INTRODUCTION
Influenza can cause direct and secondary complications leading to primary-care visits, hospitalizations or deaths, imposing an enormous health economic burden on society, especially in high-risk populations such as the elderly [Reference Nichol1]. Age and underlying conditions, such as cardiopulmonary disease, are well-known risk factors for serious influenza-related complications [Reference Hak2]. The primary method for preventing influenza – vaccination – should therefore aim at reducing the burden of post-influenza complications in persons who would benefit most from vaccination [Reference Smith3].
The rate of distribution of vaccine in South Africa in 1995 was estimated to be 12·5/1000 population. At this time the rate was low compared to around 145/1000 in the United States and Canada [Reference Martin and Schoub4]. However, no recent published information is available on the rate of distribution of influenza vaccine in South Africa and the impact of influenza vaccination in reducing severe outcomes in African countries is unknown.
Although randomized placebo-controlled trials (RCTs) would be the preferred method for studying clinical effects of the influenza vaccine, because of the global recommendations on influenza vaccination, placebo-controlled trials, are no longer possible on ethical grounds. However, post-marketing observational studies such as cohort- and case-control studies incorporating clinical endpoints relevant to the individual patient can be used to estimate vaccine effectiveness, providing that there is sufficient control for confounding in these studies [Reference Szucs5]. Nested case-control studies that can be conducted on large medical databases can provide an indication of influenza vaccination effectiveness and is a cost-effective alternative to full-cohort analyses [Reference Hak6]. In a nested case-control study cases of a disease or other outcomes that occurred in a defined cohort are identified and for each case a specified number of matched controls are selected from among those in the cohort who have not developed the disease by the time of disease occurrence in the case [Reference Ernster7]. Time-matching is an essential feature of this design. A cohort member who serves as a control may later become a case and a cohort member may be selected as a control for more than one case [Reference Ernster7]. This selection of controls is sometimes described as ‘risk set sampling’ or density sampling and in this instance the controls will estimate the exposure odds in the study base and the results will estimate the rate ratio from the cohort [Reference Pearce8].
Previous studies have evaluated the efficacy, effectiveness and cost-effectiveness of influenza vaccination in the elderly population, mostly in the northern hemisphere [Reference Gross9, Reference Rivetti10]. No recent published data are available on the effectiveness of vaccination in the elderly or any other population in most African countries including South Africa.
This nested case-control study carried out in an elderly population in South Africa could, for the first time, provide valuable information in this setting on the rate of vaccination as well as on the effectiveness of the vaccination to decrease hospitalizations or death, which can often be associated with influenza-related complications. We further explored whether influenza vaccination is also effective in the elderly with high-risk medical conditions.
MATERIALS AND METHODS
Setting and study population
The study was performed in a population of elderly members of a private medical funding organization in South Africa. Medihelp Medical Scheme is a non-profit organization with the core responsibility of health-funding administration as well as the application of managed health-care principles. In 2004, the scheme managed the health-care funds of 45 700 community-dwelling and institutionalized elderly (aged ⩾65 years) living in South Africa. Updated electronic data is kept on membership details, health-care claims and managed health-care interventions of members. As data integrity may have financial implications for the organization, a high level of data integrity is ensured by means of ongoing internal audit, including trend analysis and sampling for forensic auditing. Data for this study was retrospectively obtained from the Medihelp database. Ethical approval was obtained from the Faculty of Health Sciences Research Ethics Committee at the University of Pretoria.
Members continuously enrolled between January 2003 and August 2004 who were aged >65 years on 1 January 2004 and who had access to influenza vaccination during the periods February–May of each year were included in the study. In all, 45 522 elderly members were included in the baseline study cohort.
Selection of cases and controls
The South African health-care industry utilizes the International Classification of Diseases, tenth revision (ICD-10), published by the World Health Organization (WHO). The codes from ICD-10 were used to identify cases within the study cohort of elderly for the purpose of this study. To enable comparison with previous similar published studies using the ninth revision (ICD-9 codes), a cross-map, developed for the South African market, was used to crosslink the codes of the ninth revision (ICD-9 codes) to the relevant ICD-10 codes.
The influenza sentinel surveillance programme of the National Institute for Communicable Diseases in South Africa was designed to observe season timing and determine circulating strains. The influenza season in Gauteng started during the last week of May and ended during the second half of July [11]. Although there is no correlation between the number of isolates and season severity, based on mortality data and absenteeism, it did, however, appear that the season was mild to moderate during the South African 2004 winter [11].
Three types of cases were identified from the cohort data collected during the influenza season of May–July 2004: (1) members who were hospitalized for acute respiratory conditions (influenza or pneumonia) (n=227); (2) those hospitalized for non-elective cardiovascular conditions (n=428); and (3) those who died during, or within 2 weeks after the influenza season from any cause (n=627).
For identification of hospitalizations as a result of acute respiratory conditions, data on claims was classified as follows: hospitalizations for influenza (ICD-10 codes J10.0, J10.1, J10.8); and hospitalizations for pneumonia (ICD-10 codes J12.0–J12.9, J13–14, J15.0–J15.9, J16.8, J17.0–J17.8, J18.0, J18.8). Hospitalizations for cardiovascular conditions were classified as follows: diseases of the mitral and aortic valves (ICD-10 codes I05.0–I05.8, I06.0–I06.8, I07.9, I08.0), rheumatic heart conditions (ICD-10 codes I09.0–I09.8), ischaemic heart disease (ICD-10 codes I20.0–I20.9, I21.1–I21.4, I23.8, I24.0–I24.8, I25.0–I25.9), cardiomyopathies and heart failure (ICD-10 codes I42.1–I42.9, I43.0, I43.2, I50.0–I50.9, I51.0–I51.9).
Patients who were hospitalized once for a respiratory condition or cardiovascular condition were not eligible to become a case for a second time if they were again hospitalized during the study period. Patients who died during the study period were counted as all-cause mortality cases regardless of possible prior hospitalizations. For each of the cases, four control subjects were randomly chosen from the cohort, making use of the incidence density sampling technique in Stata version 9.2 (StataCorp., College Station, TX, USA), matching on the date on which the case was identified.
Influenza vaccine
According to the WHO, during the 2004 influenza season, the most commonly used influenza A H3N2 vaccine strain in South Africa was A/Wyoming/3/02, an a/Fujian/411/02-like virus, while the B viruses included either the B/Shangdong/7/97 or B/Brisbane/32/02 strains [11]. The majority of viruses isolated were influenza A H3N2 viruses (97·3%). Results of the phylogenetic analysis of representative South African influenza A H3N2 HA1 sequences, however, showed that the 2004 isolates exhibited genetic drift from the A/Fujian/411/02 and A/Wyoming/3/02 strains and the majority were grouped with the more recent A/Wellington/1/2004 reference strain [11].
Exposure status
Vaccination status was positively confirmed if a patient claimed benefits for any of the available influenza vaccines between 1 February and 30 April 2004.
Covariates
At baseline, information on the following potential confounding covariates for each of the cases and controls was obtained from the electronic database: age, gender, prior health-care consumption variables, comorbidity and severity of disease-indicating variables, medication-utilization variables, and vaccination status of the patient in 2003 or of a family member of the patient in 2004 or 2003 (Table 1).
Table 1. Baseline characteristics for cases and controls
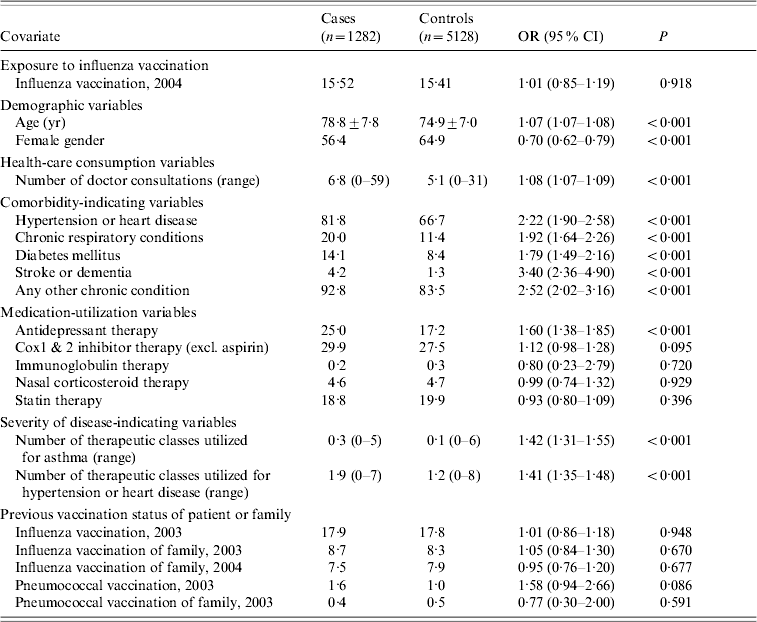
OR, Odds ratio; CI, confidence interval.
Data are percentage of patients, unless otherwise indicated.
Sample size calculations
Sample-size calculations performed with Win Episcope 2.0 (University of Utrecht, The Netherlands) prior to the study were based on an estimated vaccination rate in the controls of 20%. The number of cases that would give a statistical power of 80%, to detect an odds ratio (OR) of ⩽0·65 based on earlier studies in elderly persons, if the case:control ratio was 1:4, at a 95% level of confidence, was 393.
Data analysis
Using the nested case-control data, univariate analysis was applied using Stata version 9.2 software. Baseline characteristics for cases and controls were compared using χ2 and Student's t test for categorical and continuous variables. The study controls were compared in terms of exposure status, and we assumed variables that differed between those who received and those who did not receive the vaccine as potential confounders. Multivariate conditional logistic regression analysis was used to assess the association of vaccination status with the combined endpoint after adjustments for the covariates, i.e. age, gender, number of doctor consultations during 2003, hypertension and/or heart disease, chronic respiratory condition, diabetes mellitus, stroke and/or dementia, other chronic condition, utilization of antidepressant therapy, number of therapeutic classes utilized of medication for hypertension and/or heart disease, and number of therapeutic classes utilized of medication for asthma therapy. To explore potential differences in effects of vaccination we also analysed the components of the combined outcome separately, i.e. hospitalization for a respiratory (influenza and pneumonia) illness; hospitalization for a cardiovascular condition; all-cause mortality; and hospitalization for either respiratory (influenza and pneumonia) illness or a cardiovascular condition. For these analyses, all the controls selected initially were used each time to increase the power of the analysis, and ORs for the different component endpoints were determined with unconditional logistic regression analysis.
In addition, the patients identified as cases in the study were categorized into high-risk patients and low-risk patients. Patients with hypertension or heart disease, a chronic respiratory condition, diabetes, rheumatological condition, dementia and stroke, and patients utilizing cytostatic drugs were classified as high-risk patients and the rest of the patients were classified as low risk. Subgroup analysis was performed with unconditional logistic regression analysis to assess the association of vaccination with the endpoints in each of the two subgroups. Post-estimation statistics including the Hosmer–Lemeshow goodness-of-fit statistic was used to assess the fit of the models. Finally, the vaccine effectiveness was calculated with the formula: 1 – OR×100%.
Post-hoc sensitivity analysis was carried out to establish the potential effect of an unknown unmeasured confounder indicating healthy user bias on the findings in this study [Reference Nichol, Wuorema and Von Sternberg12].
RESULTS
The vaccination rate in this elderly population was 15·4% prior to the influenza season of 2004. The percentage of people vaccinated in the high-risk subgroup was 16·9% and in the low-risk subgroup 1·2%.
In the 45 522 elderly subjects there were 627 deaths from any cause, 428 hospitalizations for cardiovascular conditions, and 227 hospitalizations for acute respiratory conditions. In total, 1282 (2·8%), of this elderly population had an outcome during the influenza season. The incidence rates for the different outcomes were 561 deaths from any cause, 382 cardiovascular hospitalizations and 202 respiratory hospitalizations per 10 000 person-years. The incidence rate of the combined outcomes in this study was 1145/10 000 person-years. In the study population, the number of outcome events in the high-risk group was 1107 (23·2%), while the number of outcome events in the low-risk group was 175 (10·7%).
A comparison between the baseline characteristics of the cases and controls for the combined outcomes (all-cause mortality and hospitalizations) is presented in Table 1.
In the main analyses the average age of the 5128 controls that were randomly chosen from the cohort was similar between vaccinated and unvaccinated persons and there were slightly more females than males in the unvaccinated group. Vaccinated controls were more likely to have high-risk conditions and they had higher numbers of doctor consultations but similar rates of hospitalization than the unvaccinated controls during the baseline as well as the outcome period (Table 2).
Table 2. Description of controls in terms of influenza vaccination status
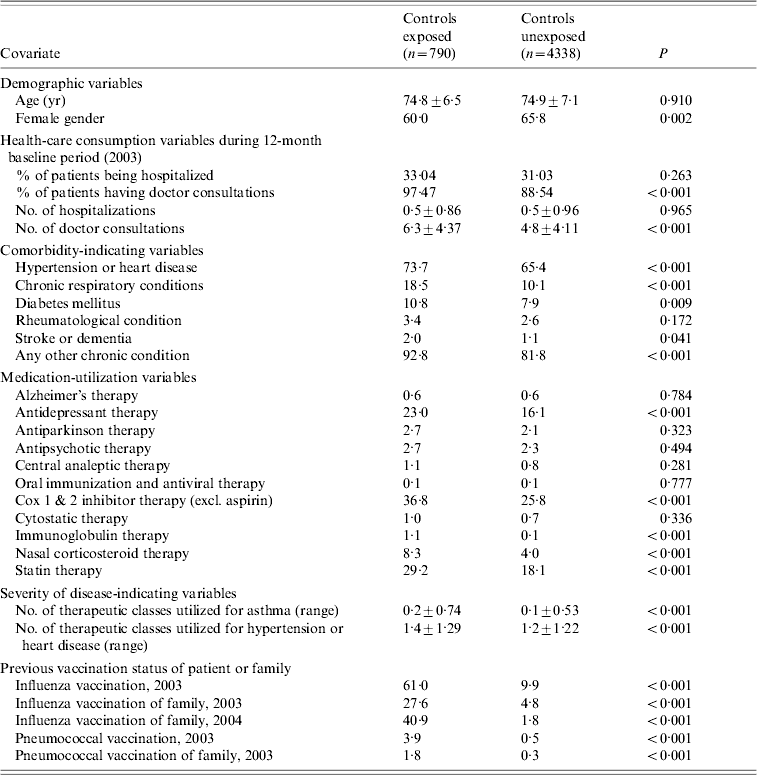
Data are percentage of patients, unless otherwise indicated.
The effect of adjustment for confounding after addition of each variable changed the unadjusted OR of 1·01 from the null for the combined endpoint as shown in Table 3.
Table 3. Unadjusted and adjusted odds ratios (combined outcomes)
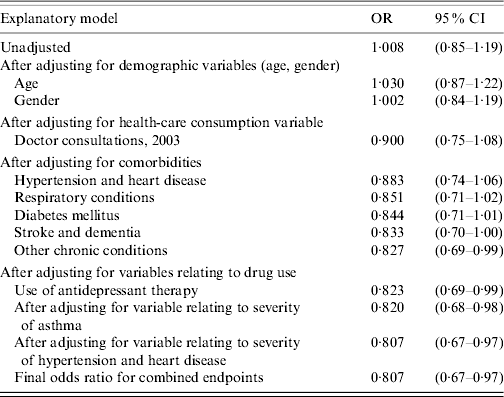
OR, Odds ratio; CI, confidence interval.
After adjustments for all measured confounders, influenza vaccination was associated with a statistically significant reduction in the combined primary outcome of a hospitalization for acute respiratory condition or a cardiovascular condition, or of death from any cause of 19·3% [95% confidence interval (CI) 3·1–32·9].
When analysed separately, influenza vaccination was associated with a statistically significant reduction in all-cause mortality of 23·6% (95% CI 1·0–41·0) and similar reductions in hospitalizations, although non-statistically significant, of 14·6% (95% CI −12·8 to 35·4) and 13·8% (95% CI −24·6 to 40·3), respectively, for cardiovascular and acute respiratory conditions (Table 4).
Table 4. Estimates of vaccine effectiveness
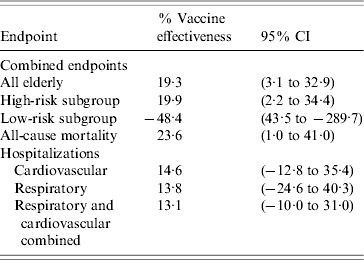
CI, Confidence interval.
When analysing according to risk status, influenza appeared to be associated with a statistically significant reduction in the combined outcome of 19·9% (95% CI 2·2–34·4) in the high-risk elderly. All-cause mortality was reduced statistically significantly in this subgroup (27·1%, 95% CI 3·6–44·8, see Table 4).
Post-hoc sensitivity analysis of the impact of a potential unmeasured confounder indicating healthy user bias in the dataset revealed that such a confounder could have theoretically caused these findings (Table 5).
Table 5. Sensitivity analysis to quantify the potential effects of an unmeasured confounder on the measured vaccine effectiveness
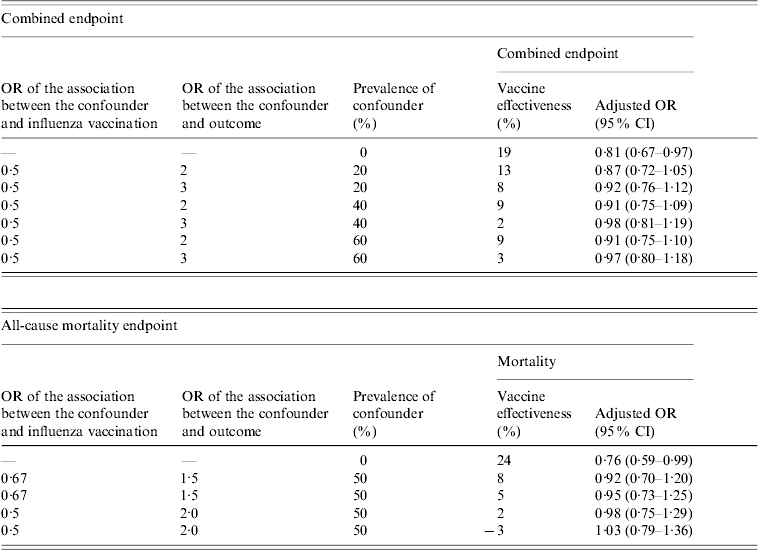
OR, Odds ratio; CI, confidence interval.
DISCUSSION
After full adjustment for measured confounders, the present study indicated that the use of influenza vaccination was associated with a decrease in serious complications in the elderly from South Africa. Such an association appeared most significant in the subgroup of elderly with high-risk medical conditions in which the largest number of outcome events occurred. However, the sensitivity analysis indicated that potential unmeasured healthy user bias could also have caused these findings hence the data are inconclusive regarding the benefits of such vaccination in the elderly in South Africa.
In this study the incidence rates of outcomes were higher than in similar previous studies [Reference Hak2, Reference Hak13]. The rate of vaccination (15·4%) in the medically insured elderly population in South Africa was found to be lower than the rate generally reported in studies carried out in countries in the northern hemisphere (>50%) [Reference Hak2, Reference Nichol, Wuorema and Von Sternberg12–Reference Looijmans-Van den16] which may in part explain these high figures. Importantly, the potential for selection bias in this study was largely prevented by defining a baseline cohort prior to the study season from which both cases and controls were drawn. Finally, our study size was adequate to detect a statistically significant effect of at least 35% and since our number of cases was more than needed, we were able to find an even lower statistically significant effect of 19%. However, the sample size provided inadequate power to reliably estimate vaccine effectiveness in the low-risk subgroup of elderly patients.
A meta-analysis of the efficacy of influenza vaccination in 1995 showed a reduction in the risk for pneumonia, hospitalization and death in elderly persons during influenza epidemic seasons if the vaccine strain was identical or similar to the epidemic strain [Reference Gross9]. In a study of elderly patients with chronic lung disease, it was shown that vaccination was associated with a 52% reduction in hospitalizations for pneumonia/influenza and a 70% reduction in the risk of death [Reference Nichol, Baken and Nelson15]. Similar to this study, studies elsewhere also showed a higher rate of reduction in all-cause mortality than in hospitalizations [Reference Nichol, Wuorema and Von Sternberg12–Reference Hak13, Reference Looijmans-Van den16, Reference Nordin17]. It was previously postulated that an unrecognized sub-population of under-vaccinated, frail elderly people in the cohort studies could have caused an overestimation of vaccine effectiveness for a non-specific outcome such as all-cause mortality [Reference Simonson18]. In the present study it was observed that influenza vaccination was associated with a greater reduction in all-cause mortality than in the combined endpoint. However, since the 95% confidence intervals of the effect estimates in the more specific component endpoints were large, any claims considering differences in vaccine effectiveness estimates between more specific and less specific outcomes can not be made in this study.
Some subsequent studies showed increased benefits of influenza vaccination in elderly patients with high-risk conditions. One study reported that vaccination of high-risk elderly prevented 18·0/1000 patients from death or hospitalization for pneumonia/influenza but prevented only 3·8/1000 patients from the same outcomes in the healthy elderly [Reference Hak2].
In a recent review carried out by the Cochrane Collaboration, the authors assessed studies on the effects of inactivated influenza vaccines on community-dwelling elderly. After adjustment for confounding, outcomes of risk of hospitalization or death from influenza/pneumonia and hospitalization for all respiratory diseases were reduced significantly by 41%, 26% and 29% respectively [Reference Rivetti10]. In our study the effectiveness of the influenza vaccination to decrease all-cause mortality in the elderly was 23·6% and the point estimates of vaccine effectiveness for reducing hospitalizations for acute respiratory conditions indicated reductions of about 14%. This somewhat lower effectiveness estimates as compared with the previous studies may be explained by the lower vaccination rate, a suboptimal match between the vaccine and the circulating virus strains, and the relatively low level of influenza activity in 2004 in South Africa [Reference Hak2, Reference Nichol, Wuorema and Von Sternberg12].
In our study, even though many variables were measured, it was not possible to measure and control for all the potential confounding variables such as smoking status, functional status and socioeconomic status. Differences in these variables in the study population may have led to an unequal balance of average risk of exposure to influenza vaccination, or outcomes between the comparison groups, causing potential bias in the results. In non-randomized studies, differential vaccine uptake and the resulting confounding bias might explain some of the established high effectiveness of influenza vaccines in preventing all-cause mortality [Reference Jefferson19]. In another study the influence of bias due to confounding by health status was assessed by following a large population-based cohort for a period of 8 years including periods before, during and after the influenza season. The researchers concluded that differences in health-care status between vaccinated and unvaccinated groups, leads to bias in estimates in influenza vaccine effectiveness against all-cause mortality and other non-specific outcomes. This finding, however, does not mean that there is no effect of vaccination against serious complications of influenza infection [Reference Jackson20]. Although many important confounders were measured and adjustments led to change of the vaccine estimates from the null, a strong unmeasured confounder such as functional status might theoretically have led to an overestimation of the actual effectiveness. This potential has been confirmed with post-hoc sensitivity analysis. On the other hand, the influenza activity was rather mild in our season of study and hence to reach firm conclusions, longer-term follow up of the effectiveness in larger cohorts is urgently needed to enhance the knowledge base of the effectiveness of influenza vaccines in South Africa.
In the absence of large randomized controlled trials that may eliminate confounding and bias, this study provides the first data on the effectiveness of influenza vaccination in this setting. Although this study could not confirm the effectiveness of influenza vaccination in the elderly population in South Africa during the influenza season of 2004, until more data is available, the recommendation remains that all individuals aged >65 years should be vaccinated against influenza every year.
ACKNOWLEDGEMENTS
We acknowledge Medihelp Medical Scheme for the data used in the analysis. The help with the sensitivity analysis by Dr R. H. H. Groenwold is greatly acknowledged. The participation of Dr E. Hak was financially supported by the Netherlands Scientific Organization through a VENI post-doctoral research grant (grant no. 916.56.109).
DECLARATION OF INTEREST
None.







