Child social environment, according to social epidemiological studies, typically refers to shared features of child's social ecology, over and above individual-level exposure. Social environmental factors may include urbanicity, neighbourhood socio-economic status (e.g. income distribution, levels of unemployment), residential segregation, levels of crime, local building condition or the amount of public parks etc. in an urban context, but it may also include neighbourhood social cohesion, availability of a social support network or of mental health services (Gayer-Anderson & Morgan, Reference Gayer-Anderson and Morgan2013).
Recently, genotype by environment interaction (G × E) studies have moved their focus towards those features of social environment that could moderate the effects of genetic factors on mental disorders both in youths and in adults (Table 1).
Twin studies have suggested that the heritability of many phenotypes is modified by social environmental characteristics. That is, genetic influences on a phenotype become attenuated whenever external factors limit personal choice (e.g. the social constraint which once limited the use of tobacco in women) or provide so much of a ‘social push’ encouraging problematic behaviour that the importance of genetic factors diminish (Raine, Reference Raine2002). For example, in boys, the heritability of adolescent antisocial behaviour has been shown to vary by social context, being higher in a socio-economically advantaged environment where the social risk factors that push or predispose an adolescent to behave antisocially are lacking (Tuvblad et al. Reference Tuvblad, Grann and Lichtenstein2006). Similarly, the heritability of adolescent substance use and rule-breaking behaviour turned out to be higher in urban environments than in rural environments (Legrand et al. Reference Legrand, Keyes, McGue, Iacono and Krueger2008). Contrarily, according to the stress-diathesis model, genetic vulnerabilities should increase in the presence of adversities, e.g. affiliation with delinquent peers has been shown to moderate genetic influences on adolescent conduct problems, with genetic effects accounting for more of the variance in problem behaviour when individuals were exposed to higher levels of peer antisocial behaviour (Button et al. Reference Button, Corley, Rhee, Hewitt, Young and Stallings2007).
Even though it is not currently clear how broader social environment and genes interact to produce complex behaviour (constraining/eliciting v. diathesis/stress) on the basis of this evidence, measured genotype–phenotype association studies recently moved their attention towards the effect of the broader social environment in children and adolescents too.
In 2004, Kaufman and co-workers, for the first time, examined social support indices together with genetic factors in predicting depression in maltreated children. This study demonstrated that risk for depression associated with the short (S) allele of the serotonin transporter polymorphism (5-HTTLPR) and stressful life events was moderated by social support quality and availability. These results were confirmed in a later study (Kaufman et al. Reference Kaufman, Yang, Douglas-Palumberi, Grasso, Lipschitz, Houshyar, Krystal and Gelernter2006) that revealed a gene by gene interaction between brain-derived neurotrophic factor (BDNF) and 5-HTTLPR, and a moderating role of positive social environmental factors. These data suggested that the negative sequelae associated with early stress are not inevitable. Risk for negative outcomes may be modified by both genetic and environmental factors, with the quality and availability of social supports among the most important environmental factors in promoting resilience, even in the presence of genotypes otherwise expected to predispose to mental illness.
Subsequently, Sjöberg et al. (Reference Sjöberg, Nilsson, Nordquist, Ohrvik, Leppert, Lindström and Oreland2006) reported a gender-modulation on the interaction between psychosocial background variables and 5-HTTLPR. Males and females carrying the S allele of the 5-HTTLPR responded to different environmental factors. Whereas males were negatively affected by living in public housing rather than in their own owned homes and by living with separated parents, females were affected by traumatic conflicts within the family. Furthermore, the responses of males and females carrying the short 5-HTTLPR allele to environmental stress factors went in opposite directions; whereas females tended to develop depressive symptoms, males seemed to be protected from depression.
Further evidence, that G × E interactions between 5-HTTLPR and broader social environment could influence risk for depressive symptoms and that this effect is modified by gender, was reported by Uddin et al. (Reference Uddin, Koenen, de Los Santos, Bakshis, Aiello and Galea2010). In males, county-level environments modified the association between 5-HTTLPR genotype and depressive symptoms across a one-year period, even when controlling for potential family-level confounders. No G × E associations were detected in adolescent females. County-level deprivation, assessed as the proportion of households receiving public assistance, turned out to be a reliable and specific environmental risk factor as in a further study on the same sample, using building maintenance level as a measure of exposure to poor social environment, no evidence of G × E effect was found (Uddin et al. Reference Uddin, de los Santos, Bakshis, Cheng and Aiello2011).
It is worth noting that the G × E effect involving broader social environment was mainly detected among males in contrast to previous reports (for a review see Bellani et al. Reference Bellani, Nobile, Bianchi, van Os and Brambilla2013), which suggested a preponderance of G × E interactions among adolescent females when the environmental risk is measured as stressful life events. More generally, these results suggest that among adolescents, macro-social context may have differential effects by gender, such that adolescent males are more susceptible to contextual effects than their female counterparts.
One possible explanation is that the variables that affect males are associated with social status, while those affecting females are more associated with human relationships. However, the results could reflect a true difference between the sexes, which in turn might reflect a difference in the interaction between the 5-HTTLPR polymorphism and, for example, gonadal and/or adrenocortical hormones.
Finally, moderation by the broader social environment, assessed as exposure to antisocial behaviour within adolescents’ peer groups, was evaluated for the association between CHRM2, a gene encoding the muscarinic acethylcoline receptor M2 and implicated in neurocognitive process such as disinhibition on the one hand, and externalizing trajectories on the other in a population-based follow-up study (Latendresse et al. Reference Latendresse, Bates, Goodnight, Lansford, Budde, Goate, Dodge, Pettit and Dick2011). Findings suggested that CHRM2 was associated with altered developmental patterns of externalizing behaviour from early adolescence through to young adulthood, and that this association was exacerbated among those exposed to higher levels of peer group antisocial behaviour.
In conclusion, the data emerging from this novel field of G × E investigation suggest the importance of including macro-social environmental features in future research in population-based representative samples. Even though the relative risk of disease conferred by the social environment is lower than that conferred by individual-level risk factors, the pervasiveness of exposure to broader social variables suggests that their role in determining the risk of externalizing or internalizing behaviour at population level will be considerable. Furthermore, macro-social variables could play the role of potential confounders in G × E studies which limit environmental measures to individual-level features. Much more work is needed to replicate or refute the findings reported here and to understand the mechanisms underlying these observations (Sündermann et al. Reference Sündermann, Onwumere, Bebbington and Kuipers2013).
Table 1. Summary of the studies described in this review
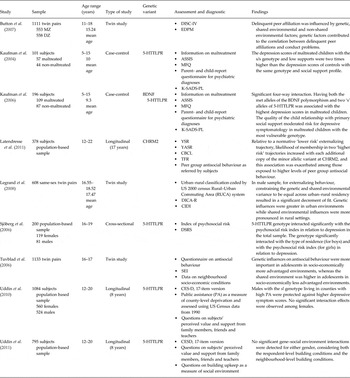
ASSIS, Arizona Social Support Interview Schedule; CBCL, Child Behavior Checklist; CESD, Center for Epidemiological Studies Depression Scale; CIDI, Composite International Diagnostic Interview; DICA-R, Diagnostic Interview for Children and Adolescents – Revised; DISC-IV, Diagnostic Interview Schedule for Children-IV; DSRS, Depression Self-Rating Scale; EDPM, Exposure to Delinquent Peers Measure; K-SADS-PL, Schedule for Affective Disorders and Schizophrenia for School Aged Children; MFQ, Mood and Feelings Questionnaire; SEI, Socio Economic Index; TRF, Teacher Report Form; YASR, Young Adult Self-Report; YSR, Youth Self-Report.



