Introduction
Silas Weir Mitchell (1864) Reference Mitchell, Morehouse and Keen1 originally described the features of causalgia and coined that term from his experience with nerve injuries in the American Civil War as the burning neuropathic pain (NeP) after traumatic nerve injury. When obvious traumatic nerve injury pain is complicated by a number of other symptoms and signs, it can be referred to as complex regional pain syndrome type II with nerve injury (causalgia) (CRPS II) (Supplementary Appendix A). Reference Harden, Bruehl and Perez2 We describe in the Results section abbreviated examples of previously published cases Reference Watson, Stinson, Dostrovsky, Hawkins, Rutka and Forrest3,Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 to illustrate these two forms of causalgia treated by neurectomy, as well as the surprising, unpublished, long term, and follow-up of one of these patients.
Except for trigeminal neuralgia, surgical procedures such as nerve resection and amputation for NeP such as causalgia aimed at peripheral nervous system denervation are regarded by many, if not most, of the community of thoughtful, experienced pain clinicians as not helpful, particularly in the long term. This is probably in part because of a perceived risk of aggravating a central component (anesthesia dolorosa), and thus, these operations have been largely abandoned.
In this article, we discuss mainly, but not exclusively, the pain literature regarding nerve resection surgery and amputation for causalgia. It is particularly critical to report results regarding the long-term efficacy and safety of any controversial, innovative, and especially initially successful procedures. We report, to our knowledge, the unpublished, unique pathological examination of an amputated limb from a previously reported patient surgically treated by nerve resection with CRPS type II (causalgia) Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 with pathological and serological evidence, suggesting an unrecognized immunological disturbance (immune deficiency and autoimmunity). These immune abnormalities influenced a subsequently successful medical treatment by immunotherapy with this refractory patient. These conditions may account for treatment failures in some patients with CRPS. We review in this article the evidence for immunological abnormalities and immunotherapy with causalgia but also CRPS generally and not exclusively CRPS II, since the specific CRPS II literature is sparse and there are clinical similarities between CRPS I and CRPS II.
Methods
A literature search from 1947 (Pubmed, Cochrane, MEDLINE, EMBASE, and CINAHL Plus) was carried out for articles, reviews, books, and book chapters regarding the surgical treatment of causalgia and CRPS II (causalgia) by nerve resection and by amputation. We reviewed the surgical literature, but the focus was mainly on the pain literature as it was thought more likely to be precise regarding the terms causalgia and CRPS II. Although randomized controlled trials (RCTs) are the gold standard, from our previous studies we recognized that these were probably less likely or unlikely and that most reports would be reviews or of cases and case series. Thus, although the scope of this search was narrow (restriction to the terms “causalgia,” “CRPS,” and “CRPS II” and to “nerve resection” and “amputation”), the search was also broad for a range of study types.
Because of a need for diagnostic precision, there was the exclusion of publications using terms other than causalgia, CRPS, and CRPS II (i.e., neuroma pain, reflex sympathetic dystrophy, and deafferentation pain) as well as other interventions (i.e., sympathectomy, dorsal root ganglion stimulation, dorsal column stimulation, and motor cortex stimulation).
Where the term “CRPS” is used in discussing an article we mean CRPS generally, as used in that particular article. We use CRPS I and CRPS II when the article specifically stated that this is the subject of the article.
An amputated limb (Figure 1) from a CRPS II causalgia patient Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 (case 2 HG in the Results section here) was examined in detail by a general pathologist (AF), dermatopathologists (KN, MS, and MAD), and a neurosurgeon (RM) the latter with a special interest and experience with peripheral nerve surgery. Because of the pathological and serological findings, we also searched these databases for immune abnormalities (immune deficiency and autoimmunity) and immunotherapy in patients with causalgia and CRPS, CRPS I and CRPS II.
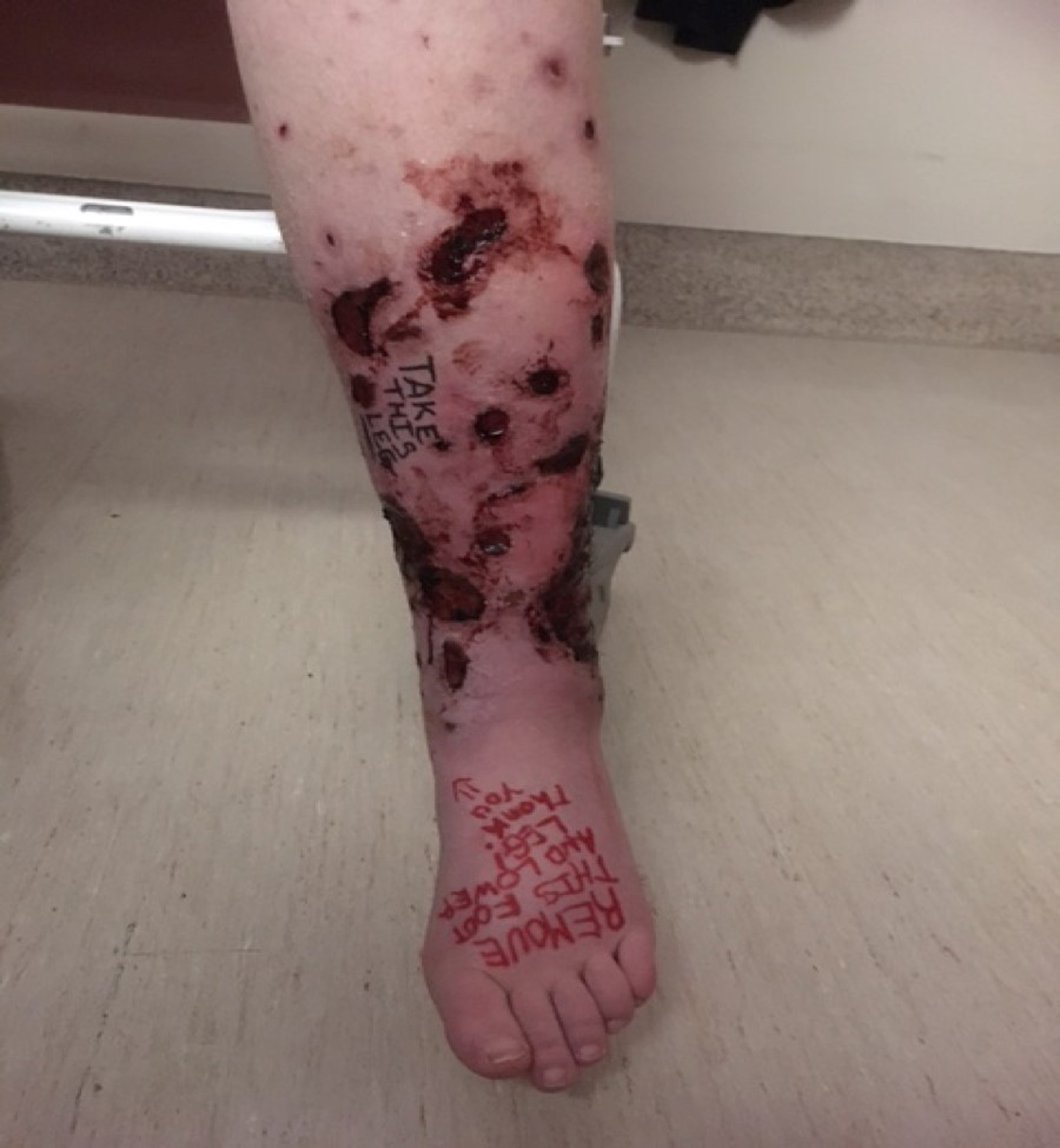
Figure 1: HG: the leg on the morning of amputation.
Results
No books or book chapters were found on the surgical treatment of causalgia by nerve resection or amputation. Six reviews of the treatment of CRPS were identified Reference Hord and Oaklander5–Reference Taylor, Noor and Urits10 only one of which mentioned amputation. Reference Duong, Bravo, Finlayson and Tran9 The comprehensive review of CRPS by Stephen Bruehl (2015) Reference Bruehl8 does not discuss nerve resection or amputation. Six cases and case series were located regarding nerve resection. Reference Watson, Stinson, Dostrovsky, Hawkins, Rutka and Forrest3,Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4,Reference Mitchell11–Reference Stovkis, van der Avoort, Van Neck, Hovius and Coert14 Twelve reports were located regarding the role of autoimmunity in CRPS, Reference Blaes, Schmitz and Tschernatsch15–Reference Alexander, Aradillas and Schwarzman26 and one of them about the association of autoantibodies with B cell immune deficiency. Reference Salzer, Santos-Valente and Klaver25 We discuss two of these articles Reference Blaes, Tschernatsch and Braeburn17,Reference Goebel, Bisla and Carganillo18 on the treatment of CRPS with intravenous immunoglobulin (IVIg) and one by plasma exchange. Reference Alexander, Aradillas and Schwarzman26 Eight cases or case series were found regarding amputation Reference Bodde, Dijkstra and den Dunnen27–Reference Ayyaswamy, Saeed, Anand, Chan and Shetty34 for causalgia and CRPS II. One of our published patients Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 had pathological evidence supporting autoimmunity, serological evidence of low immunoglobulins IgG and IgA, and positive tests for an autoimmune process with positive anti-SSA and anti-RO autoantibodies, despite other negative tests for autoimmune disorders (e.g., negative ANA (antinuclear antibody) and anti-DNA antibodies). In summary, there is in the literature some evidence for the successful treatment of causalgia by nerve resection and amputation; however, some failures of surgical treatment may be accounted for by treatable underlying immune abnormalities.
We report here the previously unpublished, very long-term follow-up of two previously published cases of causalgia treated successfully initially by nerve resection as follows.
Case 1, CHReference Watson, Stinson, Dostrovsky, Hawkins, Rutka and Forrest3 uncomplicated causalgia
A 14-year-old male presented with a 14-month history of constant right infraorbital, severe, burning pain, with electric shocks and pain on dynamic touch (allodynia) due to intractable causalgia after a fractured right orbit. He was housebound and refractory to all analgesics. The infraorbital nerve was sectioned proximal to the injury, grafted, and relocated into the buccal fat pad. He was able to return to school and a normal life and has been pain-free for 19 years.
Case 2, HGReference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 CRPS type II causalgia (with nerve injury)
A 19-year-old female was seen with a 13-year history of intractable CRPS type II after a left leg injury. The superficial peroneal and sural nerves were resected. Successful relief lasted for 4 years and 4 months. Unfortunately, incipient left leg gangrene (Figure 1) and the return of the severe left leg pain at 4 years and 4 months required amputation. The pathological examination of the amputated limb plus additional blood tests led to a successful immunotherapy at 3 years follow-up. The pathological findings in our patient Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 suggested autoimmunity and serology B cell immunodeficiency. Following amputation, her skin lesions became extensive and spread to hair, ears, and mouth, and she was unable to wear a prosthesis and was reclusive and bedridden with stump and phantom pain. She continued to have frequent upper respiratory infections and episodes of bronchitis and pneumonia for over 2 years and 4 months. Because of her immunological status (immune deficiency and autoimmunity), she was commenced on once weekly subcutaneous human immune globulin 20% solution. After 2 years, she stated that there was a “huge difference”; in that she could use her prosthesis full time, no longer required strong analgesics, no longer had frequent respiratory infections, and her skin lesions improved markedly (Figure 2). At 3 years (March 2023) follow-up, her skin lesions only remained as scars. At this time, HG stated “I feel so much better I will happily do this (have the immunotherapy) for the rest of my life.”

Figure 2: HG: 2 years after treatment with immune globulin HG was able to wear her prosthesis, and walk, had a reduction in the need for strong analgesics, no longer had frequent upper respiratory infections and pneumonia and had marked improvement in her generalized skin lesions (at 3 years after amputation she continued with marked improvement and only scarring at the sites of her previous generalized skin rash).
The unique, previously unreported pathological examination of the lower limb from the amputation on December 4, 2017 carried out on HG (case 2)
Howard et al.Reference Howard, Singleton and Soulakvslidze38 carried out a meta-analysis and validation of a histopathology scoring system for amputations for CRPS in 22 patients. They concluded that for various reasons (infrequent reporting of diagnostic criteria, lack of routine stains, and lack of clinical pathological correlation) that “it is quite difficult to write a meaningful systematic review of CRPS histology at this time.” The strengths of our amputated limb pathology (HG, case 2) are with the clinical features, diagnostic precision, surgical details, laboratory and pathological findings, and very long-term follow-up. The amputated leg (preoperatively in Figure 1) of our patient (case 2 HG) with CRPS type II was examined by three different specialists (general pathology, dermatopathology, and neurosurgery) in order to determine any possible cause pathologically for the deterioration in the patient’s condition.
On the general pathological examination (by AF) in addition to the extensive ulceration of the skin, there was evidence of underlying vascular abnormalities, with the presence of venous thrombosis and chronic changes in the cutaneous and subcutaneous small vessels. Associated chronic changes were seen with dermal and pannicular fibrosis and calcification.
The case was reviewed in consultation with dermatopathology (KN, MS, and MD), and the pathology addendum report findings were that the vascular changes were thought to be possibly due to a vasculopathic process. Potential etiologies suggested included anti-phospholipid antibody syndrome, lupus, and others, but diagnostic features were not seen. The findings reported could also be associated with Behcet’s disease. None of the above specific pathologies was found. Repeated blood work after amputation found a normal or negative erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ANA, anti-DNA antibodies, lupus inhibitors, antiphospholipid, and anti-cardiolipid antibodies, but anti-SSA/Ro autoantibodies were positive. HG was found to have B cell deficiency on repeated testing (IgG 2.25 and 3.34, range 6.8–18.0; IgA 0.06 and 0.07, range 0.6–4.20, with normal IgM x2).
Additionally, the frozen limb was dissected shortly after the amputation on December 4, 2017 by a neurosurgeon (RM) with a special interest in peripheral nerve surgery and nerve pathology. No obvious neuromas were found at the sites of the implantation of the nerves. All the nerve specimens of the leg appeared normal on microscopy. The lack of neuroma formation is supportive evidence that the original nerve resection operation was successful and had prevented neuroma formation. This evidence supports the prolonged relief (4 years and 4 months) resulting after nerve resection.
Discussion
Neurectomy
With regard to nerve resection surgery for causalgia, Silas Weir Mitchell (1872) documented causalgia in a civil war soldier (case 47, page 292), Reference Mitchell11 and the effects of resecting the median nerve with an initial aggravation of the pain, and then moderation in the distribution of the resected nerve. However, the pain persisted severely in another (ulnar) nerve’s territory.
Noordenbos and Wall (1981) Reference Noordenbos and Wall12 reported seven patients with severe causalgia with long-term follow-up unrelieved by resection. This article by two highly respected authors in the field of pain may have contributed to a negative view of this form of treatment as there is a general view in the community of pain physicians and surgeons that further denervation (other than in trigeminal neuralgia) does not generally help NeP such as causalgia and runs the risk of aggravating a central component (anesthesia dolorosa).
Only a few other reports of nerve resection surgery for causalgia and CRPS II have appeared in the pain literature in the past three decades. Inada et al (2005) Reference Inada, Morimoto, Moroi, Endo and Nakamura13 reported two cases of the successful surgical relief of upper extremity causalgia (digital nerve injury) by nerve resection. Follow-up was 2 years in one patient and 30 months in the other. Watson et al (2007) Reference Watson, Stinson, Dostrovsky, Hawkins, Rutka and Forrest3 (see abbreviated case 1 CH in the Results section) reported a 19-year-old male with severe, intractable right infraorbital nerve causalgia after a fractured right orbit. Thirteen months after injury, the pain remained severe and intractable and the nerve was resected proximal to the fracture, grafted and placed in the buccal fat pad with immediate relief. Long-term follow-up after publication found that he has been pain-free for 19 years. Stovkis et al (2010) Reference Stovkis, van der Avoort, Van Neck, Hovius and Coert14 reported 34 patients operated on for “neuroma pain” (but which included CRPS II) by nerve resection and relocation. Postoperative satisfaction rates were 36% (4/11) with CRPS II patients and 65% 15/23 in non-CRPS patients. Watson et al (2014) Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 (case 2 HG in the Results section) reported a 19-year-old female, with successful relief by nerve resection for 4 years and 4 months after 13 years of intractable (CRPS) type II (causalgia) affecting the peroneal and sural nerves of the left leg. Severe leg pain recurred and incipient gangrene (Figure 1) required amputation (unpublished long-term follow-up in the Results section). The pathological examination of the amputated limb (see Results section) suggested autoimmunity and serological testing revealed B cell deficiency as well as autoimmunity. She responded to immunotherapy, was markedly better at 2 years follow-up (Figure 2) and is pain-free at 3 years post-amputation.
Nerve grafting, entubalation, and relocation of the proximal nerve stump after neuroma resection often lead to a favorable outcome, as reported in the literature and anecdotally based on the experience of one of us (RM). In recent years, the surgical management of painful neuromas, where nerve grafts are not possible, has evolved to provide a more suitable environment for axons to reside or grow and not reform a painful neuroma. After neuroma resection, instead of burying the proximal stump of an injured nerve into an innervated muscle, the nerve is placed and sutured within a freshly harvested small piece of (denervated) muscle, a technique termed regenerative peripheral nerve interface (RPNI) (Santosa et al 2021). Reference Santosa, Oliver, Cederna and Kung35 Alternatively, the proximal stump of the injured nerve after neuroma resection is micro-surgically repaired to an adjacent small muscle nerve, a procedure termed targeted motor reinnervation (TMR) (Janes et al 2021). Reference Janes, Fracol, Dumanian and Ko36 Both of these procedures seemingly have improved pain outcomes in patients with new or recurrent neuromas, as compared to historical case series, although studies comparing the two methods are still lacking. The efficacy of TMR for managing CRPS II is being explored, and a small case series (Shin et al., 2022) shows promising results. Reference Shin, Haffner, Chang and Kleiber37
Autoimmunity and immunotherapy
Twelve references were found regarding autoimmunity associated with CRPS. Reference Blaes, Schmitz and Tschernatsch15–Reference Alexander, Aradillas and Schwarzman26 Eight of these references are to research, suggesting autoimmunity in CRPS against nervous system targets in humans and in animal research. One reference Reference Salzer, Santos-Valente and Klaver25 found autoimmunity associated with immunodeficiency (B cell deficiency) caused by protein kinase C delta, but it is unknown how often this combination of immunological findings occurs in patients with CRPS II or other NeP as with HG (our case 2 in Results). Two reports stated that some patients responded to treatment with immunoglobulin. Reference Blaes, Tschernatsch and Braeburn17,Reference Goebel, Bisla and Carganillo18 Blaes et al (2004) Reference Blaes, Tschernatsch and Braeburn17 commented on “some” uncontrolled cases of theirs and of others with improvement by IVIg. Goebel et al (2017) Reference Goebel, Bisla and Carganillo18 reported that a parallel design, RCT of 108 eligible patients with moderate to severe CRPS of 1–5 years duration in which two doses of low dose (0.5 g/kgm of body weight) of IVIg separated by 6 weeks was not effective in relieving moderate/severe CRPS of 1–5 years duration. They referred to their previous open cases and a small RCT of low-dose IVIg which, in both studies, showed that 25% had “profound pain relief of greater than 50%.” They discussed 5 other reports of CRPS one with high dose (2 g/kgm) IVIg for a total of 25 patients which indicated efficacy. They stated that “in addition, other authors have indicated that they have been using IVIg successfully in their patients.” Goebel et al., (2017) further said that “it is not known why the results from the current RCT differ so markedly from those of earlier studies.” Why are these results inconsistent? Perhaps only one type of CRPS may respond (only CRPS I or CRPS II). It may be that a full dose (2 g/kgm) could be required and/or a shorter dosing interval.
In our patient with CRPS II (case 2 HG in Results), successful treatment was with 3 years of a weekly infusion of subcutaneous human immune globulin 20% solution. One retrospective study found that of 34 patients with CRPS who received plasma exchange therapy, 91% reported a significant reduction in pain, with 45% having sustained pain relief with additional weekly treatments. Reference Alexander, Aradillas and Schwarzman26 Sahbaie et al. (2022) Reference Sahbaie, Li, Guo, Kingery and Clark39 studied a mouse model of CRPS, which may have some relevance to the human condition; in that they found that modulation of autonomic activity after limb injury may limit pain-supporting autoantibodies and may reduce chronic pain which may be relevant to our human patient (case 2 HG). Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 The role of immunodeficiency and/or autoantibodies as cause or effect is unclear. Although it is possible that the amputation itself resulted in improvement, the marked, but persistent improvement in pain and function in our patient Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 did not occur until soon after the immunotherapy was started. The most dramatic features were that HG became able to wear her prosthesis, walk using it, had no need for strong analgesics, experienced a lack of respiratory infections, and as well had marked improvement of her generalized skin lesions (Figure 2) at 2 years and only skin scars at 3 years follow-up (see Results section).
Amputation
Of six reviews Reference Hord and Oaklander5–Reference Taylor, Noor and Urits10 of the treatment of CRPS, only one specifically referenced amputation for CRPS II. Reference Duong, Bravo, Finlayson and Tran9 Two reviews of amputation for causalgia were found. Reference Bodde, Dijkstra and den Dunnen27,Reference Ayyaswamy, Saeed, Anand, Chan and Shetty34 Bodde et al., 2011 Reference Bodde, Dijkstra and den Dunnen27 reviewed 26 studies of amputation for CRPS with Level IV evidence concluding that whether to amputate or not for intractable CRPS was “an unanswered question.” Ayyaswamy et al (2019) Reference Ayyaswamy, Saeed, Anand, Chan and Shetty34 reported a systematic review of 11 studies of quality of life (QOL) after amputation for intractable CRPS with a total of 96 patients and 107 amputations finding that 66/107(62%) had improvement in QOL regarding functional status and general health. They concluded that amputation could be considered to improve QOL but considered that the evidence was limited with risk of CRPS recurrence and of phantom limb pain. Other papers regarding amputation included cases and case series, but none of these specified the type of CRPS. However, we decided to review these articles regardless because of the similarities between CRPS I and II and because of the lack of such data in the latter. Midbari and Eisenberg (2017) Reference Midbari and Eisenberg32 commented that none of the published papers on amputation for CRPS had been published in the pain literature, and that the issue was the reluctance of the pain medicine community to consider amputation for intractable patients. The recent literature on amputation for CRPS consisted of single-case reports Reference Kashy, Abd-Elsayed and Farag30,Reference Goebel, Lewis, Phillip and Sharma33 and case series. Reference Bodde, Chrier, Krans, Geertzen and Dijkstra29,Reference Midbari and Eisenberg32 One case report by Goebel (2018) Reference Goebel, Lewis, Phillip and Sharma33 described recurrence of pain 2 years after amputation. Kashy et al Reference Kashy, Abd-Elsayed and Farag30 reported a successful outcome in a patient with CRPS I but provided no long-term data. In one case series, Krans-Schreuder (2012) Reference Krans-Schreuder, Bodde, Chrier and Dijkstra28 reported that 18/21 patients said they would recommend amputation to other patients with CRPS I. Midbari et al 2016 Reference Midbari, Suzan and Adler31 reported consistently better results with CRPS in an amputated group of 19 patients versus 19 control unamputated patients and stated that 13/19 of the amputated group said they would recommend the operation to other intractable CRPS patients.
Strengths of this review are that we gather in one location some otherwise disparate information for clinicians regarding nerve resection and amputation for medically intractable CRPS II (causalgia). These data provide some guidance regarding these possible surgical options in some of these refractory patients. We also describe the first detailed pathology of an amputated limb from a patient with CRPS type II which led to a successful treatment related to immune abnormalities. Limitations of this review are that some reports of the surgical procedures for peripheral nerve injury pain may have been deliberately excluded as no concise terms, methods, or outcomes were discussed.
In conclusion, surgery involving nerve resection and relocation by grafting, if necessary, may be more successful in uncomplicated causalgia Reference Watson, Stinson, Dostrovsky, Hawkins, Rutka and Forrest3 (case 1 CH in Results) involving a traumatic injury to a single sensory nerve accessible proximal to the injury. A surgeon skilled in nerve reconstruction and familiar with the techniques described in a previous article Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 is essential in the authors’ view. Although there is a small literature on the effect of amputation for CRPS generally, there are no good data to guide a decision regarding this. Surgery alone may be less beneficial for CRPS type II, such as with HG our case Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 with an unrecognized, underlying but treatable condition (immune deficiency and autoimmunity) which may yield only temporarily positive results after nerve resection surgery. Caution is necessary in extrapolating from a single case treated successfully by immunotherapy such as our patient HG case 2 (see Results section) Reference Watson, Mackinnon, Dostrovsky, Bennett, Faran and Carlson4 with intractable CRPS II (causalgia).
Summary
-
1. Causalgia and complex regional pain syndromes types I and II (causalgia) are poorly understood and difficult to treat, but both may be neuropathic and traumatic in origin.
-
2. Because of the clinical similarities between CRPS types I and II, it is plausible that a successful treatment for CRPS type II may be applicable to both conditions.
-
3. Nerve transection and amputation have been reserved in the past for medically intractable cases of CRPS II and may result in benefit in some patients. Nerve resection may be more successful with nerve grafting, relocation, and the novel surgical approaches described and referenced in this article.
-
4. There is a small inconsistent literature on autoimmunity and immunotherapy with CRPS generally (CRPS I and II).
-
5. Surgical and other treatment failures may be, in some instances, a result of an unrecognized but treatable, underlying immunological condition (autoimmunity and immune deficiency).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2023.260.
Acknowledgments
Dr A Franko (AF) carried out the general pathological examination. Dermatopathology examination was carried out by Dr K Naert (KN), Dr M Schneider (MS), and Dr M Abdi Daoud (MD). The assistance of Dr Judy Watt Watson and Emily Watson has been invaluable in the preparation of this manuscript.
Statement of authorship
CPNW collected the data and wrote the manuscript. RM examined the amputated limb regarding the nerves from the previous nerve resection/relocation surgery. RM and DWN revised the manuscript for intellectual content, and all authors approved the final manuscript.
Competing interests
The authors have no conflicts and nothing to disclose. Specifically, there are no other non-author contributions, no technical help, no financial or material support, or financial arrangements needing to be disclosed.




