The metabolic syndrome (MetS) is a cluster of interconnected physiological, biochemical, clinical and metabolic factors that directly increase the risk of atherosclerotic cardiovascular disease (CVD), type 2 diabetes mellitus and all-cause mortality( Reference Kaur 1 ). The prevalence of the MetS is increasing worldwide along with obesity, sedentary behaviours, population ageing and unhealthy dietary habits, which include a high intake of simple carbohydrates and foods with high saturated fat along with a low intake of fibre( Reference Pitsavos, Panagiotakos and Weinem 2 ).
Nutrition research has traditionally focused on examining specific foods, nutrients and dietary components, without taking into account the context of food consumption. Since most foods are consumed as part of a meal, the social and environmental circumstances at mealtime have become key issues to better understand food intake( Reference Meiselman 3 ) and its influence on body weight and health status( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 , Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). Some studies have shown that what people do while eating and the environmental context at mealtime can influence what people eat and their body weight( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 – Reference Pierlot 6 ). For example, eating while distracted by watching television (TV), reading, working, listening to a detective story or music, or playing video games has been linked to increased food consumption, a higher intake of fat, fast foods and soft drinks, and a lower intake of fruits and vegetables, which can lead to a greater likelihood of becoming overweight( Reference Bellisle, Dalix and Slama 7 – Reference Higgs and Woodward 12 ). Social interactions such as eating with friends and colleagues can also be important distractions that put individuals at risk of mindless eating, increasing energy intake and weight gain( Reference Wansink 13 – Reference Raulio, Roos and Mukala 15 ). Eating with family, however, has been shown to have potential benefits because families frequently have healthier eating habits( Reference Orrell-Valente, Hill and Brechwald 16 , Reference Anderson 17 ).
Eating out and not having enough time to eat are mealtime habits that affect food intake and body weight. For instance, rushing meals has been associated with a higher consumption of soft drinks, fast food and fat as well as with a lower intake of healthy foods( Reference Larson, Nelson and Neumark-Sztainer 18 ). Skipping breakfast has been shown to increase obesity, fasting blood insulin, and cholesterol levels( Reference Smith, Gall and McNaughton 19 , Reference Timlin and Pereira 20 ), whereas eating out has been linked with an unhealthy diet, high fat intake( Reference Lachat, Nago and Verstraeten 21 ), low fruit and vegetable consumption( Reference Crawford, Ball and Mishra 22 ) and high risk of obesity( Reference Kruger, Blanck and Gillespie 23 ). One’s eating environment, which includes the general atmosphere, social context and the presence of distractors, is also related to diet and body weight, because it can inhibit the monitoring of food intake( Reference Wansink 13 ), regardless of the hunger or energy needs of the individual( Reference Bellisle, Dalix and Slama 7 , Reference Bellisle, Dalix and Airinei 24 , Reference De Castro 25 ).
Recent studies have demonstrated that some mealtime behaviours can predict risk of MetS and its components. A longitudinal study of Japanese adults found that eating speed was significantly correlated with the MetS, abdominal obesity, and low levels of HDL-cholesterol( Reference Zhu, Haruyama and Muto 26 ), whereas a cross-sectional study reported that eating quickly was associated with the MetS and abdominal obesity in Japanese people( Reference Nagahama, Kurotani and Pham 27 ). A population-based, cross-sectional study of Australian adults showed that eating distractions such as extensive TV watching and consuming a large amount of snack foods were associated with the MetS( Reference Thorp, McNaughton and Owen 10 ).
Mealtime habits are of interest because they may help elucidate how familiar, social and environmental factors can influence energy intake, and consequently the development of metabolic diseases( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). For example, a systematic review of intervention trials that examined the association between overall European dietary patterns and their relationship with the MetS showed that westernised dietary patterns were linked to a higher risk of the MetS while traditional patterns were inversely related( Reference Martínez-González and Martín-Calvo 28 ). The Mealtime Habits Quality (MHQ) scale is based on four mealtime situations (availability of time to eat, distractions while eating, environmental and social contexts of eating, and familiar or cultural eating habits). Studies conducted with the Health Worker Cohort Study (HWCS) participants in Mexico using the MHQ scale have demonstrated that certain mealtime habits can influence food intake( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 ), dietary patterns, obesity, and risk of weight gain( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). However, few studies have prospectively evaluated the influence of specific mealtime habits on key metabolic health indicators( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 , Reference Thorp, McNaughton and Owen 10 ). The aim of this study was to investigate the relationship between the MHQ scale and risk of developing the MetS or insulin resistance (IR) among the HWCS participants at the baseline (2004–2006) and follow-up (2010–2012) evaluations.
Methods
Study population
The HWCS is a prospective cohort study composed of 1640 active and retired health workers aged 20–70 years, from the Instituto Mexicano del Seguro Social (IMSS) and the Instituto Nacional de Salud Pública, both located in Cuernavaca, Morelos. Participants were initially enrolled in a baseline assessment that occurred during 2004–2006 and were followed-up from 2010 to 2012( Reference Gallegos-Carrillo, Flores and Denova-Gutiérrez 29 ). We excluded individuals who did not complete all the mealtime habits questions in both data collection periods (n 588), participants without clinical or anthropometric assessments (n 30) and those with diseases or health conditions that could be related to weight change or mealtime habits in either data collection period, such as CVD (n 13), cancer (n 31), kidney failure (n 13) and pregnancy (n 9). The remaining 956 participants (239 men and 717 women) were included in the analysis. This study was conducted in accordance with the Helsinki declaration on human studies( 30 ), and each participant signed an informed consent form before enrollment. The IMSS ethics committee approved the study protocol and informed consent form.
Assessment of Mealtime Habits Quality
The MHQ scale was used to assess the quality of mealtime habits among the HWCS participants by obtaining information about structured meals at any given time of the day, on any day of the week, without accounting for snacking. Mealtime items were categorised into two groups, recommended and undesirable for good health, on the basis of previously published studies( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 , Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). At the baseline assessment, participants were asked ‘When you eat, what do you generally do?’, and they responded about their mealtime habits with the specific binary choices: ‘yes’ or ‘no’. For the follow-up evaluation, the MHQ scale was changed to a multiple-choice response format, in order to capture a wider range of mealtime habits( Reference Spector 31 ). The two formats of the MHQ scale were constructed using the same domain content, and the consistency of the results of the two versions was measured through parallel-form reliability, which is reported elsewhere( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ).
For this study, mealtime behaviours were classified into four specific situations: (a) availability of time to eat, (b) distractions while eating, (c) environmental and social context of eating, and (d) familiar or cultural eating habits. Availability of time to eat was measured with four items at the baseline assessment (‘I take my time to finish my meal’, ‘I rush my meals to avoid exceeding the available time to eat’, ‘I eat in huge mouthfuls’ and ‘I eat slowly’). For the follow-up assessment, these items were replaced with two questions: ‘How fast do you eat your meals?’ and ‘Did you skip any meal?’, since these questions have previously been associated with a lack of time to eat( Reference Goon and Islam 32 ). Distractions while eating were determined using two items at the baseline assessment (‘I’m distracted: I talk, watch TV or read’ and ‘I take advantage of mealtimes to accomplish work activities’). These two items were combined into one question for the follow-up assessment: ‘Are you distracted when you eat, either by watching TV, working, reading, talking, or solving everyday problems?’. The environmental and social context of eating was measured with two items at the follow-up assessment: ‘Do you eat with friends, family, or colleges?’ and ‘How many times per week do you eat your main meal at home?’. Familiar or cultural eating habits were assessed at baseline with the item ‘I eat all my food, without leaving anything on the plate’, which at follow-up was changed to ‘Do you eat all your food without leaving anything on the plate?’. Moreover, food selection is a mealtime situation that has been closely related to energy intake and BMI. In this study, it was measured at baseline with the item ‘I choose what I eat’; however, in order to be more specific about the type and amount of food selected by participants, this item became two questions for the follow-up assessment: ‘Do you choose the food you eat with your health in mind?’ and ‘Do you choose the amount of food that you eat?’. Finally, the question ‘Do you enjoy eating?’ was added at the follow-up assessment, because greater pleasure with food has been associated with fewer food anxieties, fewer dieting behaviours, and a lower BMI( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ).
Exploratory factor analysis was used for both assessments to uncover underlying factors and factor loadings. During both evaluations (2004–2006 and 2010–2012), factor solution was composed of one factor, recommended mealtime habits were correlated positively and undesirable habits had a negative correlation. The scale was constructed by summing the contribution of each item, weighted by its factor loading. As the indicators had negative values, five was added to the total summarised score in order to obtain a positive score. Each participant received an individual score representing the quality of their mealtime habits, with higher scores reflecting a better quality of mealtime habits. At baseline, the MHQ scale had a mean of 5·28 points, ranging from 2·24 to 8·08 (sd 1·17), and the internal consistency was 0·84( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 ). During the follow-up assessment, the MHQ scale had a mean of 3·84 points with a range from 0·87 to 7·19 (sd 0·96), and the internal consistency was 0·60 (online supplementary Appendix: MHQ questionnaire form)( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). A panel of data for longitudinal analysis that included the two MHQ scores was created in order to derive a personal-level data set. Next, participants were classified into tertiles (with the highest tertile reflecting the recommended MHQ), and were designated a MHQ status for baseline and another one for follow-up, so we could relate MHQ status with its corresponding MetS and IR status at baseline or follow-up.
Metabolic syndrome assessment
The MetS was determined using criteria from the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) and the International Diabetes Federation (IDF). The NCEP-ATP III defines the MetS as having at least three of the following five components: (1) abdominal obesity (waist circumference ≥102 cm for men and ≥88 cm for women), (2) elevated TAG (≥150 mg/dl or 1·7 mmol/l) or specific treatment for this lipid abnormality, (3) reduced HDL-cholesterol (<40 mg/dl or 1·03 mmol/l for men and <50 mg/dl or 1·29 mmol/l for women) or taking medication for this condition, (4) elevated blood pressure (systolic ≥130 mmHg or diastolic ≥85 mmHg) or taking medication for hypertension, or (5) elevated fasting plasma glucose (≥110 mg/dl or 6.1 mmol/l) or previously diagnosed type 2 diabetes( Reference Grundy, Cleeman and Daniels 33 ).
According to the IDF definition of the MetS, a person must have abdominal obesity (≥90 cm for men and ≥80 cm for women, these cut-offs may vary based on ethnicity-specific values) or a BMI >30 kg/m2 and any two of the following four factors: (1) elevated TAG (≥150 mg/dl or 1·7 mmol/l) or specific treatment for this lipid abnormality, (2) reduced HDL-cholesterol (<40 mg/dl or 1·03 mmol/l for men and <50 mg/dl or 1·29 mmol/l for women) or specific treatment for this lipid abnormality, (3) elevated blood pressure (systolic ≥130 mmHg or diastolic ≥85 mmHg, or treatment for hypertension, or (4) elevated fasting plasma glucose (≥100 mg/dl or 5·6 mmol/l) or previously diagnosed type 2 diabetes( Reference Zimmet, Alberti and Serrano-Ríos 34 ).
Anthropometric and clinical evaluation
Waist circumference was obtained using a steel measuring tape at the high point of the iliac crest at the end of normal expiration, to the nearest 0·1 cm. Weight was assessed with participants wearing minimal clothing with a previously calibrated electronic total body composition analyser, TANITA scale, model TBF-300A. Height was measured with a conventional stadiometer. BMI was calculated as a ratio of weight (kg):height squared (m2). One blood pressure measurement was taken with an electronic digital blood pressure monitor. Participants were seated with their right arm resting at heart level. The anthropometric and blood pressure measures were obtained by nurses trained in these standardised procedures, and reproducibility of the values was evaluated (resulting in a concordance coefficient of 0·83–0·90).
Biochemical assessment
Fasting venous blood samples were collected and glucose levels were assessed by the oxidised glucose method. TAG were determined by the colorimetric method after enzymatic hydrolysis with lipases technique, HDL-cholesterol was measured by the elimination of chylomicron and subsequent catalase, and insulin sensitivity was measured using the homoeostasis model assessment and calculated from the fasting insulin and glucose measurements using the standard formula: (glucose (mmol/l)×insulin (μU/ml))/22·5( Reference Matthews, Hosker and Rudenski 35 ). All biomedical assays were performed at the IMSS laboratory in Cuernavaca, Mexico, in compliance with the procedures of the International Federation of Clinical Chemistry and Laboratory Medicine( Reference Tate, Berg and Courderc 36 ).
Demographic characteristics, smoking, energy content, and dietary patterns assessment
Demographic data were obtained through self-administered questionnaires. Smoking status was assessed using the categories proposed by the World Health Organization( 37 ): current, past and never.
Dietary patterns (DP) and energy intake were obtained using a semi-quantitative FFQ validated in a Mexican population( Reference Hernández-Avila, Romieu and Parra 38 ). This questionnaire included data on the consumption of 116 food items commonly consumed over the past year, ranging from never to ≥6 times/d. For this study, we used three major DP that were previously identified in a cross-sectional study of 9467 Mexican adults who participated in the HWCS( Reference Denova-Gutiérrez, Castañón and Talavera 39 ). Briefly, in both assessments, food items were classified into twenty-eight food groups on the basis of similarity in nutrients, lipid content profile, sugar content, proportion of dietary fibre and commonly consumed foods. Three DP were derived: the prudent or balanced diet is represented by a greater intake of processed vegetable juices, potatoes, fresh fruits, fresh vegetables and legumes and a lower intake of pastries; the Western diet is characterised by a higher intake of pastries, refined cereals, corn tortillas and soft drinks and a lower intake of wholegrain cereals, sea food and full-fat dairy products; and the high-animal protein/high-fat diet is typified by a greater intake of red meat, processed meat, margarine and eggs and a lower intake of fruits and wholegrain cereals. The factor score for each dietary pattern was constructed by summing the standardised percentage of energy intake of food groups, weighted by their factor loading. Next, a panel of data for longitudinal analysis was created, obtaining a personal-level data set of the three DP for both assessments. Individuals were classified into tertiles, with the highest tertile reflecting greater adherence to each DP. We then determined whether there were any differences in the participant’s adherence to each DP between the baseline and follow-up assessments, and the associated risk of developing the MetS or IR over time.
Depression evaluation
Depressive symptoms were assessed using a Spanish-language depression scale, derived from a twenty-item questionnaire created by the Center for Epidemiologic Studies( Reference Radloff 40 ). We defined probable clinical depression using the mean plus one standard deviation as a cut-off, which has previously been used to identify depressive symptoms in a Mexican population( Reference Gallegos-Carrillo, Flores and Denova-Gutiérrez 29 ). A continuous scale from 0 to 60 points was generated, and a score of 16 or more was used to identify participants who had depressive symptoms at the baseline or follow-up assessments( Reference Radloff 40 ).
Physical activity
Recreational physical activity (PA) was assessed using a PA questionnaire( Reference Wolf, Hunter and Colditz 41 ) that has been validated in Spanish( Reference Martínez-González, López-Fontana and Varo 42 ) and adapted for the HWCS population in Mexico( Reference Méndez-Hernández, Flores and Siani 43 ). We determined PA levels on the basis of the public health recommendations regarding the type and amount of PA needed to improve and maintain health benefits among adults: <30 and ≥30 min/d( Reference Fernández-García and Hernández-Tezoquipa 44 ).
Statistical analysis
The differences observed between the 2004–2006 and 2010–2012 assessments, with regard to demographics, lifestyle and anthropometric measures, prevalence of the MetS and its components as well as the prevalence of IR, were obtained using the McNemar test for assessing differences in two paired proportions and the t test for assessing differences in two paired means (Table 1).
Table 1 Demographic, anthropometric, and lifestyle characteristics of the HWCS participants at the baseline and follow-up evaluations (n 956: female 717, male 239) (Mean values and standard deviations)

NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; MetS, metabolic syndrome; HOMA, homoeostasis model assessment; MHQ, Mealtime Habits Quality.
* P value was calculated using the McNemar test for difference in two paired proportions, and the t test (for paired data) to assess difference of two means, both comparing baseline and follow-up assessments. Proportions and means were adjusted by age and sex. A P value ≤0·05 was considered as significant.
† BMI: ratio of weight (kg):height squared (m2); normal weight 18·5–24·9 kg/m2, overweight 25–29·9 kg/m2, obesity ≥30 kg/m2.
‡ Dietary pattern adherence: balanced dietary pattern was typified by greater intake of processed vegetable juices, potatoes, fresh fruits, fresh vegetables and legumes and a lower intake of pastries; the Western dietary pattern was characterised by a higher intake of pastries, refined cereals, corn tortillas and soft drinks and a lower intake of wholegrain cereals, sea food and full-fat dairy products; and the high- protein/high-fat dietary pattern was typified by a greater intake of red meat, processed meat, margarine and eggs and a lower intake of fruits and wholegrain cereals. Dietary patterns were categorised in tertiles; tertile 3 represents greater adherence( Reference Denova-Gutiérrez, Castañón and Talavera 39 ).
§ The MHQ score was created by summing factor loadings of each mealtime habits item, assigning each participant an individual MHQ score at baseline and another at the follow-up assessment. Categories of the MHQ: lower MHQ category (from 0·87 to 3·86 points score), middle MHQ category (from 3·87 to 5·19 score) and higher MHQ category (5·20 to 8·08 score).
The MHQ scale was constructed by summing the factor loadings of each mealtime habits item, and participants were assigned an individual MHQ score at baseline and another for the follow-up assessment. A panel of data for longitudinal analysis was then created, which included the two MHQ scores for obtaining a personal-level data set( Reference Baltagi 45 ). Participants were classified into tertiles (with the highest tertile reflecting the recommended MHQ), obtaining one MHQ status for baseline and another for follow-up, so we could relate MHQ status with its corresponding MetS and IR status at baseline or follow-up. Adherence to each DP across the MHQ categories was assessed using the Cochran’s Q test to determine whether there was a difference between the means of the three groups (Table 2). The Cochran’s Q test was also used to identify any differences in the percentage of new cases of the MetS, components of the MetS, and IR across the three MHQ categories. For this analysis, we only included participants who did not have the MetS or its components or IR at the baseline evaluation, as defined by NCEP-ATP III or IDF criteria (Table 3).
Table 2 Dietary pattern adherence across Mealtime Habits Quality (MHQ) categories, at baseline and follow-up evaluations, among Mexican adult participantsFootnote *
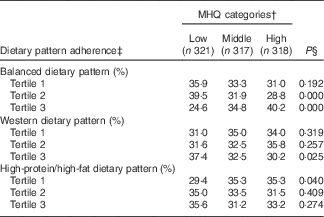
* Categories: lower MHQ category (from 0·87 to 3·86 points score), middle MHQ category (from 3·87 to 5·19 score) and higher MHQ category (from 5·20 to 8·08 score).
† The MHQ category was constructed by summing the factor loadings of each mealtime habit item, assigning each participant an individual MHQ score at baseline and another at the follow-up assessment. Individuals were classified in tertiles (highest tertile reflecting more advisable MHQ), obtaining one MHQ status for baseline and another for follow-up.
‡ Dietary pattern adherence: balanced dietary pattern (typified by a greater intake of processed vegetable juices, potatoes, fresh fruits, fresh vegetables and legumes and a lower intake of pastries; Western dietary pattern (higher intake of pastries, refined cereals, corn tortillas and soft drinks, and a lower intake of wholegrain cereals, sea food, and full-fat dairy products); and high-protein/high-fat dietary pattern (greater intake of red meat, processed meat, margarine, and eggs and a lower intake of fruits and wholegrain cereals). Dietary patterns were categorised into tertiles; tertile 3 represents greater adherence( Reference Denova-Gutiérrez, Castañón and Talavera 39 ).
§ In order to relate the MHQ status to the respective baseline or follow-up dietary pattern adherence, the Cochran’s Q test was performed to assess the difference of three probabilities. Proportions were adjusted by sex and age. A P value <0·05 was considered statistically significant.
Table 3 Change in the percentage of participants with the metabolic syndrome (MetS) components, the MetS, and insulin resistance (IR) across Mealtime Habits Quality (MHQ) categories, after 7 years of follow-up, among the HWCS participants

WC, waist circumference; NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; HOMA, homoeostasis model assessment.
* The MHQ score was created by summing factor loadings of each mealtime habits item, assigning each participant an individual MHQ score at the baseline and another at the follow-up assessment. Categories of the MHQ: lower MHQ category (from 0·87 to 3·86 points score), middle MHQ category (from 3·87 to 5·19 score) and higher MHQ category (5·20 to 8·08 score).
† To assess the difference for developing MetS components, MetS and IR across MHQ categories, Cochran’s Q test was performed to assess the difference of three probabilities. Proportions were adjusted by sex and age. A P value <0·05 was considered as statistically significant.
Finally, in order to estimate the independent effect of MHQ on the risk of developing the MetS, components of the MetS, or IR over the study period, we used generalised linear models to calculate the adjusted relative risks with binomial log-linear regression( Reference Rothman, Greenland and Lash 46 ), as well as their respective 95 % CI. Relative risks were adjusted by sex, depression, dietary patterns, PA and education level as categorical variables, while age and energy intake were continuous variables. Analyses were performed using STATA software, version 12.0.
Results
Table 1 compares the demographic, anthropometric, and lifestyle characteristics of the HWCS participants from the baseline and follow-up assessments. Participants were mainly women (75 %) and middle-aged, with a mean age of 46 years at baseline and 53 years at follow-up. Some anthropometric indicators and the MetS components significantly changed from 2004–2006 to 2010–2012, for example, the percentage of participants with normal BMI decreased from 38 to 33 %, whereas abdominal obesity increased from 48 to 58 % (using the NCEP-ATP III criteria) and from 80 to 88 % (using the IDF criteria). Furthermore, the percentage of participants with elevated glucose increased from 23 to 43 %, elevated TAG rose from nearly 43 to 55 %, the percentage of participants with low HDL-cholesterol decreased from 88 % to almost 62 % and high blood pressure increased from nearly 30 % to almost 49 %. Our findings regarding the MHQ reported at baseline and during the follow-up assessment indicate that the mean score decreased significantly from 5·28 to 3·84 points. Additionally, the percentage of participants categorised in the lower MHQ category increased significantly from nearly 15 to 52 %, and the percentage of participants in the higher MHQ category decreased from 58 to 8 % (P<0·000, for both). In terms of lifestyle characteristics, the percentage of physically active participants decreased from almost 37 to 31 %, and the prevalence of current smokers decreased from nearly 16 % to almost 11 % (Table 1).
The participants’ adherence to the three DP that were identified in the HWCS population( Reference Denova-Gutiérrez, Castañón and Talavera 39 ) was examined for each of the MHQ categories over the 7 years of follow-up, and is presented in Table 2. A higher percentage of participants with greater adherence to the balanced dietary pattern were classified in the higher MHQ category than in the lower MHQ category (40·2 v. 24·6 % respectively, P≤0·000), whereas a greater percentage of participants adhering to the Western dietary pattern were categorised in the lower MHQ than in the higher MHQ category (37·4 v 30·2 %, respectively, P≤0·025). A greater percentage of participants with lower adherence to the high-protein/high-fat dietary pattern were classified in the higher MHQ than in the lower MHQ category (35·3 v 29·4 %, respectively, P≤0·040).
Table 3 shows that a larger percentage of participants who reported more undesirable than recommended mealtime habits during the study period developed the MetS and its components, as well as IR. During the 7-year follow-up period, 25·8 and 48·3 % of individuals in the lower MHQ category developed abdominal obesity (according to NCEP-ATP III and IDF criteria, respectively), whereas only 2·5 and 5·4 % of those in the higher MHQ category developed abdominal obesity (according to NCEP-ATP III and IDF criteria, respectively). Furthermore, a greater percentage of participants in the lower MHQ category as compared with the higher MHQ category developed impaired fasting glucose (25·7 v. 3·4 %), elevated TAG (30·9 v. 2·6 %), decreased HDL-cholesterol (15·1 v. 0 %) and high blood pressure (28·5 v. 4·3 %). More participants categorised in the lower MHQ developed the MetS, 17·4 % according to the NCEP-ATP III and 20·5 % according to the IDF criteria, compared with only 1·6 and 1·8 % of those categorised in the higher MHQ, respectively. In addition, 12·5 % of the participants who were classified in the lower MHQ developed IR during the study period, compared with only 1·2 % of those categorised in the higher MHQ category.
The crude and adjusted relative risks for developing the MetS, its components or IR for each of the MHQ categories are presented in Table 4. Compared with participants in the higher MHQ category, those in the middle and lower MHQ categories had a 3·8 and 7·9 times greater risk of developing elevated glucose, respectively; 8·9 and 12·9 times higher risk of developing elevated TAG, respectively; were 6·0 and 9·5 times more likely to develop hypertension, respectively; had a 7·6 and 10·2 greater likelihood of developing abdominal obesity, respectively (considering the NCEP-ATP III criteria); an 8·5 and 9·8 higher risk of abdominal obesity, respectively (considering the IDF criteria); 7·3 and 8·8 times larger risk of developing the MetS, respectively (based on the NCEP-ATP III criteria); 10·9 and 11·1 times higher risk of developing the MetS, respectively (using the IDF criteria); and a 4·7 and 11·2 times greater risk of developing IR, respectively.
Table 4 Developing the metabolic syndrome (MetS) and insulin resistance (IR) across the Mealtime Habits Quality (MHQ) categories, after 7 years follow-up, among Mexican adults (Relative risks (RR) and 95 % confidence intervals)

NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; MetS, metabolic syndrome; IDF, International Diabetes Federation; HOMA, homoeostasis model assessment.
P values and CI of the RR were calculated by using generalised linear models, which were adjusted by sex, depression, dietary patterns (balanced, Western and high protein/fat), with recreational physical activity and level education as categorical variables and age and energy content as continues variables. * P<0·05, *** P<0·001.
† The MHQ score was created by summing factor loadings of each mealtime habits item, assigning each participant an individual MHQ score at the baseline and another at the follow-up assessment. Categories of the MHQ: lower MHQ category (from 0·87 to 3·86 points score), middle MHQ category (from 3·87 to 5·19 score) and higher MHQ category (5·20 to 8·08 score).
Discussion
The results of this prospective study indicate that certain habitual mealtime behaviours can predict the development of metabolic diseases. We found that participants who reported more undesirable mealtime habits (low MHQ) than recommended mealtime habits (high MHQ) had a >11-fold higher risk of developing the MetS or IR. Recent nutrition studies have indicated that isolated mealtime behaviours such as distractions while eating( Reference Bellisle, Dalix and Slama 7 – Reference Higgs and Woodward 12 ), not having enough time to eat( Reference Larson, Nelson and Neumark-Sztainer 18 ), skipping breakfast( Reference Smith, Gall and McNaughton 19 , Reference Timlin and Pereira 20 ), rushing meals, eating out( Reference Lachat, Nago and Verstraeten 21 ), eating quickly, and certain eating environments or social interactions during meals( Reference Wansink 13 – Reference Raulio, Roos and Mukala 15 ) can promote unhealthy diets and a higher BMI. These undesirable mealtime behaviours are also associated with a prolonged inflammatory state, as well as the development of IR, dyslipidemia, and the MetS( Reference Ahluwalia, Andreeva and Kesse-Guyot 47 ).
This study demonstrates that several inter-related recommended and undesirable mealtime habits can influence dietary intake, anthropometric status and the development of metabolic diseases. However, as the set of mealtime habits we examined cannot be disaggregated, it is difficult to directly compare our results with other studies that have evaluated the influence of isolated mealtime behaviours on risk of the MetS and its components. To better understand the impact of specific mealtime habits on anthropometric indicators and the subsequent development of metabolic diseases, we situate our results in a comprehensive theoretical framework to help explain how four mealtime situations – availability of time to eat, distractions while eating, environmental and social context of eating, and familiar or cultural eating habits – can affect food intake and risk of the MetS.
We evaluated availability of time to eat through the following four items: taking time to eat, rushing meals, skipping meals, and eating in huge mouthfuls. Other researchers have also studied the effects of eating quickly on risk of developing the MetS. For example, a longitudinal study with Japanese adults found that compared with the slow-eating group, the fast-eating group had a multivariate-adjusted hazard ratio that was 1·30 times greater for incidence of the MetS, 1·35 times higher for abdominal obesity, and 1·37 times greater for low HDL-cholesterol( Reference Zhu, Haruyama and Muto 26 ). In a cross-sectional study of Japanese adults, the likelihood of having the MetS was inversely related to eating speed – 0·79 for slow and 1·61 for fast eating among men (P<0·001) and 0·74 for slow and 1·27 for fast eating among women (P<0·001) – and eating rate was also associated with abdominal obesity( Reference Nagahama, Kurotani and Pham 27 ). Lack of time to eat is a frequent trend in many countries, for example, among young adults in the USA, 35 % of males and 42 % of females reported not having enough time to sit down and eat a meal. Eating on the run is associated with a higher intake of soft drinks, fast food and fat, as well as a lower intake of healthy foods among females( Reference Larson, Nelson and Neumark-Sztainer 18 ). Among a sample of university employees in central Mexico, 39 % reported a lack of time to eat breakfast at home and 13 % indicated that they eat street food. In some urban environments and workplaces in Mexico, it may be difficult to find healthy food options because many restaurants mostly offer fatty foods, sweetened soft drinks and, meals that lack fruits and vegetables( Reference Méndez-Hernández, Siani and Lamure 48 ).
We analysed two items that relate to distractions while eating: watching TV or reading while eating and doing office work while eating. Distractions while eating have received much attention as they have been associated with ignoring satiety signals that trigger feelings of fullness( Reference Wansink 49 ), which impairs the ability to monitor food consu mption, leading to an unintentional excess of energy intake( Reference Moray, Fu and Brill 50 ). Other studies have evaluated the impact of eating distractions on the MetS risk, including a cross-sectional study of 5682 Australian adults over the age of 35 years. They found that participants who spent ≥2 h/d watching TV and consumed three or more snack food servings per day were 1·5 and 3·6 times more likely to have the MetS, respectively, compared with those who reported low levels of TV viewing and low snack intake( Reference Thorp, McNaughton and Owen 10 ). Poor dietary habits are potential mediators associated with high levels of TV viewing that can be triggered by advertising( Reference Thompson, Spence and Raine 51 , Reference Scully, Dixon and Wakefield 52 ) and increased intake of energy-dense and nutrient-poor snack foods( Reference Cleland, Schmidt and Dwyer 53 ). TV viewing has also been shown to have an impact on subsequent meals because individuals who eat while watching TV report less vivid memories of their previous meals and consequently consume more food at the subsequent meals( Reference Bellisle, Dalix and Slama 7 ). A meta-analysis of twenty-four experimental studies provided additional evidence that watching TV reduces attention during eating, which increases food intake at the next meal( Reference Robinson, Aveyard and Daley 9 ). In the present study, we also examined other distractions such as reading, doing office work or engaging in tasks, which have been demonstrated to be associated with a greater likelihood of becoming overweight/obese( Reference Ogden, Coop and Cousins 8 – Reference Higgs and Woodward 12 , Reference Moray, Fu and Brill 50 ). Other sources of distractions examined by the scientific literature are listening to stories or music, playing video games, social interactions, and eating environment, which can all lead to impaired awareness of food consumption, mindless eating, increased energy intake, and risk of weight gain( Reference Ogden, Coop and Cousins 8 , Reference Wansink 13 – Reference Raulio, Roos and Mukala 15 ). The environmental and social contexts of eating were also assessed as part of the current study by taking into account the number of times participants ate with friends, family or colleagues, and the number of times they ate their main meal at home. One’s eating environment, which includes social interactions and distractions, can make people more vulnerable to mindless eating, resulting in increased meal size and energy intake( Reference Wansink 13 ), regardless of hunger( Reference Bellisle, Dalix and Slama 7 , Reference Bellisle, Dalix and Airinei 24 , Reference De Castro 25 ). Other studies have shown that meals eaten with others tend to be larger and longer in duration compared with meals eaten alone, regardless of the relationship of the eating companion or the time of day, with similar effects occurring at morning, noontime and evening meals( Reference De Castro 25 ). Furthermore, a systematic review of twenty-nine nationally representative studies or large cohorts reported that eating away from home is associated with a higher total energy intake, energy content from fat and lower intake of micronutrients, particularly vitamin C, Ca and Fe( Reference Lachat, Nago and Verstraeten 21 ).
The familiar and cultural customs associated with eating were also examined using the following three items: eating with family, friends or colleagues, eating at home, and eating all the food without leaving anything on the plate. The scientific literature provides evidence that culture is one of the major determinants of what we eat, reflecting unwritten social rules such as eating all the food one is served, which is usually done to show appreciation for the food( Reference Wansink 49 ). However, due to the enormous serving sizes that are offered at restaurants, this custom has become an unhealthy habit that contributes to overeating( Reference Ouwehand and De Ridder 54 ). Furthermore, the largest contributors to both adult and child BMI seem to include the familiar mealtime habits of the people with whom one eats, as well as the location and duration of the meals( Reference Anderson 17 ).
The results of this prospective study suggest that individuals who are engaged in more undesirable than recommended mealtime behaviours have a >10-fold risk of developing the MetS or IR. Other observational studies report an inverse relationship between the MetS and a greater adherence to healthy diets, such as the Mediterranean diet, ‘Dietary Approach to Stop Hypertension’ or diets based on guidelines such as the ‘Healthy Eating Index’ in the USA or the ‘Programme National Nutrition Santé’ in France( Reference Wansink 13 , Reference Martínez-González and Martín-Calvo 28 , Reference Wansink 49 – Reference Thompson, Spence and Raine 51 ). A prospective study with 3232 participants from a large European cohort demonstrated that higher adherence to a Mediterranean diet reduced risk of the MetS to 0·47 (95 % CI 0·32, 0·69) and 0·50 (95 % CI 0·32, 0·77) using the updated Mediterranean score and the Mediterranean Diet Score, respectively( Reference Kesse-Guyot, Ahluwalia and Lassale 55 ). A systematic review of intervention trials that focused on European Dietary Patterns and the MetS showed that the Western dietary pattern, which includes high servings of saturated fatty acids and simple carbohydrates, is associated with a higher risk of the MetS. Conversely, more traditional dietary patterns that are characterised by a high intake of vegetables, fruits, wholegrain cereals and fish are associated with a reduced risk of the MetS( Reference Martínez-González and Martín-Calvo 28 ).
Other studies with the HWCS participants have shown that the quality of mealtime habits is associated with dietary patterns( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 , Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). A cross-sectional study of 5240 HWCS participants between the ages of 20 and 70 years found that a Western dietary pattern that includes a high intake of pastries, refined cereals, corn tortillas and soft drinks, as well as a lower intake of wholegrain cereals, sea food and full-fat dairy products, is associated with a significantly greater risk of high fasting glucose (OR 1·67), low HDL-cholesterol (OR 1·55) and the MetS (OR 1·56)( Reference Denova-Gutiérrez, Castañón and Talavera 39 ). A longitudinal study of 837 HWCS participants reported that the healthiest MHQ category is related to a greater adherence to the balanced dietary pattern (higher intake of fresh fruits and vegetables, legumes and a lower intake of pastries), and a lower adherence to the protein/fat dietary pattern (greater intake of red meat, processed meat, margarine and eggs and a lower intake of fruits and wholegrain cereals). In contrast, the least healthy MHQ category is related to a higher adherence to the aforementioned Western dietary pattern( Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). Moreover, a cross-sectional study with 7472 HWCS participants found that individuals who were classified in the healthier MHQ category were more likely to report adherence to a balanced dietary pattern than to the Western pattern( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 ).
The MHQ scale was constructed with self-reported mealtime behaviours, which might not capture all possible variations at mealtime. Since food intake is affected by the meal of the day, the day of the week, the specific eating context, and the people who are present, we believe that using the MHQ scale to assess mealtime habits has several benefits. First, the MHQ scale is a tool that generalises people’s daily life experiences regarding meals, attempting to provide a general picture of mealtime habits. Second, the original MHQ scale was composed of binary response items for the baseline assessment, which were changed to multiple-choice responses for the follow-up assessment, allowing for a wider variety of possible mealtime behaviours to be studied. Third, the MHQ scale has been demonstrated to be a comprehensive, reliable and valid instrument because of its structure, its ability to predict dietary patterns, body weight status and the MetS, as well as its demonstrated consistency with other Mexican populations, including adolescents( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 – Reference Pierlot 6 ).
Conclusions
In previous studies, the quality of mealtime habits has demonstrated the ability to predict dietary patterns, anthropometric status, and risk of gaining weight( Reference Dosamantes-Carrasco, Méndez-Hernández and Denova-Gutiérrez 4 , Reference Dosamantes-Carrasco, Méndez-Hernández and Flores 5 ). The present study provides evidence that certain meal situations such as availability of time to eat, distractions while eating, the environmental and social contexts of eating, and familiar or cultural eating habits are associated with the development of the MetS and/or IR. Our results also support the idea that mealtime habits could be a key issue in nutrition research, since most foods are consumed as part of a meal, making the meal an appropriate area of study for concerns about food intake and its consequences on health( Reference Meiselman 3 ). Moreover, the MHQ scale could be used as a part of health promotion interventions that target mealtime behaviours, in order to help demonstrate how these strategies could improve diet quality, thus reducing the risk of weight gain and metabolic diseases.
Acknowledgements
The authors acknowledge the Health Worker Cohort Study participants and everyone who contributed to this project and thank them for their time and commitment.
This study was funded by the ‘Consejo Nacional de Ciencia y Tecnología’ (CONACyT) (National Science and Technology Council). The numbers of grant are: year 2002, number 7876; year 2008, number 87783. Y. N. F. was supported by NIH/NCI K07CA197179 for her work on this study.
The contribution of each author to this research study is as follows: P. M.-H. and L. D. D.-C. were involved in the design of the study, statistical analysis and writing of the manuscript. C. S., B. R.-P., L. C.-P., R. P., M. M.-G. and Y. N. F. helped in drafting the manuscript. E. R.-L. and E. S.-M. assisted with the statistical analysis and contributed to writing the manuscript. J. S. helped with the study design, and has led the Health Worker Cohort Study as its Principal Investigator. All authors have approved the final version of this manuscript to be published, have made critical comments during the preparation of the manuscript, and fully accept responsibility for this study.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516003329







