
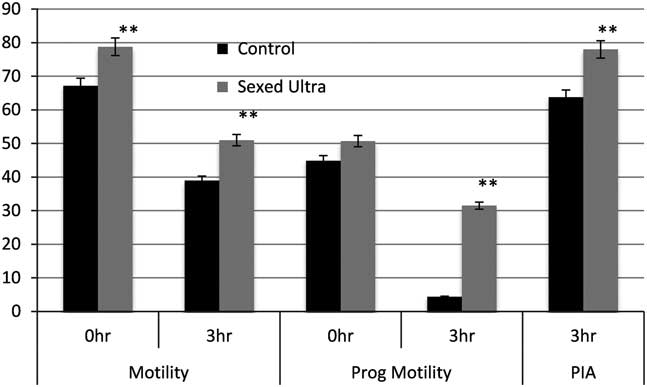
Figure 2 Comparison of SexedULTRA™ and XY (control) methods on in vitro semen quality tests. Sperm motility and progressive motility were determined using computer-assisted semen analysis and percentage intact acrosome (PIA) was determined using differential interference contrast microscopy (n=12 bulls). **Bars with superscripts differ (P<0.001). Data from Gonzalez-Marin et al. (Reference Gonzalez-Marin, Lenz, Gilligan, Evans, Gongora, Moreno and Vishwanath2016).
Table 1 Field fertility results of SexedULTRA™ inseminated in heifers

Data adapted from Vishwanath (Reference Vishwanath, Sedoglavich, Evans, Morad and Sharpe2014) and Lenz et al. (Reference Lenz, Gonzalez-Marin, Gilligan, DeJarnette, Utt, Helser, Hasenpusch, Evans, Moreno and Vishwanath2016).
a,bWithin trial, rows with different superscript letters differ (P<0.01).
Table 2 Effect of increasing sperm dose rates with SexedULTRA™ process on 56-day non-return rates (NRR)

SU=SexedULTRA™; Conv=conventional semen.
Trial conducted with German Genetics International (n=5 bulls), Lenz et al. (Reference Lenz, Gonzalez-Marin, Gilligan, DeJarnette, Utt, Helser, Hasenpusch, Evans, Moreno and Vishwanath2016).
a,b,cValues with superscript letters within column differ (P<0.001).
Are sex-sorted sperm different? Oviductal binding, timing of insemination and sperm heterogeneity
It is plausible that the sperm resulting from sex-sorting are physiologically different to unsorted sperm and therefore may require a different set of principles in an AI programme than apply to the use of conventional sperm. It has been suggested that the interaction between the process of sex-sorting and subsequent cryopreservation is possibly quite different to what happens with conventional sperm (Seidel, Reference Seidel and Garner2012). Evidence from field trials indicates that the fertility achieved with fresh (i.e. non-cryopreserved sex-sorted semen is only slightly less than that achieved with fresh unsorted semen, suggesting that the actual process of sex-sorting itself is not quite as damaging as may have been assumed previously. Data from large-scale field trials in New Zealand indicate that fresh sex-sorted sperm at a concentration of 1 million has a relative fertility of 93% to 97% of that of conventional sperm at a concentration of 2 million (Table 3, Xu, Reference Xu2014). Calving statistics from AI with sex-sorted or conventional semen were analyzed in 2011 and 2012 and the difference between sex-sorted and conventional was similar to difference in NRR of approximately −3% (Xu, Reference Xu2014). The SexedULTRA™ technology in many ways seemed to have reduced the effect of the interaction between the sex-sorting process and subsequent cryopreservation, an effect which was quite evident in the XY Legacy process where the sexed product did not respond to a dose rate increase and fertility was consistently lower at ~75% of that of conventional.
Table 3 18 to 24-day non-return rate (NRR) of fresh sex-sorted (1 million) or conventional semen (2 million)

SS=sex-sorted semen; Conv=conventional semen; Insems=insemination.
Data from Xu (Reference Xu2014). All inseminations in lactating dairy cows.
One question that arises is whether sex-sorted sperm is physiologically different and whether they behave any differently in vivo? Functional studies in vitro found the sorting process results in a more advanced membrane state, which resembles in vitro capacitation (chloro tetracycline analysis and protein tyrosine phosphorylation, Bucci et al., Reference Bucci, Galeati, Tamanini, Vallorani, Rodriguez-Gil and Spinaci2012). These capacitation-like changes were more prominent in bull spermatozoa than boar spermatozoa (Bucci et al., Reference Bucci, Galeati, Tamanini, Vallorani, Rodriguez-Gil and Spinaci2012). Sorted spermatozoa also show altered motility characteristics, velocity and amplitude of lateral head displacement as assessed by computer-assisted sperm analysis (bull, Suh et al., Reference Suh, Schenk and Seidel2005; sheep, de Graaf et al., Reference de Graaf, Evans, Maxwell, Downing and O’Brien2006), and ability to penetrate cervical mucus (de Graaf et al., Reference de Graaf, Evans, Maxwell, Downing and O’Brien2006) compared with non-sorted spermatozoa. Sorted ram spermatozoa also bind in fewer numbers to oviduct epithelial cell monolayers in vitro (de Graaf et al., Reference de Graaf, Evans, Maxwell, Downing and O’Brien2006) and detach more rapidly than non-sorted spermatozoa (Hollinshead et al., Reference Hollinshead, Gillan, O’Brien, Evans and Maxwell2003). In a recent study, the binding of sex-sorted porcine sperm to oviduct cells was reduced by more than half compared with unsorted controls. However, the percentage of sperm that bound to purified soluble glycans (bi-SiaLN and suLex) and the location of binding was similar between control and sex-sorted sperm (Figure 3, Winters et al., Reference Winters, Nettenstrom, Lopez, Willenburg, Vishwanath, Bovin and Miller2017). The plausible theory is the changed ability of sex-sorted sperm to bind to oviduct cells reflects partial capacitation and that sex-sorted sperm perhaps need less time to complete capacitation in the oviduct than non-sorted sperm (Winters et al., Reference Winters, Nettenstrom, Lopez, Willenburg, Vishwanath, Bovin and Miller2017). In a somewhat related observation, in field trials with beef cattle, changing the timing of insemination significantly improved conception rates with sex-sorted sperm (Thomas et al., Reference Thomas, Locke, Vishwanath, Hall, Ellersieck, Smith and Patterson2017). Delaying insemination closer to the time of ovulation lifted conception rates to be almost at par with conventional semen. From the oviduct binding studies in pigs and from the fixed time insemination trials with cattle, it is logical to deduce that the sex-sorting process does alter the physiology of sperm and perhaps they do not need to spend quite so much time in the female reproductive tract to become fully capacitated and functional for fertilization.

Figure 3 Binding to oviduct cell aggregates was reduced in sorted samples. (a) Example of sperm bound to an oviduct cell aggregate. (b) Four sample groups included: Y-bearing sperm, X-bearing sperm, XY an equal mixture of sorted X- and Y-bearing and the control (C) containing sperm that was not sorted (n=5). *Significant difference compared to the control (P<0.05). Means±SEM. Data from Winters et al. (Reference Winters, Nettenstrom, Lopez, Willenburg, Vishwanath, Bovin and Miller2017).
Another important aspect to consider in evaluating relative fertility of sex-sorted and conventional semen is physiological heterogeneity. It may be that a semen sample has distinct sub-populations of sperm that would be physiologically ready for fertilization at different times post insemination. In other words, sub-populations of inseminated sperm capacitate at different times after insemination and are then functionally ready for fertilization. This diversity within the sperm population of an ejaculate allows some flexibility from the time the sperm enters the female reproductive tract to the time when ovulation occurs and a competent population of sperm are available for fertilization. The variation in fertility of an individual semen sample or amongst multiple semen samples from the same individual is attributed to this diversity in a sperm population within an ejaculate (Rodriguez-Martinez, Reference Rosenfeld2006). If this heterogeneity in the sperm population is altered, it may lead to sub-fertility or enhanced fertility depending on the time of insemination relative to oestrus onset. Fertility estimates at various times of insemination is a sort of proxy estimate of heterogeneity. Fertility at each time point is a product of the number of sperm units capacitated and available for fertilization and the probability of fertilization. As an illustration of this notion, in trials with fresh encapsulated sperm compared with fresh conventional sperm, it has been suggested that the encapsulation process altered the heterogeneity of the sperm population rendering the majority of the sperm fertile in a narrow time window (Nebel et al., Reference Nebel, Vishwanath, McMillan and Saacke1993 and Reference Nebel, Vishwanath, Pitt, Mcmillan and Saacke1995; McMillan and Vishwanath, Reference McMillan and Vishwanath1994; Vishwanath et al., Reference Vishwanath1997). There is tacit evidence that there could be a similar effect of sex-sorting on sperm heterogeneity. A report by Thomas et al. (Reference Thomas, Lock, Poock, Ellersieck, Smith and Patterson2014 and Reference Thomas, Locke, Vishwanath, Hall, Ellersieck, Smith and Patterson2017) in beef heifers shows that sex-sorted semen has better fertility and is almost comparable to that of conventional semen when inseminated at later times in a split time AI protocol. It was also noted that conventional semen performed equally well at both the early and later times of insemination indicating that there are distinct sub-populations of sperm that were available for fertilization at both the early and later times. A similar observation has been noted in red deer where insemination times after a CIDR programme markedly affected the pregnancy outcome with sex-sorted frozen semen but not with conventional semen (Luis Anel-Lopez et al., Reference Anel-Lopez, Garcia-Alvarez, Parrilla, Del Olmo, Maroto-Morales, Fernandez-Santos, Ortiz, Soler, Martinez, Vazquez and Garde2017, personal communication). The early times of insemination with sex-sorted semen gave the highest pregnancy rate (82% average) compared with the later time (15% average) whereas fertility with conventional semen at a higher dose rate was consistently between 70% and 90% at the four time periods of insemination. This is another piece of evidence that the heterogeneity of the sperm sample has been altered with the sex-sorting process.
Meta-analysis: performance of sex-sorted semen over the last decade
The first analysis on the long-term usage of sex-sorted semen in the USA was compiled by USDA researchers and presented at the American Dairy Science Meeting in 2016 (Hutchison and Bickhart, Reference Hutchison and Bickhart2016). Tracking the performance of sex-sorted semen across 8 years, a positive improvement in overall fertility of sex-sorted semen was observed soon after the introduction of the SexedULTRA™ technology (Figure 4). In 2013, there was a sharp decline in the difference in conception rates between conventional and sex-sorted semen coinciding with the global introduction of SexedULTRA™ technology. In the same period, sexed-semen utilization in heifers increased from 9.4% in 2007 to 30.7% in 2015 (Hutchison and Bickhart, Reference Hutchison and Bickhart2016) and sexed-semen utilization in cows increased from 0.2% in 2007 to 1% in 2015.

Figure 4 Holstein inseminations from 2007 through to 2015: 5 963 876 heifer inseminations (1 323 721 to sexed semen) and 42 232 502 cow inseminations (253 586 to sexed semen). Mean conception rates for sexed semen increased due to improved technology (42% in 2007 compared with 49% in 2015). Comparable conception rates for heifer conventional inseminations were 56% and 59% for 2007 and 2015, respectively. Conception rates for sexed-semen inseminations in cows were 26% in 2007 and 30% in 2015 compared with 30% and 32% for conventional inseminations during the same years. Adapted from Hutchison and Bickhart (Reference Hutchison and Bickhart2016).
A similar exercise was undertaken with ST partnering herds (Figure 5, Heuer et al., Reference Heuer, Kendall, Sun, Deeb, Moreno and Vishwanath2017). Conception rates were estimated for conventional and sexed semen using field data from 63 commercial dairy farms. The total numbers of inseminations in the data set were 2 214 246 for conventional and 343 154 for sexed semen from 2508 Holstein sires. The percentage of sexed semen used in those herds increased from 8% in 2012 to 23% in 2015. A linear mixed model was fitted to the data that included an interaction term between year-month of insemination and semen type, the insemination number (1 to 3) and the age of the service sire at the time of insemination. Random effects included the service sire and a herd-year-season of insemination effect. The model was fitted separately for heifers and cows, whereas the cow model included the lactation number (1 to 3) as an additional fixed effect. Least square means (LSM) on the interaction between year-month and semen type were used to describe the changes in conception rates over time while averaging over the remaining fixed effects. The cow conception rates show strong seasonality which is consistent over years with any semen type. A seasonality effect was not observed in heifers. The LSM conception rate in cows in January 2012 was 0.38 whereas sexed semen reached 0.25. In heifers this difference in conception rates was even more pronounced with 0.58 using conventional and 0.38 with sexed semen. In June 2014, conventional and sexed semen conception rates were almost at the same level for the first time in cows (0.34 and 0.33). Data obtained through ST partnering Holstein and Jersey herds between 2012 and 2016 have shown very similar results to those compiled by the USDA. The proportion of inseminations using sexed semen has increased over the years, but the rate of sexed semen utilization was particularly more pronounced after 2013 and after the introduction of SexedULTRA™ (Heuer et al., Reference Heuer, Kendall, Sun, Deeb, Moreno and Vishwanath2017). Sexed semen use is becoming more common in Holstein cows, especially in first and second lactation cows, and has virtually displaced the use of conventional semen in Jersey heifers and cows. When conception rates were evaluated using a subset of the data (lactation 0 to 2, service 1 to 3), mean conception rates for sexed semen have increased and the differences from conventional semen have decreased consistently after the introduction of SexedULTRA™ in all female categories in both Holstein and Jersey cattle. Conception rates of >90% of that obtained with conventional semen can be obtained with SexedULTRA™ semen (Figure 5). From the encouraging results obtained in the German Genetics trial (Table 2), STgenetics adopted the higher dose of 4 million sperm as a new product in 2015 (SexedULTRA-4M™). Data on relative fertility of conventional and sex-sorted in ST partnering herds in both Holstein and Jersey showed an additional increase in conception rates since the introduction of the higher dose rate sex-sorted semen. Relative conception rates above 90% are now common in both Jersey and Holstein heifers and, in Holstein and Jersey cows, the fertility of conventional and sex-sorted semen is reaching equivalence particularly from the early part of 2016 (Figure 5, Heuer et al., Reference Heuer, Kendall, Sun, Deeb, Moreno and Vishwanath2017). SexedULTRA-4M™ with the higher dose rate of 4 million sex-sorted sperm per straw was introduced to the market in 2017.

Figure 5 Conception rates obtained with STgenetics sexed and other conventional semen in ST partnering herds. Only inseminations from 2012 through 2016 with confirmed outcomes from lactations 0 to 2 and service number 1 to 3 were included. Holstein data includes 122 876 STgenetics inseminations. Jersey data includes 222 262 STgenetics inseminations. *Conventional and sexed differ; ○Conventional and sexed do not differ. Adapted from Heuer et al. (Reference Heuer, Kendall, Sun, Deeb, Moreno and Vishwanath2017).
Sex-sorted semen: other species
Ovine
This was the first species where comparable if not superior fertility of sex-sorted semen to conventional semen was first demonstrated (de Graaf et al., Reference de Graaf, Evans, Maxwell and O’Brien2007b). Sex-sorted, frozen-thawed ram spermatozoa are superior in fertility to that of non-sorted, frozen-thawed controls when inseminated in superovulated ewes (de Graaf et al., Reference de Graaf, Evans, Maxwell and O’Brien2007b). In non-superovulated ewes a low dose of 1 million motile sex-sorted sperm showed equivalent fertility to conventional (de Graaf et al., Reference de Graaf, Evans, Maxwell and O’Brien2007b). Ram sperm when frozen before and following sex-sorting result in similar or higher fertilization and/or lambing percentages compared with non-sorted spermatozoa (de Graaf et al., Reference de Graaf, Beilby, O’Brien, Osborn, Downing, Maxwell and Evans2007a and Reference de Graaf, Evans, Maxwell and O’Brien2007b). It is likely that the excellent results in sheep with sex-sorted sperm could in part be due to laparoscopic inseminations (LAI) where the sperm are placed at the tips of the uterine horns. This may partially ameliorate the stresses associated with sex-sorting. With the global sheep market facing a resurgence, there is renewed interest in using sex-sorted semen as a breeding option both at the elite stud level as well as the commercial farm level.
Cervine
Information on fertility of sex-sorted deer sperm is limited but most reports point towards the resilience of deer sperm to withstand the sorting process and maintain good fertility. This is the case with both red deer and white-tailed deer. A study comparing DNA fragmentation kinetics and post-thaw motility of sex-sorted and conventional semen of white-tailed deer showed better semen characteristics for the sex-sorted sample (Kjelland et al., Reference Kjelland, Gonzalez-Marin, Gosálvez, Lopez, Lenz, Evans and Moreno2011). Fertility trials with red deer using 3×106 sex-sorted sperm on two separate ranches showed the AI pregnancy results were similar using conventional and sex-sorted sperm. Based on these results it would appear that red deer sperm withstand the sex-sorting process and there appears to be both interspecific and intraspecific tolerance by sperm to the sex-sorting process (Bringans et al., Reference Bringans, Kjelland, Lenz, Templeton, Evans, Belluzzo and Rosenstein2010). Lower fertility with Y-sorted sperm was noted in recent studies with Iberian red deer (Anel-Lopez et al., Reference Anel-Lopez, Garcia-Alvarez, Parrilla, Del Olmo, Maroto-Morales, Fernandez-Santos, Ortiz, Soler, Martinez, Vazquez and Garde2017). There are no current published reports on the application of the SexedULTRA™ method in deer semen processing. Personal communication from the sorting laboratory in Navasota, Texas confirms that there is a steady market for both frozen and fresh sex-sorted products for white-tailed deer (Jared Templeton, Sexing Technologies, personal communication, www.stgen.com). Fresh semen use as a sex-sorted product in deer has increased considerably in the recent past and amounts to many thousands of inseminations in the last 5 years. Other than reviews in the past, recent literature on the use of sex-sorted semen for elk, red deer, white-tailed deer and other Cervids is limited.
Caprine
Sex-sorting of goat sperm has also become a recent interest as the Dairy Goat industry continues to expand. Successful sex-sorting of goat semen has been achieved, and birth of kids have been reported after LAI with about 32 million sperm per insemination of either sex-sorted or conventional sperm. While fertility was lower for sex-sorted sperm, the success of the technique was demonstrated (Bathgate et al., Reference Bathgate, Mace, Heasman, Evans, Maxwell and de Graaf2013). Most caprine inseminations occur transcervically and the challenge is to be able to deliver a good fertile dose of sex-sorted semen that can be used for this purpose. Recent advances with the SexedULTRA™ procedure have been applied to goat semen processing and a commercial service offering sex-sorted semen for laparoscopic AI is available (Jared Templeton, personal communication).
Equine
Sex-sorted stallion sperm has been tested for field fertility and reported in literature since 2008. Two methods of insemination, hysteroscopic and deep uterine insemination (DUI) of sex-sorted sperm, are used and both have reported indifferent results (Lindsey et al., Reference Lindsey, Varner, Seidel, Bruemmer and Squires2005). A recent publication using a pre-sort storage period at ambient temperature, followed by sex-sorting and cryopreservation of the sex-sorted sperm showed no difference in fertility to conventional stallion sperm by hysteroscopic inseminations (Gibb et al., Reference Gibb, Grupen, Maxwell and Morris2017). Although fertilization rates with sex-sorted semen are comparable with conventional semen, the primary issues related to cryopreservation and general early embryonic death after hysteroscopic or DUI remain, and these challenges need to be overcome before sex-sorted stallion sperm become a routine option in the ART toolbox for equines.
Porcine
The application of sex-sorted sperm in the swine industry presents challenges that need to be overcome before this technology can be used commercially. Flow cytometric sperm sorting speeds are a limitation, especially for an industry that inseminates 2.5 to 3.0 billion sperm in 75 to 100 ml of extender (Knox, Reference Knox2016). Sorting speeds have improved to around 20 million cells/h, but one AI dose would require about 100 h of sorting time (Spinaci, Reference Spinaci2016). To overcome this obstacle, new insemination techniques have been used to (a) reduce the number of sperm and (b) deposit the spermatozoa closer to the site of fertilization. Laparoscopic insemination has been shown to be a plausible alternative to inseminate pigs from a sorting perspective and could be a realistic model for sexed sperm on nucleus or multiplier herds since one sex is generally preferred and fertility could be reduced if additional animals from the desired sex are produced. del Olmo et al. (Reference del Olmo, Parrilla, Sanchez-Osorio, Gomis, Angel, Tarantini, Gil, Cuello, Vazquez, Roca, Vaquez and Martinez-Emilio2014) reported a farrowing rate of 80.7% and a litter size of 10.5 with 6 million sorted sperm (1.0×106 in each oviduct and 2×106 in each uterine horn) in sows. This technique is still relatively new in terms of use with sexed sperm but has shown promising results. Deep uterine insemination is another option for inseminating reduced numbers of sexed sperm, particularly on a commercial farm. A DUI catheter, about 1.5-m long was used to bypass the cervical folds and manipulate through the length and coiled nature of the uterine horn before depositing cells in one of the uterine horns (Vazquez et al., Reference Vazquez, Roca, Gil, Cuello, Parrilla, Vazquez and Martínez2008). In hormonally synchronized sows, Vazquez et al. (Reference Vazquez, Roca, Gil, Cuello, Parrilla, Vazquez and Martínez2008) inseminated non-sorted sperm at 50×106, 200×106, 1×109 cells per dose and reported farrowing rates of 92.3%, 88.9%, 88.6%, respectively, compared with an 87.5% farrowing rate for a three billion cell, non-synchronized control. The litters sizes for each treatment were 9.41, 9.75, 9.61 and 10.02, respectively. Data from DUI trials with sexed sperm (50 to 140×106 sorted cells) have been inconsistent (Rath, Reference Rath2003; Vazquez, Reference Vazquez2003), but have verified that sex skewed litters can be produced non-invasively with very few cells. In a field trial performed by ST comparing a single DUI (600×106/dose, n=12) and LAI (10×106/dose, n=12) against a non-synchronized conventional AI control (>1.5×109/dose, n=14) there were no statistical differences in conception rate, farrowing rate and all litter measurements. However, further catheter development is needed to deposit sperm in both horns and not rely on sperm transport for bilateral fertilization.
Alternative methods to distinguish X and Y chromosome bearing sperm
Besides a size difference, the one other distinguishing feature between X and Y chromosomes are the DNA sequences that are unique to each type. Fluorescent in situ hybridization techniques can be used to specifically probe for X- or Y-sperm but requires the disintegration of the head of the sperm (Kawarasaki et al., Reference Kawarasaki, Welch, Long, Yoshida and Johnson1998). Using the same principle, functionalized gold nanoparticles were used to locate and non-invasively bind to Y-chromosome-specific sequences. The evidence is provided on the actual binding but no fertility data have been presented (Rath et al., Reference Rasmussen, Block, Seidel, Brink, McSweeney, Farin, Bonilla and Hansen2013). Numerous reports, patents, publications and trade brochures exist on alternative methods to sex-sort sperm but none have been shown to be repeatable and of commercial significance. Of note are some publications providing convincing evidence of manipulating sex ratios by nutritional (Herrmann et al., Reference Herrmann, Koschorz, Wertz, McLaughlin and Kispert1999), genetic (Roche et al., Reference Roche, Lee and Berry2006), physical (Sang et al., Reference Sang, Yang, Ham, Liang, Hua, Xiong, Huo and Yang2011) and immunological methods (Rosenfeld, Reference Rodriguez-Martinez2012). Perhaps the most recent one is a report on separation of buffalo semen by a swim-up method and validated by Real-Time-PCR (Ul-Husna et al., Reference Ul-Husna, Awan, Mehmood, Sultana, Shahzad, Ansari, Rakha, Naqui and Akhter2017).
Performance of sex-sorted sperm in IVF programmes
Historically, it was always considered that the most economical method to use sex-sorted sperm in breeding programmes would be through IVF methods where a relatively small number of spermatozoa are required. This was until low-dose inseminations with sex-sorted sperm became feasible (Seidel and Garner, Reference Seidel2002). Combined with ovum pick-up, quite a large number of in vitro fertilized embryos are currently generated for breeding companies through the use of both X- and Y-sorted sperm (www.stgen.com; www.transova.com). These companies also provide a service using previously frozen sperm which are then thawed and subjected to the sorting process (reverse sorting). Although, there are many studies that show similar rates of cleavage and blastocyst formation from embryos generated from sexed and non-sexed sperm (Xu et al., Reference Xu, Chaubal and Du2009; Carvalho et al., Reference Carvalho, Sartori, Machado, Mourao and Dode2010; Ruiz López et al., Reference Ruiz López, Camisão de Souza, Zaraza González, De Ondiz Sánchez, Jon Romero-Aguirregomezcorta, Rodrigues de Carvalho and Rath2013) others report some reduction in blastocyst yield (Bermejo-Alvarez et al., Reference Bermejo-Álvarez, Rizos, Rath, Lonergan and Gutiérrez-Adán2008). However the conclusion at this time is that the overall calving rate following transfer of in vitro produced embryos with non-sorted or sex-sorted sperm is similar (Rasmussen et al., Reference Rath, Barcikowski, de Graaf, Garrels, Grossfeld, Klein, Knabe, Knorr, Kues, Meyer, Michl, Moench-Tegeder, Rehbock, Taylor and Washausen2013; Ruiz López et al., Reference Ruiz López, Camisão de Souza, Zaraza González, De Ondiz Sánchez, Jon Romero-Aguirregomezcorta, Rodrigues de Carvalho and Rath2013).
Flow cytometry: industrialization of sperm sorting technology
Progress in the adaptation of technologies requires quite a few intersecting elements to come together such as price, efficiency, performance, ease of use and acceptance. In the case of sex-sorting, two major elements controlled the success of this technology. The first was the biology, which affected the fertility performance of the product in field applications. The second was the ease and availability of machines to sex-sort semen in various locations. The original MoFlo flow cytometers, Cytomation Inc, Fort Collins, CO, USA were expensive, bulky, had low throughput and required a great deal of technical expertise to operate (Sharpe and Evans, Reference Sharpe and Evans2009). The modern Genesis machines (Figure 6) developed by Cytonome ST (Boston, MA, USA) (www.cytonome.com) have much advanced electronics and automated features with multiple heads in one machine for parallel sorting. The new Genesis III sorters, Cytonome Inc, Boston, MA, USA use a solid-state laser, dual orthogonal detectors (at 0° and 90° to the laser), an orienting nozzle, and digital electronics to provide sorted sub-populations of X- or Y-sperm at rates of ~8000 sperm/s with ~90% purity when operating at an input event rate of 40 000 sperm/s (Sharpe and Evans, Reference Sharpe and Evans2009; Evans, Reference Evans2010; Vishwanath et al., Reference Vishwanath, Nebel, McMillan, Pitt and Macmillan2014). Single operator workstations are compact and easy to operate with integrated fluidics and all digital controls (Figure 6). The confluence of machine development and improvements in sperm biology (Genesis machines and SexedULTRA™) has accelerated the uptake of this technology in the last 5 years (Hutchison and Bickhart, Reference Hutchison and Bickhart2016).

Figure 6 The new Genesis III a modular high-speed sorting platform for parallel processing. Banks of five Genesis III machines are set up in a pod for fast throughput. The speed of sorting in a pod can reach up to 500 million cells/h.
Genomic selection and sexed semen: a powerful tool in genetic advancement in the farmed species
The recent advent of genomic selection especially in cattle and pigs has provided farmers with additional tools to fast-track genetic progress. Genomics along with sexed semen alter three components in the genetic progress equation.
![]() $\Delta G{\equals}{{I{\times}R{\times}\sigma} \over {GI}}$
, where ΔG is the progress in
genetic standard deviations per year, I the selection
intensity, R the accuracy of selection and
σ the genetic standard deviation in the population
under selection. Genomic prediction increases prediction accuracy of selection
candidates without progeny information from around 0.5 to 0.8 for most traits,
and, by being able to make accurate selection decisions very early in life, it
decreases the generation interval for sires of bulls from 6.5 to 1.75 years
(Schaeffer, Reference Schaeffer2006). According to
Schaeffer (Reference Schaeffer2006), genomic selection
alone doubles the genetic progress per year across all pathways of selection.
The biggest potential of sexed semen is on the commercial dairies directly.
Historically, there was very little or no selection on the female side in
commercial dairies. This is due to the fact that at replacement rates of
40%, accounting for calf losses and a sex ratio of 50/50,
dairy farmers need to keep every single born female simply to maintain herd
size. Therefore, genetic progress through the dam-to-dam
pathway has essentially been 0. Sexed semen offers a way of introducing
selection in that pathway for the first time by skewing the sex ratio. The
effect is exclusively in the selection intensity
(I) component. The fraction of selected females before the
sexed semen option has been (close to) 100%, resulting in very little
genetic progress. Table 4 shows an
example of how genomics and sexed semen can affect genetic progress on the
female side in commercial dairies. Everything else being equal, the use of sexed
semen can increase ΔG for example in the trait milk
yield, from 38.5 kg/year to 105.6 kg/year. Sexed semen in
conjunction with genomic selection can leverage that progress even further to
184.8 kg/year. Making use of the available technologies can,
therefore, enhance genetic progress in the dam-to-dam pathway
by a factor 3 to 5 (Heuer et al., Reference Heuer, Kendall, Sun, Deeb, Moreno and Vishwanath2017).
$\Delta G{\equals}{{I{\times}R{\times}\sigma} \over {GI}}$
, where ΔG is the progress in
genetic standard deviations per year, I the selection
intensity, R the accuracy of selection and
σ the genetic standard deviation in the population
under selection. Genomic prediction increases prediction accuracy of selection
candidates without progeny information from around 0.5 to 0.8 for most traits,
and, by being able to make accurate selection decisions very early in life, it
decreases the generation interval for sires of bulls from 6.5 to 1.75 years
(Schaeffer, Reference Schaeffer2006). According to
Schaeffer (Reference Schaeffer2006), genomic selection
alone doubles the genetic progress per year across all pathways of selection.
The biggest potential of sexed semen is on the commercial dairies directly.
Historically, there was very little or no selection on the female side in
commercial dairies. This is due to the fact that at replacement rates of
40%, accounting for calf losses and a sex ratio of 50/50,
dairy farmers need to keep every single born female simply to maintain herd
size. Therefore, genetic progress through the dam-to-dam
pathway has essentially been 0. Sexed semen offers a way of introducing
selection in that pathway for the first time by skewing the sex ratio. The
effect is exclusively in the selection intensity
(I) component. The fraction of selected females before the
sexed semen option has been (close to) 100%, resulting in very little
genetic progress. Table 4 shows an
example of how genomics and sexed semen can affect genetic progress on the
female side in commercial dairies. Everything else being equal, the use of sexed
semen can increase ΔG for example in the trait milk
yield, from 38.5 kg/year to 105.6 kg/year. Sexed semen in
conjunction with genomic selection can leverage that progress even further to
184.8 kg/year. Making use of the available technologies can,
therefore, enhance genetic progress in the dam-to-dam pathway
by a factor 3 to 5 (Heuer et al., Reference Heuer, Kendall, Sun, Deeb, Moreno and Vishwanath2017).
Table 4 Genetic progress in dam-to-dam pathway using sexed semen and genomic selection

σ is the genetic standard deviation in the population under selection, P is the proportion of animals selected as replacements, R is the accuracy of selection, I is the selection intensity, GI is the generation interval and ΔG is the progress in genetic standard deviations per year related to trait selected.
The future
It is perhaps fair to say that sexed semen has now ‘come of age’. The primary issues such as fertility and ease of availability have been mostly addressed. Incremental improvements in the sex-sorting methods and automation will further speed up the process. It is perfectly possible that one day, fertility of sex-sorted sperm could exceed the current fertility levels of conventional semen. The fact is that individual sperm cells are interrogated and the opportunity to remove the sub-fertile sperm cells or alternatively choose only the most competent cells is also a possibility. Sex-sorted semen is globally traded and is now mainstream with almost every major AI company in the world offering a sexed product. While options on machinery and technology to sex-sort sperm cells may change in the future, the primary method to stain sperm cells using the Hoechst dye and a fluorescence signal to discriminate them in their sex ratio will still remain for the foreseeable future.
Acknowledgements
The talented R&D team at Sexing Technologies and STgenetics, Dr Rick Lenz, Mike Evans, Thom Gilligan, Clara Gonzalez-Marin, Carlos Gongora, Dr Kilby Willenburg, Jared Templeton, Dr Leo Brito, Candis McLeary, Esther Maia, Sara Westberry, Dr Claas Heuer and the Production teams at Sexing Technologies, Navasota, Fond du Lac, Marysville, Ithaca, Calgary, Vienna, Europe, South America, New Zealand and Australia.












