Conjugated linoleic acid (CLA) is a collective term to describe a class of positional and geometric isomers of linoleic acid (cis-9, cis-12 C18 : 2n-6) in which the two double bonds are separated by a single C-C bond, but not by a methylene group (-CH2-). CLA isomers are intermediate products of bacterial biohydrogenation of dietary unsaturated fatty acids in the rumen, and are mainly found in fat of ruminant milk and meatReference Griinari, Bauman, Yurawecz, Mossoba, Kramer, Pariza and Nelson1.
In the last 10 years, extensive research have indicated that commercial CLA preparations, fed to laboratory animals, showed several health-related properties, including anti-adipogenicReference Evans, Brown and McIntosh2–Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen5, anti-carcinogenicReference Belury6–Reference Cho, Kim, Lim, Kim, Sung, Kim and Park8, anti-atherogenicReference Kritchevsky, Tepper, Wright, Tso and Czarnecki9–Reference Toomey, Harhen, Roche, Fitzgerald and Belton15 and anti-inflammatoryReference O'Shea, Bassaganya-Riera and Mohede16, Reference Tricon, Burdge, Kew, Banerjee, Russell, Grimble, Williams, Calder and Yaqoob17 effects. Consequently, in view of the evident health-related properties associated with CLA isomers and also in line with the concept of functional foodsReference Aggett, Antoine and Asp18, attempts have been made to enrich animal-derived foods with CLA isomers through animal nutrition strategies. Indeed, these attempts resulted in production of functional food such as CLA-enriched milk (butter, cheese), ruminant and non-ruminant meat, as well as eggsReference Azain19, Reference Raes, De Smet and Demeyer20–Reference Schmid, Collomb, Sieber and Bee23.
Except for our preliminary studiesReference Szymczyk and Pisulewski24, there have been no data available on whether eggs, enriched naturally with CLA isomers, and fed to laboratory animals, could have health-related properties similar to those described for CLA isomers fed in free forms. Therefore, the major objective of the present study was to verify the potential health-related, namely anti-atherogenic, potency of CLA-enriched eggs, fed to apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ), representing a unique and reliable model of atherogenesisReference Jawien, Gajda, Rudling, Mateuszuk, Olszanecki, Guzik, Cichocki, Chlopicki and Korbut25–Reference Jawien, Csanyi, Gajda, Mateuszuk, Lomnicka, Korbut and Chlopicki27. We fed laying hens with commercial CLA mixture in a manner that allowed production of CLA-enriched eggsReference Szymczyk and Pisulewski28, Reference Franczyk-Żarów29. Subsequently, the eggs (egg yolks) were given to apoE/LDLR− / − mice with pre-established atherosclerosis. Anti-atherogenic properties of CLA-enriched eggs were compared with those of CLA-supplemented eggs, using the following criteria: (1) serum lipid profile; (2) development of atherosclerosis; and (3) composition of atherosclerotic plaque.
Materials and methods
Production of conjugated linoleic acid-enriched eggs
The feeding conditions to obtain CLA-enriched eggs were essentially the same as described previouslyReference Szymczyk and Pisulewski28. In order to determine the optimum concentration of dietary CLA, laying hens were assigned to five modified commercial layer diets, containing sunflower and rapeseed oils (20 and 40 g/kg, respectively), and the following graded levels of CLA oil (g/kg) : 0, 2·5, 5·0, 7·5 and 10. The CLA oil (Luta-CLA® 60), obtained from BASF (Ludwigshafen, Germany), contained 600 g CLA/kg, with equal representation of two major CLA isomers (cis-9, trans-11 and trans-10, cis-12). The CLA oil was substituted for an appropriate amount of sunflower oil to obtain the required CLA concentrations. After 4 weeks of feeding, eggs were collected and egg yolks analysed for fatty acids using a Shimadzu GC–MS analyser (Model QP 5050A; Shimadzu Corporation, Kyoto, Japan) Table 1. In addition, the eggs collected after 4 weeks were hard-boiled, egg yolks were separated and their hardness was evaluated on a Stable Micro Systems texture analyser (Model TA-XT2; Leeds UK) Table 2. In view of the obtained results, the optimum dietary CLA concentration in laying hen diets, for nutritional enrichment of hen eggs, was arbitrarily chosen to be 7·5 g/kg. Consequently, the laying hens were then fed the CLA-free (0 g/kg) and CLA-supplemented (7·5 g/kg) diets to produce an appropriate amount of freeze-dried CLA-free and CLA-enriched egg yolks, to be included into AIN-93G diets fed to apoE/LDLR− / − mice.
Table 1 Effect of feeding hens with conjugated linoleic acid (CLA)-enriched diets (0, 2·5, 5·0, 7·5 and 10 g/kg) on fatty acid composition (relative %) of egg-yolk lipids after 4 weeks of experiment
(Mean values with their standard errors)
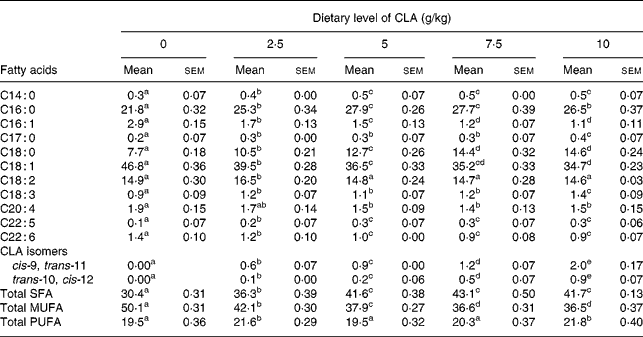
a–e Mean values within a row with unlike superscript letters were significantly different (P < 0·001; Duncan's multiple range test).
Table 2 Effect of feeding hens with conjugated linoleic acid (CLA)-enriched diets (0, 2·5, 5·0, 7·5 and 10 g/kg) and days of refrigeration on egg characteristics and egg yolk hardness after 4 weeks of experiment
(Mean values for CLA dietary level and days of refrigeration with their standard errors)
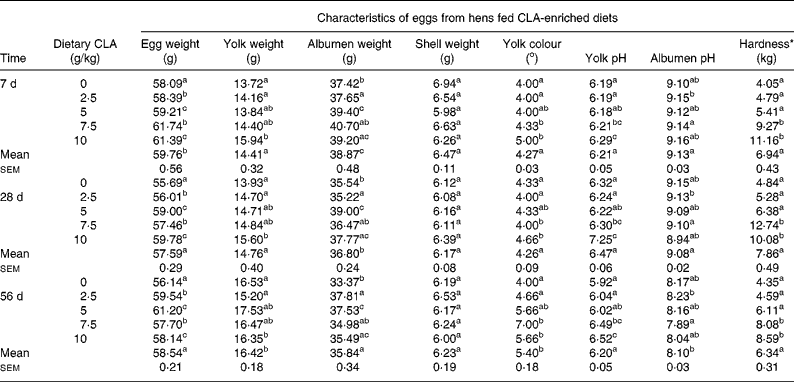
a,b,c Mean values for CLA dietary level effect within a column with unlike superscript letters were significantly different (P < 0·05; Duncan's multiple range test).
* Hardness determined for hard-boiled egg yolks using a Stable Micro Systems TA-XT2 texture analyser.
Animals and housing
The apoE/LDLR− / − mice used in the present studyReference Ishibashi, Herz, Maeda, Goldstein and Brown30 were obtained from the Cardiovascular Research Institute Maastricht, Maastricht University (Maastricht, The Netherlands) and bred in the animal house in Warszawa (Polish Academy of Science, Medical Research Centre). Animals were housed in colony cages in a temperature-controlled environment (22–25°C) with a 12 h light cycle. They had free access to food and water.
All procedures involving animals were conducted according to the Guidelines for Animal Care and Treatment of the European Union and were approved by the Local Animal Ethics Committee.
Diets and feeding
Up to the age of 4 months, the mice were fed a commercial, cholesterol-free, pelleted diet (Sniff M-Z Spezialdiäten GmbH, Soest, Germany). Diet and water, consumed ad libitum, were regularly checked and provided daily.
At the age of 4 months, mice (n 18) were randomly assigned to three experimental groups (n 6) and fed AIN-93G-based dietsReference Reeves, Nielsen and Fahey31 for the next 2 months.
The experimental diets were the following: (I) AIN-93G+ CLA-free egg-yolk powder (control); (II) AIN-93G+ CLA-free egg-yolk powder +0·1 % CLA (CLA-supplemented eggs); and (III) AIN-93G+ CLA-enriched egg-yolk powder providing 0·1 % CLA incorporated into egg-yolk lipids (CLA-enriched eggs). The detailed composition of these three experimental AIN-93G-based diets is shown in Table 3.
Table 3 Composition of the AIN-93G-based diets (g/kg) fed to apoE and LDL receptor double-knockout (apoE/LDLR−/−) mice
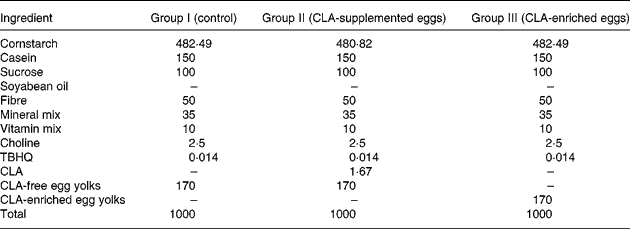
CLA, conjugated linoleic acid; TBHQ, Tert-buty lhydroquinone.
After 2 months of feeding with respective diets, the mice were deprived of food overnight, injected intraperitoneally with 1000 IU heparin (Sanofi-Synthelabo, Paris, France), and after 10 min, anaesthetized intraperitoneally with 40 mg sodium thiopental/kg (Biochemie, Vienna, Austria), and finally killed by cervical translocation.
Blood sampling and plasma lipid profile analyses
Blood samples were taken from the left ventricle of the heart, collected into test tubes and centrifuged (4000 g, 10 min) to obtain plasma samples. The samples were deep frozen ( − 80°C) and stored until further analysis. Plasma samples were analysed using commercially available kits for total cholesterol (TC; Liquick Cor-Chol 60 no. 2-204; Cormay, Lublin, Poland), LDL-cholesterol (LDL-C; no. OSR 6183; Olympus Diagnostica GmbH, Hamburg, Germany), HDL-cholesterol (HDL-C; no. OSR 6287; Olympus) and TAG (Liquick Cor-TG 30 no. 2-262; Cormay). The results are expressed in mmol/l.
Quantitation of atherosclerosis in aortic roots (cross-section analysis)
In anaesthetized mice, the thorax was longitudinally opened, the right atrium was incised and the heart was perfused by PBS (pH 7·4) through the apex of the left ventricle at a constant pressure of approximately 100 mmHg. Next, the heart and the ascending aorta were dissected. The excised heart and ascending aorta were embedded in OCT compound (CellPath, Oxford, UK) and snap-frozen. Cryosections (10 μm thick) were cut from the aortic root using a standardized protocolReference Nicoletti, Kaveri, Caligiuri, Bariety and Hansson32, Reference Elhage, Jawien, Rudling, Ljunggren, Takeda, Akira, Bayard and Hansson33. Serial sections were cut from the proximal 1 mm of the aortic root. Eight sections were collected at 100 μm intervals starting at a 100 μm distance from the appearance of the aortic valves. Sections were thaw-mounted on poly-l-lysine-coated slides and air dried. After fixation in 4 % paraformaldehyde (pH 7), sections were stained with Meyer's haematoxylin and oil red-O (Sigma-Aldrich, St Louis, MO, USA)Reference Robertson, Rudling, Zhou, Gorelik, Flavell and Hansson34. Oil red-O-stained sections were examined under an Olympus BX50 (Olympus, Tokyo, Japan) microscope and used for quantitative evaluation. Images of the aorta were recorded using an Olympus Camedia 5050 digital camera and stored as TIFF files of resolution 1024 × 768 pixels. The total area of the lesion was measured semi-automatically in each slide using LSM Image Browser 3 software (Zeiss, Jena, Germany). For each animal a mean lesion area was calculated from eight sections, reflecting the cross-section area covered by atherosclerosisReference Robertson, Rudling, Zhou, Gorelik, Flavell and Hansson34.
Quantification of atherosclerosis in descending aortas (en face analysis)
The aorta from arch to bifurcation was dissected out from surrounding tissues and fixed in 4 % formaldehyde. Then it was opened longitudinally, pinned on to brown silicone plates and stained with Sudan IV (Sigma-Aldrich). The aortic lesion area and total aortic area were measured fully automatically using specially designed algorithm and Aphelion software. Images of the aorta were recorded using a digital camera as RGB colour images. The images obtained were binarized on the basis of colour distribution in two areas of interested selected automatically at the image edges, i.e. outside the aorta. The resulting binary image was additionally filtered using mathematical morphology operations in order to remove small holes, traces of needles and other artifacts. In turn, the aortic lesion area was detected automatically using the minimum entropy criterion on grey-scale images, being a difference between the red and blue components of the initial image.
Analysis of atherosclerotic plaques composition by immunohistochemistry
For indirect immunohistochemistry, acetone-fixed cryosections of ascending aorta were used. Sections were cut and collected as described earlier. They were incubated overnight with primary antisera: Cy3-conjugated anti-smooth muscle α-actin (SMA; Sigma-Aldrich; diluted 1 : 600), rat anti-mouse CD68 (Serotec, Oxford, UK; diluted 1 : 800). Then, goat anti-rat IgG biotinylated antibodies were applied followed by DTAF-conjugated streptavidin (both from Jackson IR, West Grove, PA, USA). Sections were examined using an epifluorescence Olympus BX50 microscope equipped with appropriate filter cubes to show Cy3 (red), and DTAF (green) fluorescence for SMA and CD68, respectively. The images were registered with a Camedia 5050 digital camera.
In each section, the total area occupied by CD68-immunopositive macrophages was measured semi-automatically using LSM Image Browser software and expressed as a percentage of the whole area of atherosclerotic plaque. Atherosclerotic plaques with a continuous layer of SMA-positive cells at the internal part of the plaque were also counted and expressed as a percentage of the whole area of atherosclerotic plaque.
Statistical analysis
Results are expressed as means and their standard errors. Where appropriate, the data were subjected to one-way ANOVA generated by the Statistica version 6.1 package (StatSoft Inc., Tulsa, OK, USA), followed by post-hoc Duncan's multiple range test. The differences between treatment means were considered significant at P < 0·05. The data resulting from quantification of atherosclerosis were analysed by the non-parametric Mann–Whitney test and differences between treatment means were considered significant at P < 0·05.
Results
Effect of conjugated linoleic acid-supplemented and conjugated linoleic acid-enriched eggs on plasma lipid profile
The effect of dietary treatments on plasma lipoprotein concentrations is shown in Fig. 1. The only evident effect of dietary treatments was the reduction of plasma TC in mice fed CLA-enriched eggs as compared to the effect of CLA-supplemented eggs (P < 0·05). At the same time, dietary treatments had no significant effects on individual plasma lipoproteins. The same was true for plasma TAG.
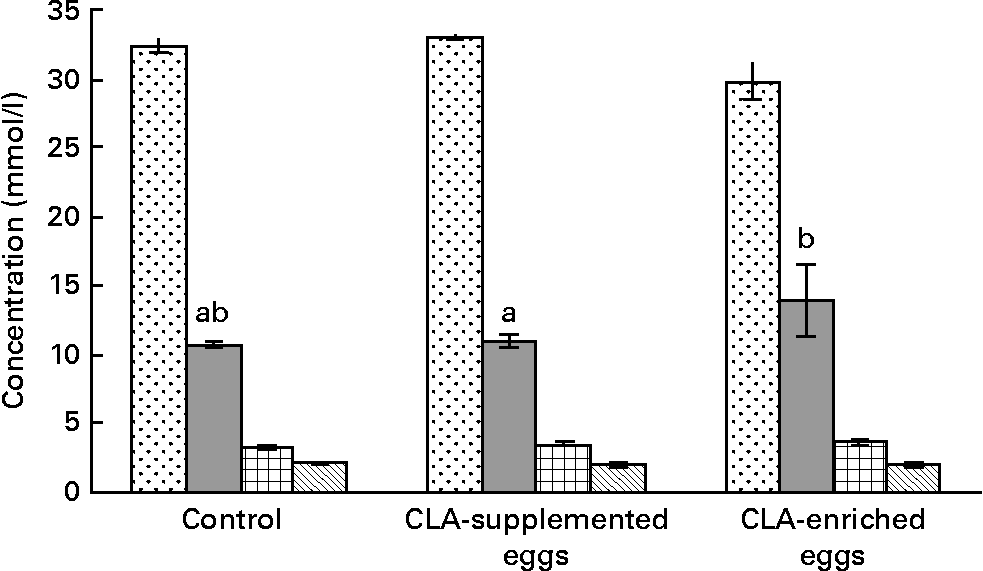
Fig. 1 Total cholesterol (![]() ), LDL-cholesterol (
), LDL-cholesterol (![]() ), HDL-cholesterol (
), HDL-cholesterol (![]() ) and TAG (
) and TAG (![]() ) concentrations in plasma in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control, conjugated linoleic acid (CLA)-supplemented and CLA-enriched eggs. Values are means with their standard errors depicted by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05; Duncan's multiple range test).
) concentrations in plasma in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control, conjugated linoleic acid (CLA)-supplemented and CLA-enriched eggs. Values are means with their standard errors depicted by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05; Duncan's multiple range test).
Effect of conjugated linoleic acid-supplemented and conjugated linoleic acid-enriched eggs on the development of atherosclerosis
Area of atherosclerosis as measured in whole aorta (en face) by Sudan IV staining was not different between experimental groups (Figs. 2 and 3). Also area of plaques as measured in aortic roots (cross-section) did not differ significantly between experimental groups (Figs. 4 and 5). However, in apoE/LDLR− / − mice fed CLA-supplemented or CLA-enriched eggs, the area of plaques tended to be lower as compared to the control group (1 075 661 v. 1 366 832 μm2 and 1 209 342 v. 1 366 832 μm2, respectively).
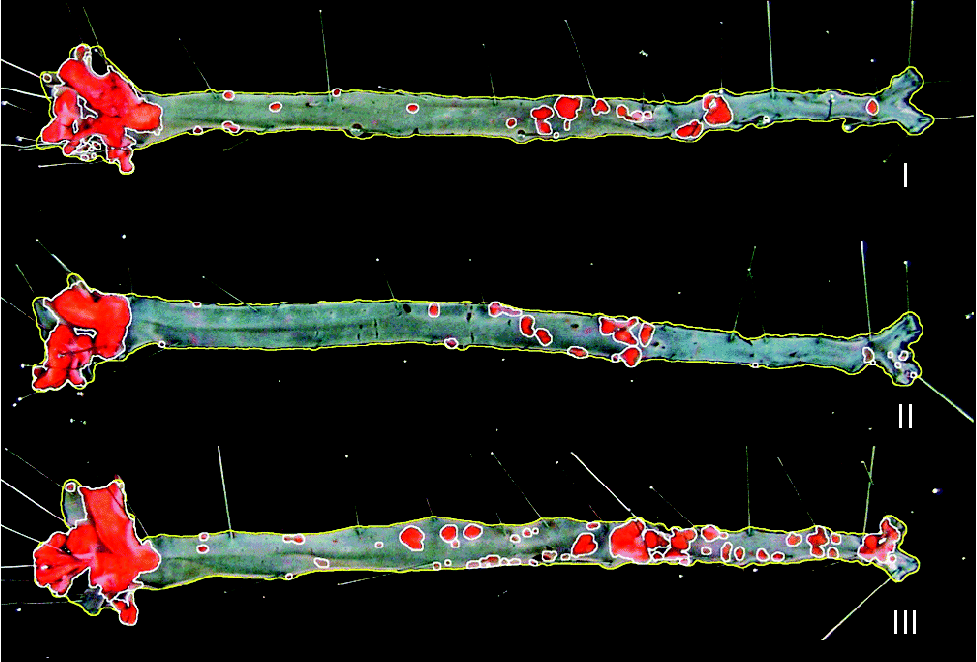
Fig. 2 Representative images of en face aorta showing atherosclerotic plaque in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control (I), conjugated linoleic acid (CLA)-supplemented (II) and CLA-enriched (III) eggs.
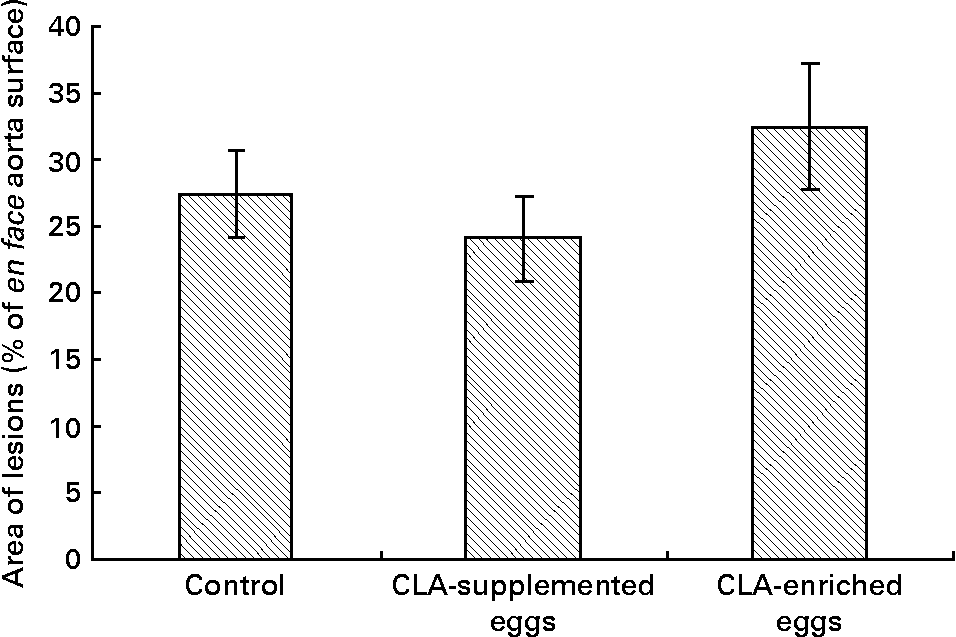
Fig. 3 Quantification (% area) of atherosclerotic plaque in entire aorta (en face) in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control, conjugated linoleic acid (CLA)-supplemented and CLA-enriched eggs. Values are means with their standard errors depicted by vertical bars.

Fig. 4 Representative images of cross-sections of aortic roots showing aortic plaque in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control (I), conjugated linoleic acid (CLA)-supplemented (II) and CLA-enriched (III) eggs.
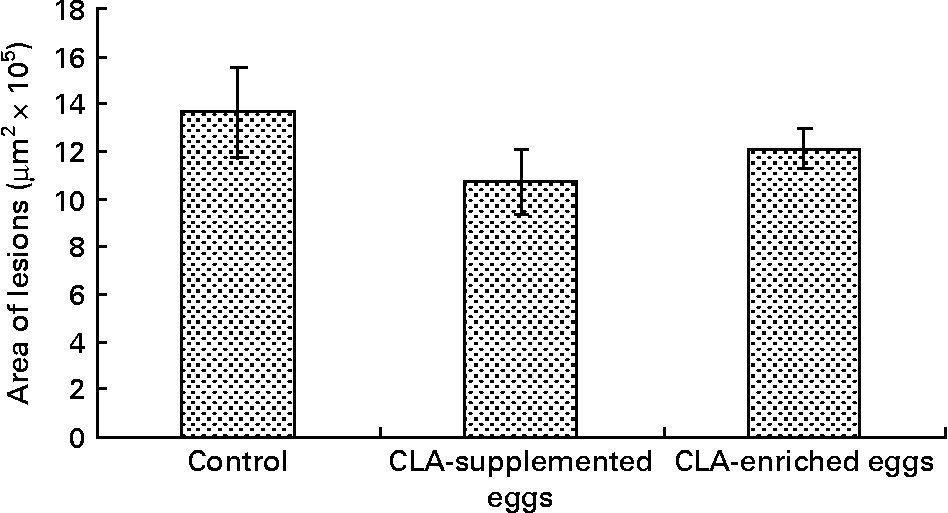
Fig. 5 Quantification (μm2) of atherosclerotic plaque area in aortic roots (cross-section) in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control, conjugated linoleic acid (CLA)-supplemented and CLA-enriched eggs. Values are means with their standard errors depicted by vertical bars.
Interestingly, despite the lack of significant effects of CLA-supplemented or CLA-enriched eggs on the size of atherosclerotic plaque, the diet containing CLA-enriched eggs had a pronounced effect (P < 0·05) on macrophage accumulation in atherosclerotic plaques as determined by immunohistochemical staining for a specific macrophage marker (CD68). Indeed, as shown in Fig. 6, the plaque area covered by macrophages in mice fed control and CLA-enriched diet was 60·9 and 32 %, respectively. In contrast, in mice fed CLA-supplemented eggs such an effect could not be seen.
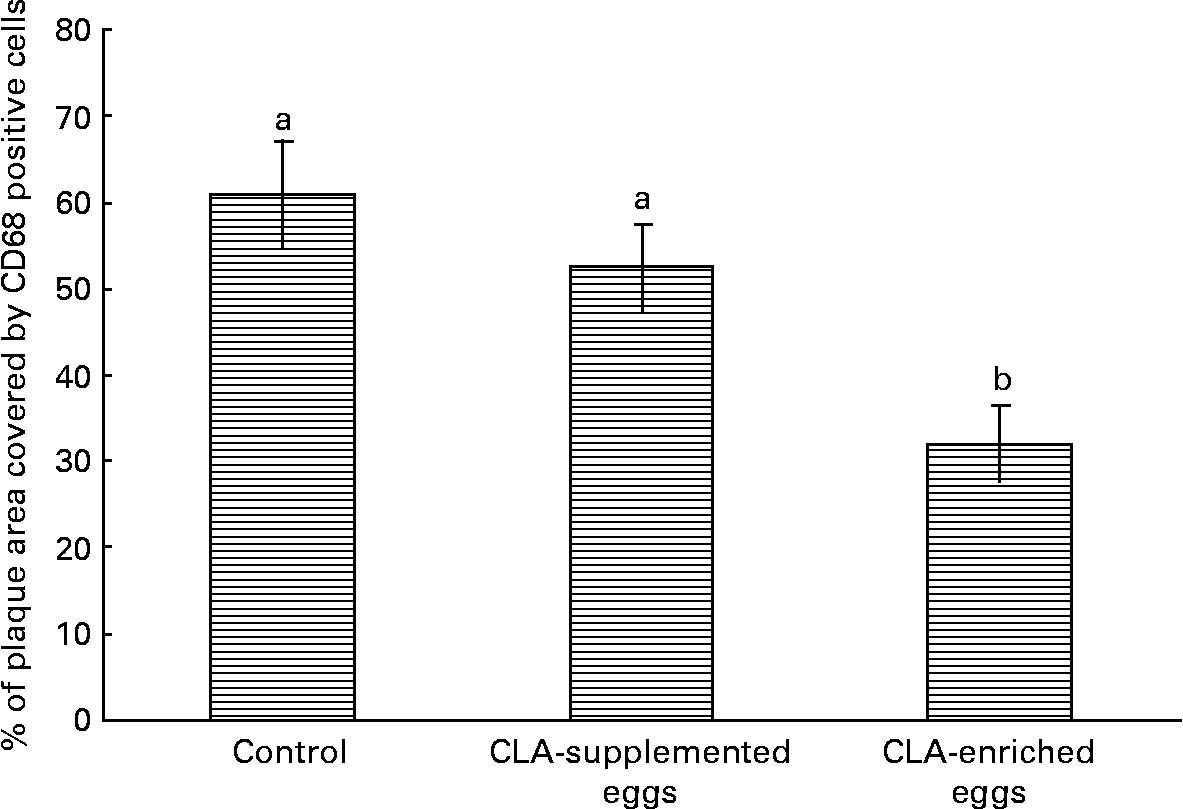
Fig. 6 Quantification (%) of macrophage accumulation in atherosclerotic plaque (cross-sections) as determined by immunohistochemical staining for a specific macrophage marker (CD68) in in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control, conjugated linoleic acid (CLA)-supplemented and CLA-enriched eggs. Values are means with their standard errors depicted by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05; non-parametric Mann–Whitney test).
The opposite changes were observed as regards area covered by SMA-positive cells in aortic plaques (Fig. 7). Indeed, the area covered by SMA-positive cells in aortic plaques was increased in mice fed CLA-enriched diet as compared to the control diet. Again, no such effect was noticed for CLA-supplemented diet. The present findings are considered to indicate that the diet containing CLA-enriched eggs, but not the one containing CLA-supplemented eggs, displays anti-inflammatory, anti-atherosclerotic effects characterized by a decreased occurrence of macrophages and increased presence of smooth muscle cells in the atherosclerotic plaques.

Fig. 7 Representative images of aortic roots (cross-sections) stained for a specific macrophage marker (CD68; green fluorescence) and anti-smooth muscle α-actin-positive cells (red fluorescence) in apoE and LDL receptor double-knockout mice (apoE/LDLR− / − ) fed control (I), conjugated linoleic acid (CLA)-supplemented (II) and CLA-enriched (III) eggs.
Discussion
The objective of the present study was to examine potential anti-atherogenic effects of eggs (egg yolks) naturally enriched with CLA isomers. In line with the concept of functional foodsReference Aggett, Antoine and Asp18, Reference Bellisle, Diplock, Hornstra, Koletzko, Roberfroid, Salminen and Saris35, Reference Roberfroid36, the present discussion will examine the eggs (egg yolks) naturally enriched with CLA isomers according to whether they: (1) can be considered conventional foods; (2) can be consumed as a part of a normal diet; (3) are composed of naturally occurring components; (4) have a positive effect on target function(s) beyond nutritive value; (5) enhance health and/or reduce the risk of disease; and (6) have scientifically based claims.
The present study clearly indicates that eggs naturally enriched with CLA isomers (as derived in the present experiment) preserve generally their composition and conventional properties (Tables 1, 2). However, it should be indicated that increasing dietary CLA concentrations (0·0–1·0 %) led to increased concentrations of CLA in egg-yolk lipids. Moreover, cis-9, trans-11 CLA was incorporated into egg-yolk lipids more efficiently than trans-10, cis-12 CLA. It should be also indicated that increasing dietary CLA concentrations altered relative proportions of SFA and MUFA in egg-yolk lipids (Tables 1 and 4). Namely the proportions of SFA (e.g. 16 : 0 and 18 : 0) were increased (P < 0·01), those of MUFA (e.g. 18 : 1n-9) decreased (P < 0·01), whereas concentrations of PUFA were not affected. Generally, when compared with the results of our earlier studies using much higher dietary concentrations of CLA (e.g. 0·0–2·0 %Reference Szymczyk and Pisulewski28), the changes noted in the present experiment were largely attenuated. Interestingly, the present inverse changes between SFA and MUFA concentrations tended to level off at the concentration of dietary CLA reaching 0·75 %. Moreover, dietary CLA had no apparent effects on sensory parameters of eggs (Table 2) during refrigerated storage (7, 28 and 56 d; 4°C). Thus, the present observations could be attributed to the period of the storage. On the other hand, dietary CLA increased consistently the firmness of hard-boiled egg yolks, notably at the concentrations of 0·75 and 1·0 % CLA, at which it tended to level off. In view of the present results we have arbitrarily chosen the dietary concentration of 0·75 % CLA as the optimum for laying hens, to enrich effectively hen egg yolks with CLA. The present results are generally consistent with the earlier findings and were thoroughly discussed and elucidated by Schäfer et al. Reference Schäfer, Männer, Sagredos, Eder and Simon37, Raes et al. Reference Raes, Heyghebaert, De Smet, Nollet, Arnouts and Demeyer38, Szymczyk & PisulewskiReference Szymczyk and Pisulewski28, and more recently by Shang et al. Reference Shang, Wang, Li, Yin and Li39 and Cachaldora et al. Reference Cachaldora, Garcia-Rebollar, Alvarez, Mendel and de Blas40.
Table 4 Effect of feeding hens with conjugated linoleic acid (CLA)-enriched diets (0 and 7·5 g/kg) on fatty acid composition (relative %) of egg-yolk lipids (egg yolks destined to be freeze-dried and fed to apoE and LDL receptor double-knockout (apoE/LDLR−/−) mice
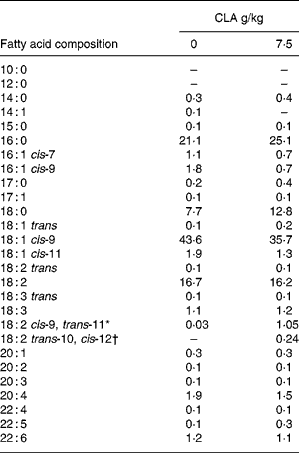
*Concentration of cis-9, trans-11 CLA isomer in freeze-dried egg yolks : 641 mg/100 g (7·5 g dietary CLA).
†Concentration of trans-10, cis-12 CLA isomer in freeze-dried egg yolks : 143 mg/100 g (7·5 g dietary CLA).
The CLA-enriched eggs, as derived in the present experiment and considered to preserve the conventional properties of hen eggs, can be consumed as a part of the normal human diet. In fact, the concept of functional foods (i.e. nutritionally enhanced eggs) has been already advocated by HaslerReference Hasler41. As emphasized by Raes et al. Reference Raes, Heyghebaert, De Smet, Nollet, Arnouts and Demeyer38, these eggs can be considered a good source of CLA isomers, supplementing those derived from ruminant products (milk and meat), naturally rich in CLA. What is more, the functional eggs, naturally enriched with n-3 PUFA, are currently commercialized in Europe and in the USA. Therefore, the consumption of functional eggs enriched naturally with both CLA and n-3 PUFA in usual human diets is fully feasibleReference Raes, Heyghebaert, De Smet, Nollet, Arnouts and Demeyer38, Reference Surai and Sparks42.
The CLA-enriched eggs (as derived in the present experiment), are becoming a good source of naturally occurring components, i.e. CLA isomers, showing several health-related properties (see Introduction). Generally, the predominant CLA isomers in ruminant fat are cis-9, trans-11 CLA, that accounts for more than 80 % of total isomers, and trans-10, cis-12 CLAReference Griinari, Bauman, Yurawecz, Mossoba, Kramer, Pariza and Nelson1. In the CLA preparations currently available, two isomers, i.e. cis-9, trans-11 and trans-10, cis-12 CLA, usually predominate and are represented in equal amounts, whereas the older CLA preparations contained also trans-8, cis-10 and cis-11, trans-13 CLAReference Kramer, Cruz-Hernandez, Deng, Zhou, Jahreis and Dugan43. Therefore, the use of a current product (Luta-CLA® 60) in the present studies decreased the risk of incorporation of CLA isomers of unknown biological activity into egg-yolk lipids. What is more, the ratio of cis-9, trans-11 to trans-10, cis-12 isomers in freeze-dried egg yolks was 80 : 20, thus reflecting the ratio of these isomers in natural sources of CLA (e.g. milk fat). This preferential incorporation of cis-9, trans-11 isomer into egg-yolk lipids has been already reported by Raes et al. Reference Raes, Heyghebaert, De Smet, Nollet, Arnouts and Demeyer38 and Szymczyk & PisulewskiReference Szymczyk and Pisulewski28.
As recommended by Aggett et al. Reference Aggett, Antoine and Asp18, the beneficial health-related effects of CLA-enriched eggs were evaluated using three types of biological marker: (1) markers of exposure to a biologically active food component; (2) markers of biological response; and (3) markers of intermediate end-point.
In the present experiment carried out on apoE/LDLR− / − mice, we have not evaluated the degree of exposure of the mice to dietary CLA isomers by measuring the CLA concentrations in body fluids. However, feeding CLA isomers to man was clearly reflected by elevated blood concentration of CLAReference Belury, Mahon and Banni44, Reference Burdge, Lupoli and Russell45. What is more, CLA isomers were most probably incorporated into egg-yolk phospholipids, and as such, could have been more efficiently absorbed, compared with their absorption from TAGReference Payet, Esmail, Polichetti, le Brun, Adjemout, Donnarel, Portugal and Pieroni46.
Among the markers of biological response, the serum lipid profile of the experimental mice was evaluated in detail. Generally, the serum lipid profile was typical for our animal model, i.e. apoE/LDLR− / − 30. However, the present studies produced equivocal results. In the mice fed CLA-enriched eggs, total plasma cholesterol was significantly (P < 0·05) decreased (Fig. 1). In contrast, concentrations of individual plasma lipoproteins were not affected and only tended to increase (LDL-C and HDL-C) or decrease (TAG). The results of early studies, using CLA commercial oils, are also inconclusive. In an early experiment of Lee et al. Reference Lee, Kritchevsky and Pariza47, CLA treatment decreased TC and LDL-C, as well as plasma TAG in rabbits. Also, in studies of Nicolosi et al. Reference Nicolosi, Rogers, Kritchevsky, Scimeca and Huth48, the hamsters fed CLA showed significantly reduced levels of plasma TC, LDL-C+VLDL-cholesterol and TAG with no effect on HDL-C. As reported by Munday et al. Reference Munday, Thompson and James49, dietary CLA did not produce significant differences in serum TC or HDL-C in mice. At the same time, the mice fed CLA showed significantly higher HDL-C : TC ratio and significantly lower TAG concentrations compared to control animals. In contrast to the present findings, increasing dietary CLA in rabbits (0·0–1·0 %) led to higher serum TC and TAG levelsReference Kritchevsky, Tepper, Wright, Tso and Czarnecki9. Interestingly, the above effects were attenuated at lower dietary CLA concentrationsReference Kritchevsky, Tepper, Wright and Czarnecki10. In studies involving healthy human subjectsReference Noone, Roche, Nugent and Gibney11, isomeric blends of CLA isomers (cis-9, trans-11 : trans-10, cis-12; 50 : 50 and 80 : 20) were used. The 50 : 50 CLA isomer blend reduced plasma TAG concentrations whereas the 80 : 20 CLA isomer blend reduced VLDL-cholesterol concentrations. More recently, isomer-specific effects of CLA were studied in detail. It was shown, that cis-9, trans-11 CLA increased plasma HDL-C as well as HDL-C : LDL-C ratioReference Valeille, Gripois, Blouquit, Souidi, Riottot, Bouthegourd, Serougne and Martin13. Opposing effects of cis-9, trans-11 and trans-10, cis-12 CLA were also reported by Tricon et al. Reference Tricon, Burdge and Kew12. Namely the former isomer decreased favourably the LDL-C : HDL-C ratio and the TC : HDL-C ratio in healthy human subjects, whereas the latter increased them.
A single attempt to verify potential anti-atherogenic properties of eggs (egg yolks) naturally enriched with CLA was also madeReference Szymczyk and Pisulewski24. The rats fed these eggs (egg yolks) showed favourable changes in plasma lipids: plasma TC and LDL-C were decreased, whereas concentrations of HDL-C were increased. No changes in serum TAG concentrations were observed and liver cholesterol was sharply decreased (~50 %) in rats fed the CLA-enriched egg yolks.
Very recently, hypocholesterolaemic effects of CLA-enriched butter on hamsters were studiedReference Lock, Horne, Bauman and Salter50, Reference Valeille, Ferezou, Parquet, Amsler, Gripois, Quignard-Boulange and Martin51. In both experiments, CLA-enriched butter altered plasma lipoprotein profiles (lower TC and LDL-C, higher HDL-C) that could be associated with a reduced risk of atherosclerosis. No such effects were noted in human subjects who were offered dairy products with naturally incorporated CLAReference Desroches, Chouinard, Galibois, Corneau, Delisle, Lamarche, Couture and Bergeron52, Reference Tricon, Burdge and Jones53.
Several mechanisms have been proposed to explain the effects of CLA isomers on plasma lipid profiles in laboratory animals and man. It was postulated that CLA may reduce the number of LDL particles by inhibition of hepatic synthesis of apoB-containing lipoproteinsReference Yotosumoto, Hara and Naka54. CLA may also increase the LDL receptor activity and thus increase the clearance rate of LDL in blood circulationReference Grundy and Denke55. According to Yeung et al. Reference Yeung, Yang and Huang56, CLA down-regulates intestinal acyl CoA:cholesterol acyltranferase activity, increases the excretion of sterols and consequently decreases serum cholesterol concentration in hamsters.
With the advent of nutritional genomics, novel mechanisms by which dietary CLA might modulate plasma lipids have been suggestedReference Fernandez and West57. CLA are considered to be ligands of nuclear transcription factors, i.e. PPAR-α and sterol regulatory element binding protein-1, which are involved in lipid hepatic metabolism. For instance, activation of PPAR-α by CLA may lead to several effects, e.g. reduction of fatty acid and TAG synthesis and VLDL production. Sterol regulatory element binding protein-1 is a regulator of genes encoding enzymes involved in both lipogenesis and cholesterol biosynthesis, thus providing a mechanism by which CLA may decrease plasma cholesterol and TAG levels.
The third type of biological marker chosen in the present experiment was development of pre-established atherosclerosis and composition of atherosclerotic plaque in apoE/LDLR− / − mice. ApoE/LDLR− / − mice represent a unique and reliable model of atherogenesis that allows quantification of anti-atherogenic activities of pharmacological toolsReference Jawien, Gajda, Rudling, Mateuszuk, Olszanecki, Guzik, Cichocki, Chlopicki and Korbut25–Reference Jawien, Csanyi, Gajda, Mateuszuk, Lomnicka, Korbut and Chlopicki27. Using this model, we assessed the functional (anti-atherogenic) effects of CLA-enriched eggs. We found that treatment of apoE/LDLR− / − mice with CLA-enriched or CLA-supplemented eggs tended to, but did not consistently, diminish the area of atherosclerotic plaque. This may be due to shorter treatment time (2 months) as compared to our previous studies (4 months)Reference Jawien, Gajda, Rudling, Mateuszuk, Olszanecki, Guzik, Cichocki, Chlopicki and Korbut25–Reference Jawien, Csanyi, Gajda, Mateuszuk, Lomnicka, Korbut and Chlopicki27 as well as the starting point of the treatment in mice, at the age of 4 months, at which apoE/LDLR− / − mice have already established atherosclerosis. Quite surprisingly, we found that CLA-enriched eggs, but not CLA-supplemented eggs, considerably attenuated inflammatory vascular response, by reducing the number of macrophages in atherosclerotic plaques. At the same time, CLA-supplemented eggs increased the abundance of smooth muscle cells in these plaques. It is well known that the intensity of vascular inflammatory response in atherosclerosis depends on the abundance and activation of macrophages as well as other inflammatory cells and is inversely associated with low abundance of homogenous area covered by smooth muscle cellsReference Elhage, Jawien, Rudling, Ljunggren, Takeda, Akira, Bayard and Hansson33. Thus, the present results support the conclusion that CLA-enriched eggs limited vascular inflammatory response. Consequently, CLA-enriched eggs exerted the anti-inflammatory and anti-atherogenic effect more effectively than CLA-supplemented eggs, at the same level of dietary intake (0·1 %). This striking difference in anti-atherogenic effects of the CLA-supplemented and CLA-enriched diets may perhaps be explained by different proportions of cis-9, trans-11 and trans-10, cis-12 CLA isomers in the CLA-supplemented eggs (50 : 50) and CLA-enriched eggs (80 : 20). Moreover, the present findings would strongly support the notion of the opposing effects of cis-9, trans-11 and trans-10, cis-12 CLA isomers in manReference Tricon, Burdge and Kew12. It was shown recentlyReference Toomey, Harhen, Roche, Fitzgerald and Belton15 that feeding an 80 : 20 isomeric blend of cis-9, trans-11 and trans-10, cis-12 CLA isomers, at a high dietary concentration of 1·0 %, to apoE-deficient mice with pre-established atherosclerosis resulted in its profound resolution. It was accompanied by reduced expression of CD68 within the aortic lesions and down-regulation of the expression of pro-inflammatory genes.
The above effects could be potentially associated with incorporation of CLA isomers into atherosclerotic plaques. Indeed, it has been shown already that dietary n-3 PUFA (derived from fish oils) were readily incorporated into atherosclerotic plaques of men, reduced macrophage numbers within the plaques and thus the probability of ruptureReference Thies, Garry, Yaqoob, Rerkasem, Williams, Shearman, Gallager, Calder and Grimble58. Very recently, Ringseis et al. Reference Ringseis, Muller, Dusterloh, Schleser, Eder and Steinhart59 clearly demonstrated that both cis-9, trans-11 and trans-10, cis-12 CLA isomers were incorporated and metabolized in human vascular endothelial cells to biologically active compounds, mediating the anti-inflammatory and anti-atherogenic actions of CLA.
In conclusion, the above evaluation of functional properties of eggs (egg yolks) naturally enriched with CLA isomers (cis-9, trans-11 and trans-10, cis-12) supports generally our hypothesis that CLA-enriched eggs can be considered a functional food. These products: preserve conventional characteristics of hen eggs; can be consumed in conventional human diets; and contain the CLA isomers that are natural and active components of ruminant fat. Moreover, the evaluation of health-related effects of CLA-enriched eggs indicated that CLA-enriched eggs reduced significantly (P < 0·05) total plasma cholesterol in the atherosclerotic mice. Also, CLA-supplemented and CLA-enriched eggs tended to lower the severity of atherosclerosis as evaluated in aortic roots. Furthermore, the mice fed CLA-enriched eggs showed significantly (P < 0·05) reduced expression of atherogenic macrophages in aortic plaques and increased expression of SMA in aortic plaques of these mice (in aortic roots). Consequently, CLA-enriched eggs could have exerted the anti-inflammatory and anti-atherogenic effect more effectively than CLA-supplemented eggs, at the same level of dietary intake (0·1 %). This effect can be considered a major functional claim of CLA-enriched eggs. To date, there have been no studies on whether CLA, fed as an integral component of eggs, has functional (i.e. anti-atherogenic) properties, similar to those of CLA isomers fed as NEFA. Interestingly, the urgent need for studies using direct measures of arterial plaques, in order to assess how nutritional intervention can influence the development of atherosclerosis, has been recently expressedReference Hamer and Steptoe60.













