Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder affecting the motor neurons controlling the voluntary muscles, leading to the gradual development of muscle weakness and atrophy (Mitchell and Borasio, Reference Mitchell and Borasio2007). Relatives have an important role in caring for a patient with ALS, which involves considerable physical, emotional, and social challenges. Relatives often dedicate many hours every day to help the patient with daily activities (Krivickas et al., Reference Krivickas, Shockley and Mitsumoto1997; Aoun et al., Reference Aoun, Connors and Priddis2012). The burden of care increases for the relatives during disease progression; moreover, due to symptoms and progression of the disease, the relatives’ lives become focused around their home and the patient, leading to a restricted social life and decreased activities (Trail et al., 2003; Hughes et al., Reference Hughes, Sinha and Higginson2005).
Quality of life (QoL) may be difficult to define, but for most individuals, QoL is associated with life satisfaction and well-being. Quality of life is subjective and multidimensional; moreover, different individuals value different aspects of life, and the meaning of QoL means different things for different individuals (Carr et al., Reference Carr, Gibson and Robinson2001). The World Health Organization's (WHO) definition of QoL is “an individual's perception of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns” [The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization, Reference Wettergren, Sprangers and Bjorkholm1995]. Despite the severity of the disease, several studies have shown that most patients with ALS have a relatively good QoL (Neudert et al., Reference Neudert, Wasner and Borasio2001; Trail et al., 2003; Chio et al., Reference Chio, Gauthier and Montuschi2004; Fegg et al., Reference Fegg, Wasner and Neudert2005; Nygren and Askmark, Reference Nygren and Askmark2006; Gauthier et al., Reference Gauthier, Vignola and Calvo2007; Roach et al., Reference Roach, Averill and Segerstrom2009; Jakobsson Larsson et al., Reference Jakobsson Larsson, Ozanne and Nordin2017). When comparing QoL in patients with ALS and their relatives, it has been found that relatives estimated their QoL as poorer than the patients (Bromberg and Forshew, Reference Bromberg and Forshew2002; Olsson et al., Reference Olsson, Markhede and Strang2010), while other studies found no difference between how patients and their relatives rated their QoL (Trail et al., 2003; Lo Coco et al., Reference Lo Coco, Lo Coco and Cicero2005; Gauthier et al., Reference Gauthier, Vignola and Calvo2007).
Few longitudinal and prospective studies have been conducted with relatives of patients with ALS with the perspective to describe individual QoL (iQoL; Gauthier et al., Reference Gauthier, Vignola and Calvo2007; Roach et al., Reference Roach, Averill and Segerstrom2009; Olsson et al., Reference Olsson, Markhede and Strang2010), and none, to our knowledge, with relatives of newly diagnosed patients with ALS and over time. This present study aims to describe iQoL from diagnosis and throughout disease progression among relatives of patients with ALS and to evaluate if iQoL correlates with the patient's physical function or the relatives’ own psychological well-being.
Methods
This was a prospective, longitudinal, and descriptive study.
Relatives of patients with probable or definite ALS, according to the El-Escorial WFN revised criteria (Brooks et al., Reference Brooks, Miller and Swash2000), who were treated by the ALS teams at three separate hospitals in Sweden, were asked to participate in the study. Inclusion criteria were being older than 18 years of age and understanding and being able to express themselves in the Swedish language. This is part of a longitudinal study with 36 patients who had been newly diagnosed with ALS (Jakobsson Larsson et al., Reference Jakobsson Larsson, Ozanne and Nordin2017); three of the patients could not identify a relative to participate in the study. A total of 33 relatives were included at the start of the study.
Data collection was performed using questionnaires and a semi-structured interview instrument. The data collection started 1–3 months after the patient was diagnosed with ALS and continued for a period of 2 years with the purpose of evaluating iQoL over time. After having given written informed consent, the relatives answered the questionnaires and the semi-structured interview were performed by the first author (BJL) during home visits or by telephone. The patient's physical function was evaluated at the same time point as the interviews.
To evaluate iQoL, the Schedule of Evaluation of Individual Quality of Life — Direct Weighting (SEIQoL-DW) was used. This is a semi-structured interview instrument to evaluate a person's iQoL by letting the respondents nominate the five most important areas (cues) of their life at present, determine the level of functioning for each cue on a visual analogue scale from worst possible (0) to best possible (100). Finally, the respondent determined the relative importance of each of these cues using a pie chart with sections, where he or she could adjust the size to reflect the relative percentage importance of the different cues. By multiplying the cue level by the cue weight for each cue and summing the values for each of the five cues, a SEIQoL-DW index score is calculated. This index can range from 0 (lowest QoL) to 100 (highest QoL) (Hickey et al., Reference Hickey, Bury and O'Boyle1996). In the present study, a modified version of the SEIQoL-DW was used (Wettergren et al., Reference Trail, Nelson and Van2003). The respondents were asked to nominate the five most important areas, both good and bad, for their overall quality of life. The definition of each area (cue) given by the respondents was documented directly on a cue definition record form. Thereafter, each area was rated on a 7-point scale regarding how well it functioned: “As bad as it could possibly be” (scored 1) and “As good as it could possibly be” (scored 7). In the original version of SEIQoL-DW, a direct weighting procedure is conducted to determine the importance of each cue. Earlier studies have found that the weighting procedure does not have an impact on the total index (Wettergren et al., Reference Wettergren, Bjorkholm and Axdorph2005, Reference Wettergren, Bjorkholm and Langius-Eklof2008). Therefore, the overall individual QoL score (SEIQoL index) was calculated by taking the sum of the ratings divided by the number of nominated areas. Finally, the relatives self-rated how they experienced their overall QoL based on the areas they nominated themselves for and how they rated each area (SR-QoL). By describing and documenting each cue nominated by the respondent, a better understanding and a clinical application will be achieved.
The Hospital Anxiety and Depression Scale (HADS) was used to evaluate the relatives’ emotional well-being. This questionnaire consists of two subscales: 7 items for anxiety (HADSa) and 7 items for depression (HADSd). The scale measures the presence and severity of symptoms of anxiety and depression in the past week. Two cut-off scores have been suggested, 8–10 = doubtful cases and ≥11 = cases (Zigmond and Snaith, Reference Zigmond and Snaith1983).
The patient's physical function was measured using the revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALS FRS-R). This functional rating scale consists of 12 questions to assess the patients’ levels of functioning: bulbar function, fine- and gross motor tasks and respiratory function. Each function is graded from 0 (total lost) to 4 (normal function); a lower score indicates more disability (Cedarbaum et al., Reference Cedarbaum, Stambler and Malta1999).
Analysis
Descriptive statistics were used to describe the demographic and clinical characteristics of the participants. For each time point, the results are based on the subgroup for patients who have survived up to that time point. The answers to the questions in the SEIQoL-DW were written down during the interview by the first author (BJL). The written answers were grouped into areas based upon the domain “the cue primarily belonged to” by two of the authors (BJL, AO). The content in each area was described qualitatively. A mixed model, with time as a continuous fixed variable with the corresponding 95% confidence interval, was used for the development of the SEIQoL-DW index and the other scales over time. Spearman's rho was used to analyze the correlation between the SEIQoL-DW, HADS, and ALS FRS-R. SPSS, version 17 (Chicago, IL, USA) was used for statistical analysis. The significance level was p ≤ 0.01 since the p-value was not adjusted for the number of tests. All tests were two-tailed. The number of dropouts for reason other than death of patients is small (n = 2) and thus taking into account dropouts due to other reasons was deemed not necessary.
Results
A total of 33 relatives were included, 24 women and 9 men. Of those, 2 relatives were missed at time point 1 (TP1) but were included at time point 2 (TP2). The sample was divided into five groups, depending on time in the study; group 1 includes relatives that only participated at the first assessment (i.e., 1–3 months after diagnosis), and group 5 includes only relatives that participated at all five assessments. Two relatives dropped out on their own initiative due to psychological distress, and the other dropouts were due to the death of patients. The characteristics of the group are shown in Table 1.
Table 1. Socio-demographic characteristics

Areas of importance for the iQoL
In total, 528 cues were nominated by the relatives. These cues were grouped into 14 areas based on the cue label and the relatives’ description of each cue. Thirty relatives nominated five cues at the different assessments, one relative nominated only one cue at the second assessment, another relative nominated four cues at the third assessment and yet another relative nominated three cues at all five assessments. The most nominated area of importance was “family” at all five time points (Table 2). This area included different family members and was described as “relations,” getting “help from other family members” but also “providing support to other family members” (Table 3). Other areas that were frequently nominated were friends, health, leisure, and hobbies. The cue level showed that most of the nominated areas were functioning well at different time points (Table 2).
Table 2. Frequencies and mean scores for indication level for each area at the five time points for the total group

% = percentage of relatives nominating the different areas (each relative could choose more than one area). CL = cue level, how each area is functioning for the relative at present time, range from 1 (as bad as it could possibly be) to 7 (as good as it could possibly be).
a 1–3 months after diagnosis.
b After 6 months.
c After 12 months.
d After 18 months.
e After 24 months.
Table 3. Descriptions of the different areas nominated by the relatives as being important for their individual quality of life

Individual QoL over time
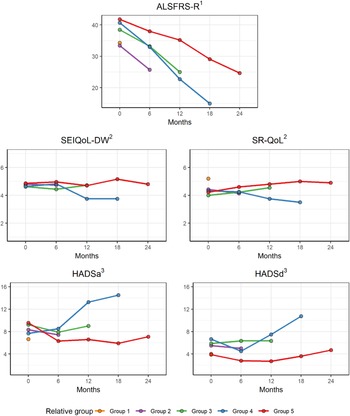
The mean score for the SEIQoL index and the SR-QoL index indicated that most relatives had a relatively good iQoL at the different assessments (Figure 1). However, when looking at each individual, the result showed that 19 relatives had scored <4 on SEIQoL and/or SR-QoL at any of the assessments, which indicated a poor iQoL. Areas that contributed to low QoL were: “family” (i.e., change relations), “own health,” “others” health” (i.e., the ALS disease), and “lack of own time” (data not shown). Even though the SEIQoL index for the total group of relatives did not change over time (p = 0.570), the mean score for both SEIQoL-DW and SR-QoL indicated a decline in iQoL in group 4 from TP2 to TP4 (Figure 1).

Fig. 1. Mean score for QoL, emotional well-being, and the patient's physical function in the different relative group during the disease progression. 1range 0–48, a lower score indicates more disabilities, 2range 0–7, higher score represents a better QoL, 3range from 0 (no distress) to 21 (maximum distress).
Relatives’ emotional well-being and patient's physical function over time
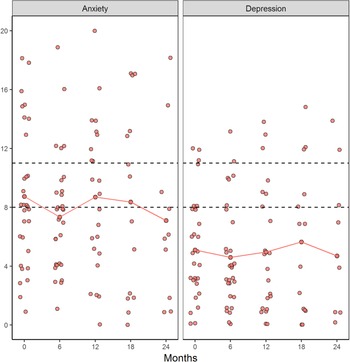
The results showed that several relatives had scores within the cut-off score for doubtful cases or cases on the HADSa (anxiety) and that some relatives had the symptoms of depression (Figure 2). When looking at each individual, the result showed that relatives in group 4 had more symptoms of anxiety and depression compared with the other groups, with score ≥10 on both HADSa and HADSd at TP3 and TP4, respectively. The ALS FRS-R mean score for the patients show an increase in function disabilities over time and that patients of relatives in group 4 had a more rapid decline in physical function between TP3 and TP4 compared with the other groups (Figure 1).

Fig. 2. Doubtful cases (score 8–10) and cases (score ≥11) on HADS two subscales at different time points.
Correlations between iQoL, emotional well-being, and the patient's physical function
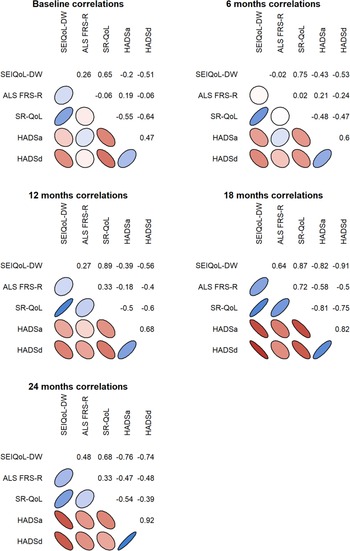
Both the SEIQoL index and the SR-QoL index correlated negatively with emotional well-being at several time points and positively with the ALS FRS-R at TP4 (Figure 3). There was also a positive correlation between the SEIQoL index and the SR-QoL index (rs = 0.77).

Fig. 3. Correlation between the relatives’ QoL, emotional well-being, and the patient's physical function during the disease progression. The shapes color scale range from dark red (strong negative correlation), white (no correlation) and to dark blue (strong positive correlation).The shape of the ellipse illustrates the strength of the correlation where a more narrow ellipse indicates a stronger relationship.
Discussion
This present study aims to describe iQoL from diagnosis through 2 years of disease progression among relatives of patients with ALS and to evaluate if iQoL correlated with the patient's physical function or the relatives’ own emotional well-being. The most nominated QoL areas were: “family” at all time points, but also “friends,” “health” (both own health and others' health), and “leisure” were areas of importance for the relatives’ iQoL, which is in accordance with previous studies (Bromberg and Forshew, Reference Bromberg and Forshew2002; Lo Coco et al., Reference Lo Coco, Lo Coco and Cicero2005; Felgoise et al., Reference Felgoise, Stewart and Bremer2009; Olsson et al., Reference Olsson, Markhede and Strang2010). The results from the semi-structured interview on the descriptions of the areas show the burden that the ALS disease has on the relatives’ life due to the increased physical and emotional demands, but also due to limited time for oneself. The relatives described worries, stress, the burden of caring, and a need to have time for relaxation and to think about their situation. Although the cue level for each area showed that most areas functioned well, there were variations for some areas between different assessments. The results show that areas such as “own time” and “social activities” had poorer functioning compared with the other areas. To some extent, these areas include a need to be able to decide over one's own time and time outside the home, which most often is limited for relatives involved in the care of patients with ALS. Earlier studies have shown that relatives often rate their QoL as relatively good, despite the impact of the disease on their social life (Galvin et al., Reference Galvin, Corr and Madden2016) and spending several hours per day to care for the patient (Murphy et al., Reference Murphy, Felgoise and Walsh2009). When looking at the mean score, the results showed that most relatives rated their iQoL as being relatively good; however, when looking at each participant, relatives in group 4 had a worse iQoL compared with the other groups. The individual descriptions for the cues showed that these relatives with poor iQoL had nominated cues that had a negative impact on their social life (i.e., lack of own time, restrictions in leisure activities, and changed family relations).
Several studies have shown that patients often rated their QoL as good and that it did not change despite the disease progression (Nygren and Askmark, Reference Nygren and Askmark2006; Gauthier et al., Reference Gauthier, Vignola and Calvo2007; Jakobsson Larsson et al., Reference Jakobsson Larsson, Ozanne and Nordin2017). These ratings and the stability of QoL over time may be explained by the response shift theory; the patient reappraised values, internal standards, and conceptualiation of QoL during the disease trajectory (Albrecht and Devlieger, Reference Albrecht and Devlieger1999; Schwartz et al., Reference Schwartz, Andresen and Nosek2007). This revaluation may help them to adjust to their situation, which may explain that most relatives rated their QoL as relatively good despite the situation. When looking at how relatives have estimated the various cues, it seems that most areas that are important also work well, but maybe it is because they are working well that they also become important and not vice versa. The individual description of the cues by relatives with a poor iQoL indicated that they had difficulties in doing this revaluation, leading to difficulties in adjusting to their situation and worse iQoL.
The ALS disease is related to both physical and psychological stress for the relatives during the disease trajectory. Earlier studies have shown that caregiver burden affects the emotional well-being of the caregivers, and that it is not uncommon with symptoms of anxiety (Burke et al., Reference Burke, Elamin and Galvin2015; Galvin et al., Reference Galvin, Corr and Madden2016). Our results showed that several relatives had scores within the cut-off score for doubtful cases to cases at the HADS and that emotional well-being correlated with both the SEIQoL index and the SR-OoL index. This correlation between emotional well-being and QoL has been found in earlier studies (Murphy et al., Reference Murphy, Felgoise and Walsh2009; O'Connor and McCabe, Reference O'Connor and McCabe2011; Burke et al., Reference Burke, Elamin and Galvin2015). When looking at the mean score for iQoL index and HADS in group 4 one year after diagnosis, these relatives had more symptoms of anxiety/depression and a poorer iQoL compared with the other groups in the late stage of the disease. Whether it is the emotional well-being that affects the iQoL or if it is the other way around is unclear.
The mean score for ALS FRS-R showed that the patients of relatives in group 4 were severely impaired between TP3 and TP4, which probably increased the burden for these relatives. Even if previous studies have shown that the patient's physical function does not seem to affect relatives’ QoL (Lo Coco et al., Reference Lo Coco, Lo Coco and Cicero2005; Felgoise et al., Reference Felgoise, Stewart and Bremer2009; Burke et al., Reference Burke, Galvin and Pinto-Grau2017), we found a correlation between iQoL and ALS FRS-R at time point 4. This result indicates that a rapid decline in physical function among the patients had a negative impact on the relatives’ iQoL. Perhaps the prospective design, with measurements from diagnosis through disease trajectory, could explain the difference between the present study and previous studies.
The strength of this study is the prospective and longitudinal design, with relatives of newly diagnosed patients with ALS, and that there were few dropouts. Felgoise et al. (Reference Felgoise, Stewart and Bremer2009) emphasized that caution must be taken when using SEIQoL-DW of groups, which the results of the present study show. When looking at the mean score of the group, we may have missed those who suffer from poor iQoL, who are the ones that need to be in focus. Felgoise et al. (Reference Felgoise, Stewart and Bremer2009) also suggested that the SEIQoL-DW might be measuring happiness rather than QoL. The findings in the present study showed a high agreement between the SEIQoL index score and the SR-QoL index score, indicating that the SEIQoL-DW is a useful instrument for assessing iQoL.
The limitation of this study is the small sample size. Another limitation is that we did not use the weighting procedure to estimate the relative importance of the cues. The reason for this was the need to be able to collect data by a telephone interview if we failed to collect data in accordance with home visits. Even though studies have shown that the weighting procedure does not have an impact on the total QoL index (Wettergren et al., Reference Wettergren, Bjorkholm and Axdorph2005, Reference Wettergren, Bjorkholm and Langius-Eklof2008), it may provide valuable information that can be useful in clinical setting when evaluating how to best support the individuals. The participants were asked to nominate areas of importance for their iQoL, both those functioning well or poorly. The results showed that most of the areas nominated were functioning well, but the present study highlights the importance of the individual descriptions of the different cues for a better understanding of how to best support the relatives to maintain a good iQoL.
Even though the present study provided information on areas of importance for relatives’ iQoL, more knowledge is needed on specific kinds of support that relatives need and how to best provide this support on an individual basis. Relatives are often central in the care for patients with ALS and dedicate many hours to help and support the patient with daily activities (Krivickas et al., Reference Krivickas, Shockley and Mitsumoto1997), focusing on the sick relative (i.e., ALS patient) and seldom on their own needs (Larsson et al., Reference Larsson, Frojd and Nordin2015). Individual and qualitatively measurements of QoL can be used to identify factors that contribute to the experience of good or bad QoL among relatives of patients with ALS.
In conclusion, our study has shown that social relations with family and friends, but also health and leisure are important for iQoL, with both negative and positive impact. Even though most relatives rated their iQoL as relatively good and stable, the result indicates that a rapid decline in the patients’ physical function and emotional distress have a negative impact on iQoL among relatives of patients with ALS. To be able to support and help relatives in their situation, health professionals need to measure both emotional well-being and iQoL, from diagnosis and throughout the disease trajectory, with the focus on the relatives’ own descriptions of perceived iQoL and those factors contributing to their iQoL. The evaluation will give the relative and health professionals insight into areas of importance and how they are functioning. This information can help health professionals to provide individual support with the aim to help relatives to obtain a good iQoL and to manage their situation.
Acknowledgments
We thank the relatives of ALS patients who participated in the study. We would also like to thank the Statisticians Marcus Thuresson and Fabian Söderdahl for their support with the statistical analysis.
Funding
The research was funded by Uppsala University, Uppsala University Hospital, Ulla-Carin Lindqvist ALS Research Foundation, and Norrbacka-Eugenia Foundation.
Conflict of interest
None.