Introduction
Mites of the family Camerobiidae (Acariformes, Prostigmata, Raphignathoidea) are recognizable by an almost round (especially when it comes to specimens mounted on microscope slides), dorsoventrally flattened idiosoma that is suspended on long, slender, stilt-like legs, mostly with long setae, which yield a characteristic appearance (Bolland, Reference Bolland1986, Reference Bolland1991). Camerobiids are free-living predators of small invertebrates (e.g., plant-associated mites and crawlers of scale insects [Hemiptera, Coccoidea]). Camerobiids hunt their prey on aboveground vegetation (including tree bark) but have also been found in the litter (Gerson et al., Reference Gerson, Smiley and Ochoa2003; Walter et al., Reference Walter, Lindquist, Smith, Cook, Krantz, Krantz and Walter2009). In the literature, more than 160 extant species have been described in seven genera: Acamerobia Fan and Walter, Reference Fan and Walter2011; Bisetulobius du Toit, Theron, and Ueckermann, Reference du Toit, Theron and Ueckermann1998; Camerobia Southcott, Reference Southcott1957; Decaphyllobius Bolland, Reference Bolland1986; Neophyllobius Berlese, Reference Berlese1886; Tillandsobius Bolland, Reference Bolland1986; and Tycherobius Bolland, Reference Bolland1986. Most species are monotypic, and the reason for this is the single, rare occurrence of these mites, which probably do not occur in larger aggregations as adults (Bolland, Reference Bolland1986, Reference Bolland2001). The historical aspect of the developing systematics hypotheses of the family and its constituent genera was summarized by Fan and Walter (Reference Fan and Walter2011).
Baltic amber is one of the richest sources of animal remains, which are exceptionally well preserved in the form of three-dimensional inclusions. Despite the long history of research on fossil organisms (Koch and Berendt, Reference Koch, Berendt and Berendt1854 published the first study that included mites), new information is continually provided on the structure of the so-called Eocene Amber Forests (Weitschat and Wichard, Reference Weitschat and Wichard2002; Seyfullah et al., Reference Seyfullah, Beimforde, Corso, Perrichot, Rikkinen and Schmidt2018). In addition, because of a diverse paleoacarofauna including the presence of minute and weakly sclerotized mites, this Lagerstätte is essential for further paleoacarological studies (Sidorchuk, Reference Sidorchuk2018).
The fossil record of the superfamily Raphignathoidea is scarce and consists of only two described species: Mediolata eocenia Kuznetsov, Khaustov, and Perkovsky, Reference Kuznetsov, Khaustov and Perkovsky2010 (Stigmaeidae) from Rovno amber and Neophyllobius succineus Bolland and Magowski, Reference Bolland and Magowski1990 from Baltic amber (Dunlop et al., Reference Dunlop, Penney and Jekel2019). None of the studied inclusions was subjected to appropriate grinding techniques (which were developed later) helpful for studying microarthropods (Sidorchuk, Reference Sidorchuk, Azar, Engel, Jarzembowski, Krogmann, Nel and Santiago-Blay2013; Sidorchuk and Vorontsov, Reference Sidorchuk and Vorontsov2018), and quality pictures of the specimens were not taken (there is only one black-and-white photograph in Kuznetsov et al., Reference Kuznetsov, Khaustov and Perkovsky2010).
This work presents descriptions of two new fossil species, Neophyllobius electrus n. sp. and N. glaesus n. sp., which were found in samples of Baltic amber (Fig. 1). These decriptions are accompanied by detailed images, line drawing interpretations, and reconstructions of their habitus. Thus, the paper expands the knowledge about the Camerobiidae mites inhabiting the extinct Eocene ecosystem where resin originated.

Figure 1. Habitus of studied inclusions: (1) Neophyllobius glaesus n. sp. (SMNG 07/36290-78a); (2) Neophyllobius electrus n. sp. (GPIH 4995a). Scale bars = 100 μm.
Materials and methods
Materials
1. Baltic amber sample with two inclusions determined by Ekaterina Sidorchuk as postlarval Camerobiidae and adult Phthiracaroidea from the Senckenberg Museum of Natural History Görlitz, Am Museum 1, 02826 Görlitz, Germany, under the collection (Sammlung Oribatida) number SMNG 07/36290-78.
2. Baltic amber with a representative of Camerobiidae from the private collection of the author and subsequently donated and deposited in the Geological–Paleontological Institute and Museum of the University of Hamburg, now CeNak—Centrum für Naturkunde, ‘Geomatikum,’ Bundesstraße 55, 20146 Hamburg, Germany, under the collection number GPIH 4995; this institution is its final deposition after preparation and study.
Amber preparation
Amber samples were cut using a handheld cutting tool (Proxxon Micromot 60/E), and then obtained pieces were polished according to methods of preparation described by Sidorchuk (Reference Sidorchuk, Azar, Engel, Jarzembowski, Krogmann, Nel and Santiago-Blay2013), using the tools introduced by Sidorchuk and Vorontsov (Reference Sidorchuk and Vorontsov2016, Reference Sidorchuk and Vorontsov2018). Because of cracks along a natural amber fissure and the small size of the preparation, some fragments were embedded in Buehler EpoThin 2 epoxy resin between two round glass coverslips. One sample that had not been embedded in the resin was placed in a test tube filled with aqueous thymol solution. All preparations are labeled following the information given in this paper.
Observations and imaging
Observations were done with a light compound microscope Nikon Eclipse Ni-U equipped with differential interference contrast (DIC) optics, 10× plan apochromatic dry, and 40× and 60× apochromatic water-immersion lens objectives. Image stacks were obtained with a Nikon DS-Ri2 microscope camera using Nikon NIS-Elements D imaging software (Nikon Corporation). All images were corrected for light, tone, noise, and sharpness using Adobe Photoshop Lightroom (Adobe Systems). Layered images were obtained by processing the focal planes with Helicon Focus Pro (Helicon Soft Ltd) rendering method A; minor retouch of the final image has been conducted to make some morphological structures visible. Drawings are interpretations of studied inclusions and were made with Adobe Photoshop (Adobe Systems) with the aid of a graphic tablet Wacom Intuos Pro on the basis of obtained pictures. Reconstructions of the species’ habitus are based on the studied material, data from the literature, and pictures of living specimens. Original images are available through Figshare data-set collections (see details provided in the Material sections of individual descriptions of species).
Measurements
All measurements are made and given herein in micrometers (μm), and they are rounded to the nearest integer. Measurements have been made with the aid of Nikon NIS-Elements D (Nikon Corporation) calibrated for used objectives. Due to the nature of preservation, measurements of organs and body parts that are oriented in three-dimensional space should be considered as minimum estimates.
Repositories and institutional abbreviations
Type specimens examined in this study are deposited in the following institutions: Senckenberg Museum of Natural History Görlitz (SMNG), Görlitz, Germany, and Geological–Paleontological Institute and Museum of the University of Hamburg (GPIH), now CeNak—Centrum für Naturkunde, Hamburg, Germany.
Systematic paleontology
Class Arachnida Cuvier, Reference Cuvier1812
Superorder Acariformes Zakhvatkin, Reference Zakhvatkin1952
Order Trombidiformes Reuter, Reference Reuter1909
Suborder Prostigmata Kramer, Reference Kramer1877
Supercohort Eleutherengonides Oudemans, Reference Oudemans1909
Cohort Raphignathina Kethley, Reference Kethley and Parker1982
Superfamily Raphignathoidea Kramer, Reference Kramer1877
Family Camerobiidae Southcott, Reference Southcott1957
Genus Neophyllobius Berlese, Reference Berlese1886
Type species
Neophyllobius elegans Berlese, Reference Berlese1886 by original designation from material collected in Italy.
Terminology
Terminology used here follows that developed in works by F. Grandjean (reviewed by Travé and Vachon, Reference Travé and Vachon1975; general terminology combined by van der Hammen, Reference van der Hammen1980). Its application to Camerobiidae follows Kethley (Reference Kethley and Dindal1990) and Fan and Walter (Reference Fan and Walter2011). The left and right sides of the body and its structures correspond to those of the dorsal view. Symbols and abbreviations used in the text are explained in the legends of the corresponding figures. Leg setae were designated with diligence on the basis of three-dimensional specimens. However, the nature of the appendages (long, slender, stilt-like) may cause difficulty in the correct determination of lateral and ventral setae; hence, the chaetotaxy of setae l and v on tibiae should be considered a simplification. Application of Grandjean's system for leg phanerotaxy has been already applied in the recent literature (e.g., Fan and Walter, Reference Fan and Walter2011; Paredes-Leon et al., Reference Paredes-León, Corona-López, Flores-Palacios and Toledo-Hernández2016; Khaustov and Abramov, Reference Khaustov and Abramov2017).
Neophyllobius electrus new species
Figures 1.2, 2–6; Table 1
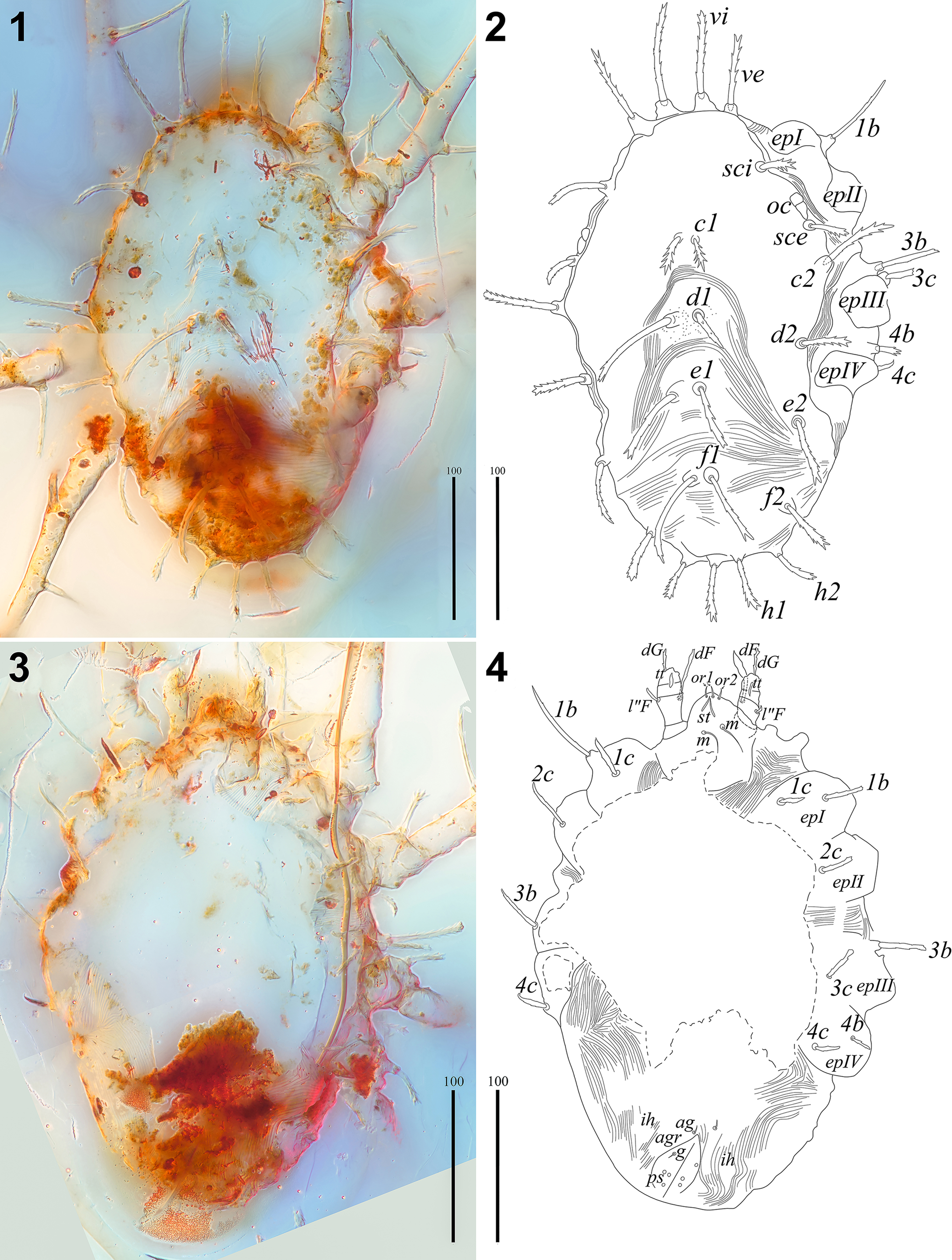
Figure 2. Neophyllobius electrus n. sp. (SMNG 07/36290-78a): (1) layered picture of the body in dorsal view; (2) line drawing interpretation of (1); (3) layered picture of the body in ventral view; (4) line drawing interpretation of (3). 1b, 1c, 2c, 3b, 3c, 4b, 4c = epimeral setae; ag = aggenital seta; agr = anogenital area; c1, c2 = idiosomal setae of row C; d1, d2 = idiosomal setae of row D; dF = dorsal seta of palpfemur; dG = dorsal seta of palpgenu; e1, e2 = idiosomal setae of row E; epI–IV = epimera of legs I–IV; f1, f2 = idiosomal setae of row F; g = genital seta; h1, h2 = idiosomal setae of row H; ih = cupule; m = subcapitular medial setae; l″F = lateral seta of palpfemur; oc = eyes; or1, or2 = oral setae; ps = pseudanal setae; sci, sce = scapular setae of prodorsum, st = cheliceral stylets; tt = palptibia + palptarsus; vi, ve = vertical setae of prodorsum. Scale bars = 100 μm.
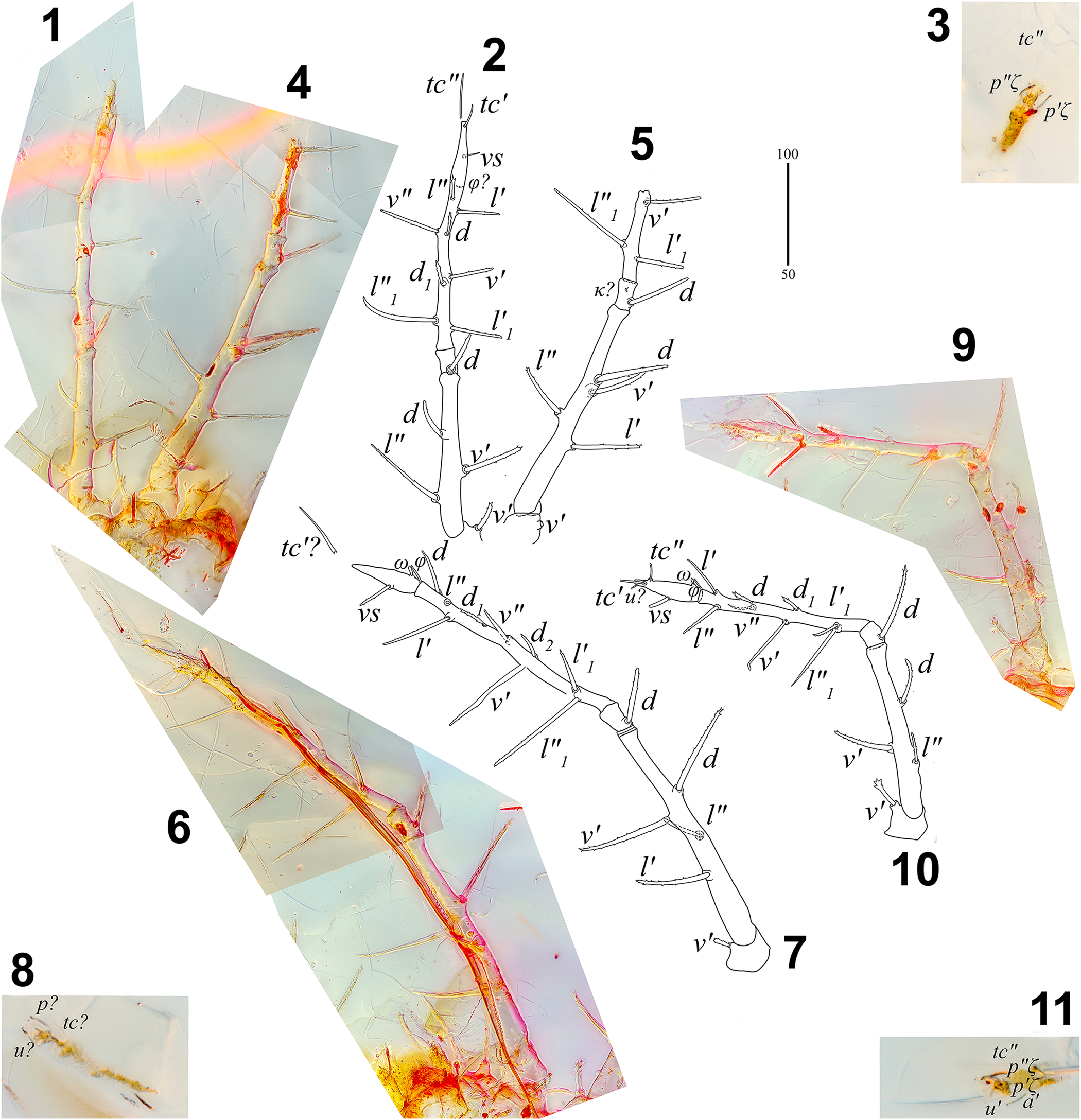
Figure 3. Neophyllobius electrus n. sp. (SMNG 07/36290-78): (1) layered picture of left leg II (SMNG 07/36290-78a); (2) line drawing interpretation of 1; (3) tarsus of left leg II (SMNG 07/36290-78b); (4) layered picture of left leg I (SMNG 07/36290-78a); (5) line drawing interpretation of (4); (6) layered picture of right leg I (SMNG 07/36290-78a); (7) line drawing interpretation of (6); (8) tarsus of right leg I (SMNG 07/36290-78b); (9) layered picture of right leg II (SMNG 07/36290-78a); (10) line drawing interpretation of (9); (11) tarsus of right leg II (SMNG 07/36290-78b). a = anterolateral seta; d, d1, d2 = dorsal seta; l, l1 = lateral seta; pζ = eupathidial proral seta; tc = tectal seta; u = unguinal seta; v = ventral seta; vs = midventral seta of tarsus; κ = minute genual seta; φ = tibial solenidion; ω = tarsal solenidion; ′ = anterior; ″ = posterior. (1, 2, 4–7, 9, 10) Scale bar = 100 μm; (3, 8, 11) scale bar = 50 μm.

Figure 4. Neophyllobius electrus n. sp. (SMNG 07/36290-78a): (1) layered picture of left leg III; (2) line drawing interpretation of (1); (3) layered picture of right leg III; (4) line drawing interpretation of (3); (5) layered picture of left leg IV; (6) line drawing interpretation of (5); (7) layered picture of right leg IV; (8) line drawing interpretation of (7). a = anterolateral seta; d, d1 = dorsal seta; l, l1 = lateral seta; tc = tectal seta; u = unguinal seta; v = ventral seta; vs = midventral seta of tarsus; φ = tibial solenidion; ′ = anterior; ″ = posterior. Scale bar = 100 μm.
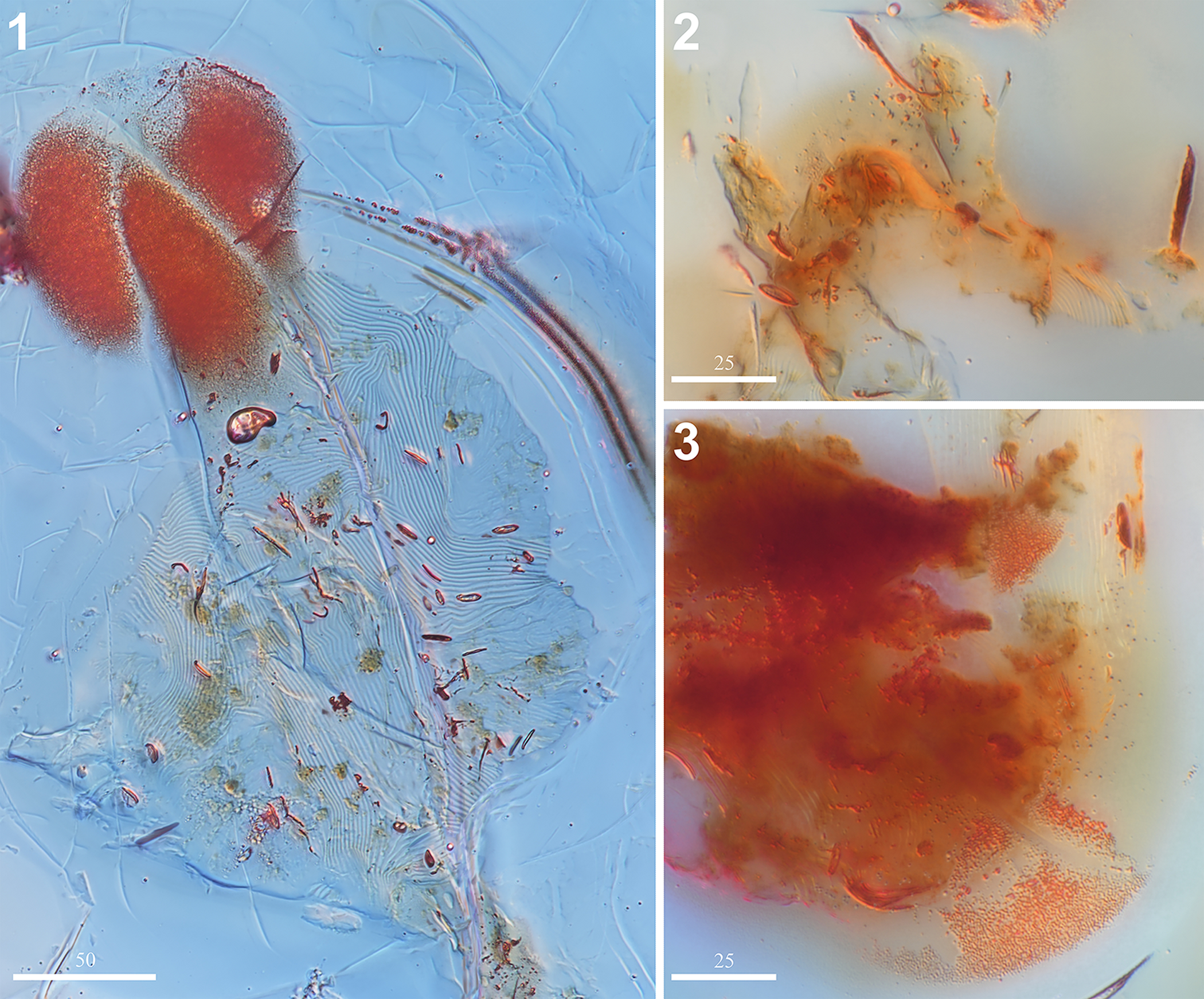
Figure 5. Neophyllobius electrus n. sp. (SMNG 07/36290-78): (1) imprint of ventral cuticle (SMNG 07/36290-78b); (2) gnathosoma in ventral view (SMNG 07/36290-78a); (3) opisthosoma in ventral view (SMNG 07/36290-78a). (1) Scale bar = 50 μm; (2, 3) scale bars = 25 μm.
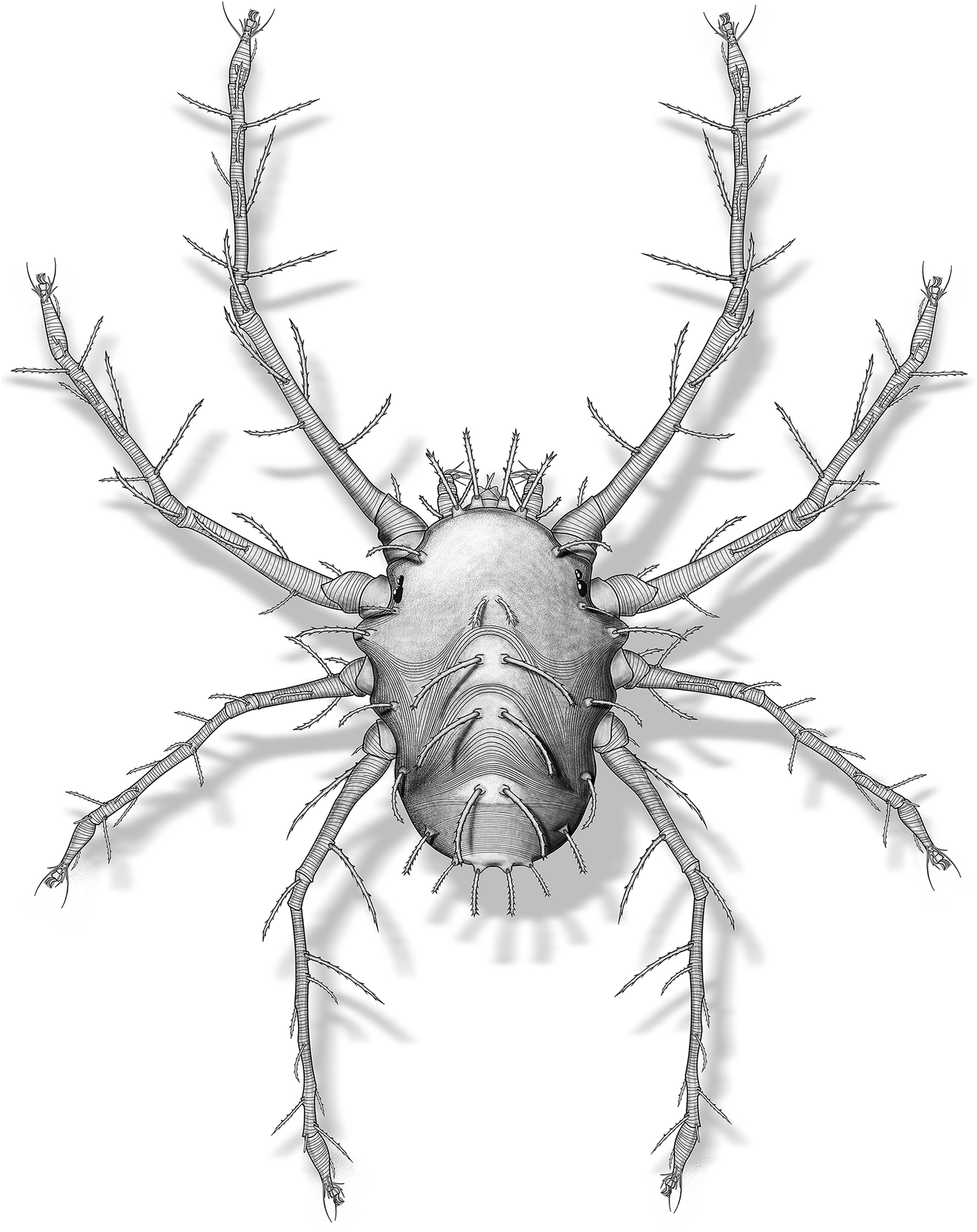
Figure 6. Neophyllobius electrus n. sp., reconstruction of adult female.
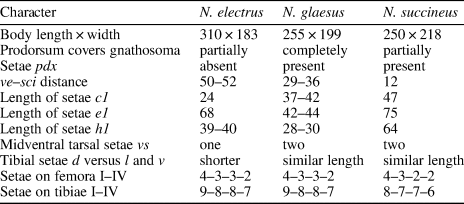
Table 1. Main differences between fossil Neophyllobius species from the Baltic amber. Units = μm.
Holotype
Adult female (Fig. 1.2) in two preparations that consist of polished amber embedded in epoxy resin between two round glass coverslips. One preparation (SMNG 07/36290-78a) contains most of the specimen's body. Another (SMNG 07/36290-78b) contains some parts of the ventral cuticle (Fig. 5.1) and partial tarsi I and II (Fig. 3.3, 3.8, 3.11). Type material is deposited in SMNG, Sammlung Oribatida, under the collection number 07/36290-78. Data from the label: Baltischer Bernstein, coll. Wunderlich, Ankauf 2007. Camerobiidae (postlarv) Phthiracaroidea (ad) det. Sidorchuk. See Zmudzinski (Reference Zmudzinski2020a) for original series of pictures of the specimen.
Diagnosis
Peritremes with at least one loop. Fourteen pairs of barbed idiosomal setae present. Setae vi and ve on anterior margin of prodorsum directed forward; together with c2, d1, e1, and f1 longer than other idiosomal setae; ratio c1:c2:d1 = 1:2:2.8. Setae d on genua I–IV barbed and relatively short (I, 51–60 μm; II, 77 μm; III, 25–42 μm; IV, 47 μm). Each of tarsi I–IV with only one midventral seta vs. Leg phanerotaxy formulae (trochanter to tarsus, tarsal setae estimated, κ setae not counted): (I) 1–4–1–9(φ)–9(ω); (II) 1–3–1–8(φ)–9(ω); (III) 1–3–1–8(φ)–7; (IV) 1–2–1–7(φ)–7.
Occurrence
Single inclusion within the sample of Baltic amber, middle Eocene 48–38 Ma (Weitschat and Wichard, Reference Weitschat and Wichard2002).
Description of inclusion
Gnathosoma (Fig. 5.2) situated ventroterminally on the idiosoma, partially covered from above by the anterior part of the idiosomal prodorsum, 61 μm long (measured from its base to the tip of the infracapitulum) and 62 μm wide basally, cuticle striated only on palps. Stylophore stumpy, partially visible from dorsal view. Peritremes present but hardly visible. Cheliceral stylets retracted, visible inside the stylophore, each 17 μm long. Subcapitulum smooth, without any ornamentation, with simple subcapitular setae: medial m (10–13 μm long; distance m–m 9 μm) situated on its middle third, and short oral or1 (5–6 μm) and or2 (4 μm; visible only on the left side), almost on the tip of the infracapitulum. Palps directed anteriad, their total length 52–63 μm. Palptrochanters without any setae. Palpfemora, each with lateral setae l″ 8–10 μm long and dorsal setae d 25–29 μm. Palpgenua with dorsal setae d 14–17 μm. Each palptibia with a longitudinal sclerotized structure (most likely a complex of palptibial setae and claw + palptarsus).
Idiosoma (Fig. 2) oval, 310 μm long and 183 μm wide (measured at the level of the bases of setae c2). Cuticle partially striated, except prodorsum, midlateral dorsum, around the setae d1, e1, and f1, and epimeral (coxal) fields. Dorsal idiosoma with fourteen pairs of barbed, relatively thick setae set on cuticular tubercles. Each tubercle single (not coupled). Setae vi (63–73 μm long; distance vi–vi 26 μm) and ve (53–56 μm; ve–ve 69 μm) directed forward and set on the largest protuberances situated on the anterior margin of prodorsum. Setae sci (22–38 μm; sci–sci 112 μm) and sce (26–27 μm; sce–sce 170 μm) on the lateral margins of prodorsum. Between them, just above the level of sce on each side, two eyes present, anterior one (8.5 μm in diameter) a little smaller than the posterior one (10.5 μm). Four pairs of setae in the central part: c1 the smallest (24 μm long; distance c1–c1 12 μm) with distinctly marked barbs and d1 (66 μm; d1–d1 19 μm), e1 (68 μm; e1–e1 16 μm), and f1 (51–64 μm; f1–f1 18 μm) much longer, gently bent backward, and similar in length. Setae c2 (50 μm; c2–c2 192 μm), d2 (35–36 μm; d2–d2 159 μm), e2 (44–45 μm; e2–e2 141 μm), and f2 (34–35 μm; f2–f2 100 μm) situated on the lateral margins. Setae h1 (39–40 μm, h1–h1 17 μm), and h2 (28–31 μm; h2–h2 66 μm) terminate the idiosoma. Extremities of epimera visible from the dorsal side; the dorsolateral cuticle passes between second and third epimera. Supplementary distances: vi–ve 22–25 μm; ve–sci 50–52 μm; sci–sce 55 μm; c1–c2 92–95 μm; d1–d2 73–79 μm; e1–e2 71–72 μm; f1–f2 43–52 μm; h1–h2 18–25 μm; c1–d1 55–62 μm; d1–e1 46–50 μm; e1–f1 66 μm; f2–h2 32 μm; e2–f2 59 μm; d2–e2 53 μm; c2–d2 58 μm; sce–c2 32–34 μm. Coxal fields grouped into two areas: I partially fused with II, and coxal field III partially fused with IV. Epimeral setae slightly barbed and set on cuticular protuberances: 1b 25–58 μm long; 1c 18–25 μm; 2c 21–30 μm; 3b 35–46 μm; 3c 26–33 μm; 4b 11–17 μm; 4c 20–22 μm. The anogenital area (Fig. 5.3) visible, but difficult to discern its details. A pair of short, simple, smooth aggenital setae ag situated just above its anterior margin. Genital valves with one pair of short, simple, smooth genital setae g (seta visible on the right side, only the base of seta visible on the left side). Three bases of pseudanal setae ps1–3 present on the right, but only two bases discernible on the left. Oval breaks in the striation situated in the proximity of anogenital region visible (probably areas of simple cupules ih).
Legs (Figs. 3, 4) stilt-like, first and fourth pairs longer than second and third pairs; all setae barbed and situated on tubercles except those on tarsi (smooth and simple except slightly barbed midventral setae vs). Legs I, the total length of the right one (Fig. 3.6): 548 μm; left one (Fig. 3.4) deficient (lacking tarsus and half of tibia). Trochanters 51 μm long, each with single ventral seta v′ 8–12 μm. Femora elongated 222–223 μm, each with four setae, from distal to proximal, d 70–90 μm; v′ 66–88 μm; l″ 41–54 μm; l′ 67–71 μm. Genua 30–37 μm, each with single seta d 51–60 μm situated on proximal third, probably a minute seta κ on the left one present. Right tibia elongated 206 μm, with nine setae, from distal to proximal, d 41 μm; l″ (only base visible), l′ 65 μm; d1 29 μm; v″ 36 μm; v′ 86 μm; d2 22 μm; l′1 49 μm; l″1 99 μm; and one rod-like distal solenidion φ 19 μm; left one incomplete (91 μm) with three setae v′ 59 μm; l′1 51 μm; and l″1 89 μm. Right tarsus 64 μm, not completely preserved, with one ventral seta vs and one clavate proximal solenidion ω, partially preserved setae tc′ and at least one p and one u. Legs II (Fig. 3.1, 3.9), total lengths 403–429 μm. Trochanter 42–47 μm with single seta v′ 30–32 μm. Femora elongated 142–177 μm, each with three setae, from distal to proximal, d 36–45 μm, v′ 55–59 μm, and l″ 36–71. Genua 30–31 μm, each with single seta d 48–77 μm situated on the proximal third. Tibiae elongated 150–164 μm, each with eight setae, from distal to proximal, l′ 41–42 μm; l″ 40 μm; d 18–19 μm; v″ 55–56 μm; v′ 25–44 μm; d1 22–23 μm l′1 30–53 μm; l″1 50–68 μm; and one rod-like distal solenidion φ 14–18 μm. Tarsi 62–64 μm, each with one ventral seta vs and tectal setae (tc); eupathidial proral setae (pζ) visible on the left tarsus; one unguinal seta u′, anterolateral a′, and two eupathidial proral setae (pζ) preserved on the right tarsus; one clavate proximal solenidion ω visible on the right tarsus only. Legs III (Fig. 4.1–4.4), total lengths 345–397 μm. Trochanters 35–40 μm, each with single seta v′ 23–24 μm. Femora elongated 111–116 μm, each with three setae, from distal to proximal, d 17–32 μm; l′ 50–55 μm; l″ 51–52 μm. Genua 32–34 μm, each with single seta d 25–42 μm situated on the proximal third. Tibiae elongated 154–169 μm, each with eight setae, from distal to proximal, l″ 31–36 μm; l′ 32–42 μm; l″1 32–38 μm; d 20–21 μm; v′ 25–29 μm; v″ 27 μm; l′1 18–21 μm; d1 14–16 μm; and one rod-like distal solenidion φ 9–12. Tarsi 44–57 μm, no setae preserved on the left one; on the right one single slightly barbed ventral seta vs and probably (tc), a, and u present; ambulacra (claws + tenant-hair empodium) preserved on each tarsus. Legs IV (Fig. 4.5–4.8), total lengths 364–485 μm. Trochanters 34–42 μm with single ventral seta v′ 18–27 μm. Femora elongated 90–160 μm, each with two lateral setae, from distal to proximal, l″ 75–89 μm and l′ 44–95 μm. Genua 40–43 μm, each with single seta d 47 μm situated on the proximal third. Tibiae elongated 190–217 μm, each with seven setae, from distal to proximal, l″ 44–46 μm; l′ 36 μm; v′ 19–21 μm; d 29–37 μm; l′1 22–32 μm; v″ 17–31 μm; l″1 81–91 μm; and one rod-like distal solenidion φ 10 μm. Tarsi 44–58 μm, each with single ventral seta vs and pair of tectal setae (tc); ambulacra preserved on the right tarsus only. Phanerotaxy formulae (trochanter to tarsus, tarsal setae estimated, κ setae not counted): (I) 1–4–1–9(φ)–9(ω); (II) 1–3–1–8(φ)–9(ω); (III) 1–3–1–8(φ)–7; (IV) 1–2–1–7(φ)–7.
Etymology
The specific epithet electrus is an adjective derived from the Latin noun electrum in the nominative, which is translated into amber.
Remarks
N. electrus n. sp. is morphologically similar to the extant species N. meyerae Bolland, Reference Bolland1991 by the presence of only one midventral seta on each tarsus I–III. However, it differs from this species by having an additional third seta on femora III, probably l″ (N. meyerae has only two setae); lack of setae pdx (present in N. meyerae); a slightly different idiosomal pattern, especially in terms of vi–ve distance; and significant differences in length between individual pairs within medial and lateral rows of idiosomal setae (there are only slight differences in N. meyerae). There are also some similarities between the new species and the Recent camerobiid N. panici Bolland, Reference Bolland1991. These two species have only one midventral seta vs on each tarsus IV, four setae on femur I, three setae on femur II, and significant differences in length ratio between the median setae. N. electrus n. sp. differs from this species by the presence of only one midventral seta on each tarsus I–III (two setae present in N. panici), lack of setae pdx (present in N. panici), having additional third setae on femora III, probably l″ (N. panici has only two setae), shorter setae d on genua (in N. panici seta d is at least three times longer than the genu), and idiosomal setae barbed (in N. panici these setae are nodular). By the presence of three setae on femora III, two setae on femora IV, and the longest setae d1 among the idiosomal setae, N. electrus n. sp. is also similar to the other extant species N. trisetosus De Leon, Reference De Leon1958. The new species differs by the presence of only one midventral seta on each tarsus I–IV (N. trisetosus has two midventral setae on each tarsus I–IV) and by the absence of setae pdx (which are present in N. trisetosus).
N. electrus n. sp. is morphologically similar to the fossil species N. glaesus n. sp. described herein. Both species have relatively short dorsal setae d of genua I–IV and the same chaetotaxy formulae of femora 4–3–3–2 and tibiae 9–8–8–7. N. electrus differs from N. glaesus by the presence of fourteen pairs of idiosomal setae (setae pdx present in N. glaesus), ve–sci distance 50–52 μm (29–36 μm in N. succineus), gnathosoma partially covered by the prodorsum (in N. glaesus prodorsum completely covers it), the presence of only one midventral seta vs on each tarsus (N. glaesus has two setae vs on each tarsus), and shorter dorsal setae d on tibiae than lateral l and ventral v setae (N. glaesus has these setae similar in length). A comparison of all three fossil Neophyllobius species from Baltic amber is summarized in Table 1.
The original sample of amber had a natural fissure filled with air that passed through the inclusion. Hence, to preserve and polish the specimen, the sample was separated into two preparations (see Materials). Nevertheless, the mite imprint in the resin is comparable in quality to modern specimens mounted on microscope slides, even thin striae on the legs and the idiosoma, and clavate tarsal solenidia are clearly visible. Residues of the cuticle, internal organs, and probably food remnants form orange-brown artifacts that hinder the visibility of certain structures such as the anogenital area. Left leg I is incomplete (whole tarsus and a half of tibia missing); those parts were probably lost before embedding in the resin. Interestingly, the apotelae of the legs are poorly preserved, so claws as relatively strongly sclerotized rigid structures should be better remained.
Neophyllobius glaesus new species
Figures 1.1, 7–13; Table 1

Figure 7. Neophyllobius glaesus n. sp. (GPIH 4995a): (1) layered picture of the body in dorsal view; (2) line drawing interpretation of (1); (3) layered picture of the body in ventral view; (4) line drawing interpretation of (3). 1a–c, 2c, 3a–c, 4a–c = epimeral setae; ag = aggenital seta; agr = anogenital area; c1, c2 = idiosomal setae of row C; d1, d2 = idiosomal setae of row D; dF = dorsal seta of palpfemur; e1, e2 = idiosomal setae of row E; epI–IV = epimera of legs I–IV; f1, f2 = idiosomal setae of row F; h1, h2 = idiosomal setae of row H; ih = cupule; m = subcapitular medial setae; l″F = lateral seta of palpfemur; oc = eyes; or1 = oral seta; pdx = fifteenth pair of idiosomal setae on prodorsum; ps1–3 = pseudanal setae; sci, sce = scapular setae of prodorsum; tt = palptibia + palptarsus; vi, ve = vertical setae of prodorsum. Scale bars = 100 μm.

Figure 8. Neophyllobius glaesus n. sp., leg I (GPIH 4995a): (1) layered picture of right one in dorsal view; (2) line drawing interpretation of (1); (3) layered picture of right one in ventral view; (4) line drawing interpretation of right tarsus in ventral view; (5) layered picture of left one in dorsal view; (6) line drawing interpretation of (5); (7) layered picture of left one in ventral view. a = anterolateral seta; bv = basiventral seta of femur; d, d1, d2 = dorsal seta; dTI = dorsal seta of right tibia I; l, l1 = lateral seta; pζ = eupathidial proral seta; tc = tectal seta; u = unguinal seta; v = ventral seta; vs1, vs2 = midventral setae of tarsus; φ = tibial solenidion; ω = tarsal solenidion; ′ = anterior; ″ = posterior. (1–3, 5–7) Scale bar = 50 μm; (4) scale bar = 25 μm.

Figure 9. Neophyllobius glaesus n. sp., leg II (GPIH 4995a): (1) layered picture of right one in dorsal view; (2) line drawing interpretation of (1); (3) layered picture of right one in ventral view; (4) layered picture of left one in dorsal view; (5) layered picture of left one in ventral view; (6) line drawing interpretation of (5). a = anterolateral seta; bv = basiventral seta of femur; d, d1 = dorsal seta; l, l1 = lateral seta; p = proral seta; tc = tectal seta; u = unguinal seta; v = ventral seta; vs1, vs2 = midventral setae of tarsus; φ = tibial solenidion; ω = tarsal solenidion; ′ = anterior; ″ = posterior. Scale bar = 50 μm.

Figure 10. Neophyllobius glaesus n. sp., leg III (GPIH 4995a): (1) layered picture of left one in dorsal view; (2) layered picture of left one in ventral view; (3) line drawing interpretation of (2); (4) layered picture of right one in dorsal view; (5) line drawing interpretation of (4); (6) layered picture of right one in ventral view. a = anterolateral seta; d, d1 = dorsal seta; ev = basiventral seta of femur; l, l1 = lateral seta; tc = tectal seta; u = unguinal seta; v = ventral seta; vs1, vs2 = midventral setae of tarsus; φ = tibial solenidion; ′ = anterior; ″, posterior. Scale bar = 50 μm.

Figure 11. Neophyllobius glaesus n. sp., leg IV (GPIH 4995a): (1) layered picture of left one in dorsal view; (2) line drawing interpretation of (1); (3) layered picture of left one in ventral view; (4) layered picture of right one in dorsal view; (5) line drawing interpretation of (4); (6) layered picture of right one in ventral view. a = anterolateral seta; d = dorsal seta; l, l1 = lateral seta; tc = tectal seta; u = unguinal seta; v = ventral seta; vs = midventral seta of tarsus; φ = tibial solenidion; ′ = anterior; ″ = posterior. Scale bar = 50 μm.

Figure 12. Neophyllobius glaesus n. sp. (GPIH 4995): (1) opisthosoma in ventral view (GPIH 4995a); (2) gnathosoma in ventral view (GPIH 4995a); (3) left tarsus of leg II (GPIH 4995b); (4) opisthosoma in dorsal view (GPIH 4995a). AM = ambulacrum (claws + tenant-hair empodium); dTI = dorsal seta of tibia; h2 = hypertrophied seta; lTI = lateral seta of tibia; tc = tectal seta; vs1, vs2 = midventral tarsal setae; vTI = ventral seta of tibia; ′ = anterior; ″ = posterior. Scale bars = 25 μm.

Figure 13. Neophyllobius glaesus n. sp., reconstruction of adult female stase.
Holotype
Adult female (Fig. 1.1) in two polished pieces of amber. One piece contains an almost complete specimen, and it is placed in a tube with a thymol aqueous solution (GPIH 4995a). Another contains the incomplete left tarsus II (Fig. 12.3) and is embedded in epoxy resin between two round glass coverslips (GPIH 4995b). Type material is deposited in GPIH under collection number GPIH 4995. See Zmudzinski (Reference Zmudzinski2020b) for original series of pictures of the specimen.
Diagnosis
Fifteen pairs of idiosomal barbed setae present (a pair of pdx present). Setae vi and ve on anterior margin of prodorsum directed forward; prodorsum rectangular and covers gnathosoma from above. All idiosoma setae similar in length (except f2 and h1, h2, which are slightly shorter). Setae d on genua I–IV barbed and relatively short (I, 37–48 μm; II, 32–35 μm; III, 26–32 μm; IV, 48–50 μm). Each of tarsi I–IV with two midventral setae vs1–2. Leg phanerotaxy formulae (trochanter to tarsus, tarsal setae estimated, κ setae not counted): (I) 1–4–1–9(φ)–10(ω); (II) 1–3–1–8(φ)–10(ω); (III) 1–3–1–8(φ)–8; (IV) 1–2–1–7(φ)–8.
Occurrence
Single inclusion within the sample of Baltic amber, middle Eocene 48–38 Ma (Weitschat and Wichard, Reference Weitschat and Wichard2002).
Description of inclusion
Gnathosoma (Fig. 12.2) situated ventrally on the idiosoma, between epimera I, wholly covered from above by anterior prodorsum, 46 μm long (measured from its base to the tip of the infracapitulum) and 48 μm wide basally. Stylophore stumpy, peritremes poorly visible, and cheliceral stylets indiscernible due to position of gnathosoma and the fossilized body remains. Subcapitulum slightly striated, with simple medial subcapitular setae: m (13–15 μm long; distance m–m 22 μm) situated on its middle third, and a pair of short oral setae or on the tip of the infracapitulum, another pair of oral setae indiscernible. Palps directed downward, their total length 30–34 μm. Individual articles hardly visible, best-visible palpfemora and sclerotized structures (most probably tibial setae and claw + tarsus). Palpfemora, each with dorsal setae d 24 μm and lateral setae l″ 9–13 μm visible on the right article. Palpgenua with dorsal setae d 19 μm.
Idiosoma (Fig. 7) oval, 255 μm long and 199 μm wide (measured at the level of the bases of setae c2), opisthosoma wider than prodorsum. Cuticle striated, except anterior prodorsum, around the setae c1, d1, e1, and f1 and epimeral (coxal) fields. Dorsal idiosoma with fifteen pairs of barbed setae set on small cuticular single (not coupled) tubercles. Prodorsum rectangular; anterior margin concave. Setae vi (49–53 μm long; distance vi–vi 44 μm) and ve (41–53 μm; ve–ve 83 μm) directed forward. Setae sci (31–35 μm; sci–sci 90 μm) and sce (34–37 μm; sce–sce 117 μm) on the lateral margins of prodorsum. Between them, on each side, two eyes present, anterior one (4 μm in diameter) a little smaller than the posterior one (7.5 μm). Five pairs of setae of similar length in the central part present: pdx (34–38 μm long; distance pdx–pdx 14 μm), c1 (37–42 μm long; distance c1–c1 21 μm), d1 (41 μm; d1–d1 16 μm), e1 (42–44 μm; e1–e1 17 μm), and f1 (41 μm; f1–f1 13 μm). Lateral setae c2 (52–53 μm; c2–c2 143 μm), d2 (33 μm; d2–d2 105 μm), e2 (42–54 μm; e2–e2 103 μm), and f2 (24–33 μm; f2–f2 113 μm) situated closer to the center (not on the margins). Setae h1 (28–30 μm, h1–h1 16 μm) situated on more-sclerotized unstriated cuticle, and h2 (23–25 μm; h2–h2 59 μm) terminate the idiosoma; left seta h2 hypertrophied (Fig. 12.4; see Discussion). Extremities of epimera visible from the dorsal side; the dorsolateral cuticle passes between second and third epimera. Supplementary distances: vi–ve 23–24 μm; ve–sci 29–36 μm; sci–sce 39–40 μm; sce–c2 35–36 μm; vi–pdx 34–37 μm; pdx–c1 31–36 μm; c1–c2 73–80 μm; c1–d1 42–43 μm; d1–e1 34–36 μm; e1–f1 42–43 μm; f1–h1 56–58 μm; d1–d2 53–57 μm; e1–e2 48–54 μm; f1–f2 63–65 μm; h1–h2 23 μm; f2–h2 35–40 μm; e2–f2 50–51 μm; d2–e2 29 μm; c2–d2 38–43 μm. Coxal fields grouped into two areas: I partially fused with II, and coxal field III partially fused with IV. Epimeral setae slightly barbed and set on small cuticular protuberances: 1a 13 μm long (only the base of seta visible on the right side); 1b 11–14 μm; 1c 19–21 μm; 2c 18–25 μm; 3a 5–10 μm; 3b 17–19 μm; 3c 18–19 μm; 4a 8 μm; 4b 14–16 μm; 4c 17 μm; setae 3a and 4a situated in the intercoxal region, distance 3a–3a 49 μm; 4a–4a 55 μm. The anogenital area (Fig. 12.1) visible, but hard to discern its details. A pair of short, simple, smooth aggenital setae ag situated just above its anterior margin. Genital valves with one pair of short, simple, smooth genital setae g (seta visible on the right side, only base of seta visible on the left side). Three bases of pseudanal setae ps1–3 present on the right valve but only two bases discernible on the left valve. Oval breaks in the striation situated in the proximity of anogenital region visible (probably areas of simple cupules ih).
Legs (Figs. 8–11) stilt-like, first and fourth pairs longer than second and third pairs, all setae barbed and situated on small tubercles except those on tarsi (simple and smooth except slightly barbed midventral setae vs). Legs I (Fig. 8), the total length of the right one 412 μm; left one deficient (lacking tarsus and a half of tibia). Trochanters 33–36 μm long, each with single ventral seta v′ 6–10 μm. Femora elongated 157–162 μm, each with four setae, from distal to proximal, d 48–49 μm; l′ 25–27 μm; l′1 25–38 μm; bv″ 9–12 μm. Genua 34–38 μm, each with single seta d 37–48 μm situated on the proximal third. Right tibia elongated 147 μm, with nine setae, from distal to proximal, d 42 μm; l′ 39 μm; l″ 44 μm; v″ 43 μm; d1 46 μm; v′ 36–44 μm; d2 34–41 μm; l″1 33–36 μm; l′1 40–42 μm; and one rod-like distal solenidion φ 21 μm; left one incomplete. Right tarsus 37 μm with the complete set of setae visible: tectal (tc), eupathidial proral (pζ), anterolateral (a), unguinal (u), midventral vs1–2, and one proximal clavate solenidion ω; apotele (ambulacral stalk, claws, tenant-hair empodium) preserved. Legs II (Fig. 9), the total length of the right one 300 μm, left one incomplete, tarsus and part of tibia as a separate preparation (GPIH 4995b). Trochanter 29 μm with single seta v′ 9–12 μm. Femora elongated 114–116 μm, each with three setae, from distal to proximal, d 38–40 μm; l′ 21–27 μm; and bv″ 15 μm. Genua 25–27 μm, each with single seta d 32–35 μm situated on the proximal third. Tibiae elongated 86 μm, each with eight setae, from distal to proximal, d 39 μm; l′ 21 μm; l″ 28 μm; v′ 41 μm; v″ 20 μm; d1 31 μm; l″1 35–49 μm; l′1 23–26 μm; and one rod-like distal solenidion φ 12 μm. Right tarsus 45 μm, with two midventral setae vs1 and vs2, tectal (tc), unguinal (u), at least one anterolateral (a) and one proral (p) and one proximal clavate solenidion ω + complete apotele; on the left tarsus tectal and midventral setae preserved along with apotele. Legs III (Fig. 10), total lengths 383–399 μm. Trochanters 32–34 μm, each with single seta v′ 14–18 μm. Femora elongated 114 μm, each with three setae, from distal to proximal, d 32 μm; l′ 23–24 μm; ev″ 21 μm. Genua 28–32 μm, each with single seta d 26–32 μm situated on the proximal third. Tibiae elongated 163–170 μm, each with eight setae, from distal to proximal, l′ 45 μm; d 39–40 μm; l″ 46–48 μm; v1 39–41 μm; v2 36–43 μm; l″1 28–42 μm; d1 24–27 μm; l′1 32 μm; and one rod-like distal solenidion φ 13 μm. Tarsi 52–53 μm, each with complete set of setae: vs1–2, (tc), (a), (u), and apotele. Legs IV (Fig. 11), total lengths 460 μm. Each trochanter 31–37 μm with single ventral seta v′ 13–16 μm. Femora elongated 136–146 μm, each with two lateral setae, from distal to proximal, l″ 24–29 μm and l′ 35–36 μm. Genua 30–34 μm, each with single seta d 48–50 μm situated on the proximal third. Tibiae elongated 191 μm, each with seven setae, from distal to proximal l′ 45 μm; d 34 μm; l″ 30 μm; v1 32 μm; v2 40 μm; l″1 43 μm; l′1 32 μm; and one rod-like distal solenidion φ 11 μm. Tarsi 64 μm, each with preserved apotele and probably complete set of setae. Phanerotaxy formulae (trochanter to tarsus, tarsal setae estimated, κ setae not counted): (I) 1–4–1–9(φ)–10(ω); (II) 1–3–1–8(φ)–10(ω); (III) 1–3–1–8(φ)–8; (IV) 1–2–1–7(φ)–8.
Etymology
The specific epithet glaesus is an adjective derived from the Latin noun glaesum in the nominative, which is translated into amber.
Remarks
N. glaesus n. sp. is morphologically very similar to the Recent species N. hypoleanae Bolland, Reference Bolland1991. Both species have relatively short dorsal setae d of genua I–III; setae c1 are shorter and d1 longer than interval to setae next behind them; setae bv″ (most proximal) of femora I are distinctly shorter than setae d (most distal); epimeral setae 1a–c are different in length; number of setae on femora I–IV are 4–3–3–2, respectively; each tarsus of legs I–IV has two midventral setae vs; dorsal setae d of palpfemur are only slightly longer than lateral setae l″. However, N. glaesus has short dorsal setae d on genua IV (N. hypoleanae has these setae at least two times longer than the length of the genu); distances between setae ve and sci are distinctly greater (in N. hypoleanae bases of ve are situated just in front of sci); setae e1 45 μm long and f1 41 μm are distinctly shorter (these setae in N. hypoleanae are 60 μm long); and setae f1 do not reach level of bases of setae h1 (in N. hypoleanae setae f1 reach behind bases of setae h1).
N. glaesus n. sp. is morphologically similar to the fossil species N. succineus Bolland and Magowski, Reference Bolland and Magowski1990. Both species have a relatively short dorsal setae d of genua I–IV; fifteen pairs of idiosomal setae (setae pdx present) without significant differences in their lengths; two midventral setae vs on each tarsus; and the dorsal setae d of tibiae similar in length to the lateral l and ventral v setae. The new species differs from N. succineus by the presence of three setae on femora III (N. succineus has only two of them); chaetotaxy formulae of tibiae I–IV is 9–8–8–7 (8–7–7–6 in N. succineus); distinctly shorter idiosomal setae e1 42–44 μm and h1 (28–30 μm) (75 and 64 μm, respectively, in N. succineus); distance ve–sci 29–36 μm (12 μm in N. succineus); and gnathosoma wholly covered by the prodorsum (in N. succineus prodorsum covers it partially). A comparison of all three fossil Neophyllobius species from Baltic amber is summarized in Table 1.
Unlike the inclusion of N. electrus, almost the entire individual was preserved inside the preparation. The cuticle imprint is translucent, especially on the legs and in the middle of the idiosoma. Residues of the cuticle, internal organs, and probably food remains form brown artifacts within the inclusion. Their highest concentration occurs on the edges of the idiosoma forming a dense border. This makes it difficult to interpret some structures, especially on the ventral opisthosoma. The piece containing the tarsus and part of the tibia of left leg II broke off and was embedded in epoxy resin to preserve such minute amber fragment. Left tarsus I had already been cut or polished in the sample.
Discussion
Common remarks for studied inclusions
The most recent and valid diagnosis of Neophyllobius has been proposed by Fan and Walter (Reference Fan and Walter2006). There is little doubt that the fossil specimens belong to this genus. Although the preservation does not reveal an exact number of peritremal loops, there is at least one in each inclusion because some peritremal structures are visible microscopically. Palptrochanters are nude, palpfemora have two setae on each, and each palpgenu has one seta. Idiosoma bears 14 or 15 pairs of setae. Genital valves have one pair of setae g. Anal valves have probably three pairs of pseudanal setae ps1–3. Solenidion ω is present on each basal half of tarsi I and II. Phanerotaxies of legs are also typical, as in Recent representatives of Neophyllobius.
There is noticeable sexual dimorphism in the family Camerobiidae (already present in nymphs, see Bolland, Reference Bolland2001). As in other raphignathoid families, males have a sclerotized aedeagus, and the genital opening is situated terminally or dorsoterminally. In addition, there are two solenidia φ on each tibia I–II, and tarsal solenidia ω are enlarged and/or elongated. The lack of these features excludes the possibility that the specimens are males. There are also other sex differences such as the length and position of some leg setae, but they are objectively comparable only if there are two sexes available to study. Camerobiid protonymphs are easily distinguished by the absence of setae on the epimera of legs IV and the nude trochanters IV (seta v′ not developed yet). These setae are present on the studied specimens. Bolland (Reference Bolland1983) stated for the first time that there are two nymphal stases within the family. However, deutonymphs are more difficult to distinguish from adults without reference material of all stases. Deutonymphs have generally longer setae d on the genua. Specimens of each new fossil species have relatively short genual setae, were found solely (larvae and nymphs are more commonly found in small aggregations), and probably have well-developed genital valves and complete chaetotaxy. Hence, the specimens are considered in this work as adult females, which seems to be the most probable determination of stase and sex. It is important for the reader to be aware of the difficulty of interpreting the fossil material, especially if it is present only in the form of sole individuals of rare mite groups.
Asymmetric hypertrophy of seta h2 in N. glaesus
Mostly neglected, François Grandjean's works on deviations and their possible evolutionary significance (summarized and extended in Grandjean, Reference Grandjean1971, Reference Grandjean1972, Reference Grandjean1973 but unfinished) echo even with individual discoveries, as is the case with the inclusion of N. glaesus n. sp. According to Grandjean, deviations can be divided into vertitions, which are important in evolutionary terms and reflect the changes that may occur in future generations permanently, and anomalies, which are more rare, random changes that are not of evolutionary significance (Grandjean, Reference Grandjean1971). Deviations are most often asymmetrical and include the absence of some idionymous organ (any holotrichous seta is an example of such), its doubling, change of size, or different location. Other scholars have also studied morphological abnormalities, mostly in terms of teratological singularities but also in the context of regulatory genes (e.g., Southcott, Reference Southcott1997; Weigmann, Reference Weigmann, Sabelis and Bruin2010; Bingül et al., Reference Bingül, Doğan and Doğan2017). Abnormalities occurring among populations of Tycherobius stramenticola Bolland, Reference Bolland1986 and T. polonicus Bolland, Reference Bolland1986, mostly in the form of asymmetrical absence of setae, were indicated (Koç and Akyol, Reference Koç and Akyol2007). The inclusion of N. glaesus n. sp. has a hypertrophied (i.e., enlarged, thickened) left seta h2 (Fig. 12.4). The right one is similar to other idiosomal setae. There are no species of Neophyllobius with setae h2 distinctly thicker than other idiosomal setae, and this kind of asymmetry has not been reported for Camerobiidae; hence, the observed deviation is more likely to be an anomaly rather than a vertition. It would be interesting to study the microanatomical structure of such an abnormality, whether enlargement and thickening are associated with more intense cuticle deposition, cell proliferation, or all of these factors and whether the abnormality is affected by any individual mutation or is an error in ontogenetic development. Note also that hypertrophic setae occur in both acariform (e.g., Fernandez et al., Reference Fernandez, Coineau, Theron and Louwrens2014) and parasitiform mites (e.g., Mašán and Fenďa, Reference Mašán and Fenďa2014) and mostly have some functions in sexual behavior. Hypertrophic setae may have originated at first from rare, random deviations (enlarging, thickening), which having a genetic background, were subject to sexual selection.
The shape of the prodorsum and position of setae h1, h2
Specimens mounted on microscope slides, especially those without highly sclerotized cuticular structures, lose valuable information about the exact shape of their bodies. The flexible cuticle, which is additionally often softened before final preparation and flattened under two microscope slides (for the essential preparation techniques, see Evans, Reference Evans1992; Walter and Krantz, Reference Walter, Krantz, Krantz and Walter2009), gives only an approximate shape of the body. In nature, it is subject to other forces and factors shaping the habitus, for example, through muscle attachments and its compositional structure (Alberti and Coons, Reference Alberti, Coons, Harrison and Foelix1999). A solid preparation made by the forces of nature, as inclusions in amber can be called, preserves the almost intact appearance of a once-living animal. Most of the studies on Camerobiidae were carried out on the basis of just such flattened individuals mounted on microscopic slides (some recent examples: Bolland, Reference Bolland2001; Akyol, Reference Akyol2013; Zeity and Gowda, Reference Zeity and Gowda2013; Khanjani et al., Reference Khanjani, Hoseini, Yazdanpanah and Masoudian2014). This process generally causes the gnathosoma to be unnaturally situated slightly on the ventral part of the body but usually at the same level as the idiosoma. Scanning electron microscopic techniques were used for the first time by Bolland (Reference Bolland1986) and then by Fan and Walter (Reference Fan and Walter2006) to image protonymphal Decaphyllobius gersoni Bolland, Reference Bolland1986 and adult Tycherobius stipula Walter and Fan, Reference Fan and Walter2006 among Camerobiidae. Images from their papers clearly show that the prodorsum forms a well-distinguishable part of the anterior idiosoma in the shape of a trapezoid, partially protecting the gnathosoma from above. Khaustov and Abramov (Reference Khaustov and Abramov2017) also used a scanning electron microscope, but the entire individual was not imaged. The studied inclusion of N. glaesus n. sp. has a similarly shaped prodorsum at the edges of which are placed vertical setae directed forward, and it almost entirely covers the gnathosoma. The specimen of N. electrus n. sp. has a round prodorsal idiosoma, but it distinctly forms a fold over the gnathosoma. Although no comprehensive studies have been conducted on the behavior of camerobiids, it can be hypothesized that the prodorsum may serve a protective function for the gnathosoma, and its shape may be taxonomically important. This feature dates back to at least the middle Eocene, from which Baltic amber probably originates (Weitschat and Wichard, Reference Weitschat and Wichard2002).
Bolland and Magowski (Reference Bolland and Magowski1990) indicated that the terminal situation of setae h1 in N. succineus is rather exceptional because, in extant species, these setae are situated rather on the dorsal side of the idiosoma. In N. electrus the same case can be found—setae h1 are placed marginally terminating the idiosoma. However, in N. glaesus, setae h1 are located clearly on the dorsal surface of the posterior idiosoma at some distance from the end of the body. So it seems that when interpreting this feature, the microscopic preparations of Recent specimens do not distort the true image of the setae situation. It is not excluded that the terminal location of the h1 setae may constitute a plesiomorphic character state in the genus Neophyllobius, or at least this state could have been more common in the Eocene species.
Brittleness of legs in Camerobiidae
Bolland and Magowski (Reference Bolland and Magowski1990) stated that legs of camerobiids are difficult to manipulate and are prone to breaking off; hence, the complete specimen of N. succineus is a quite curious finding. The studied inclusions have an almost complete set of legs. In N. glaesus the tarsus of left leg I had been polished before the sample reached the author. In N. electrus, a tarsus and a half of the tibia of left leg I are lacking, and this loss most likely happened before the mite was trapped in leaking resin. Through these observations, the following questions arise. Since the legs of camerobiids are long and prone to breaking, is there a mechanism to regenerate lost parts by adding tissues after molting? Is there a mechanism for regeneration in adults, and if not, how does limb loss affect survival and thus the fitness of individuals? It seems that the ability of limb regeneration among Prostigmata is the weakest and with the highest mortality rate compared with other higher groups of mites (Rockett and Woodring, Reference Rockett and Woodring1972) and takes place during molting. New, more-comprehensive studies of the regeneration capacity carried out on different representatives of prostigmatan mites may bring novel discoveries.
Lengths of genual setae d
A curious feature among the representatives of the genus Neophyllobius that has never been discussed is a pattern of lengths of dorsal genual setae d. These setae may be short, with length equal to or slightly longer (two or three times) than that of the genu, or they may be very long, with lengths exceeding the combined length of the genu and the following tibia. Intermediate forms are probably present. However, we require more detailed studies and measurements of this character issuing precise criteria (e.g., setae reaching to half of the tibiae are common among species). N. bequartiodendri Bolland, Reference Bolland1991, N. texanus McGregor, Reference McGregor1950, and N. trisetosus De Leon, Reference De Leon1958 are examples of species with short setae d on each genu I–IV. N. euonymi Bolland and Ripka, Reference Bolland and Ripka2000, N. curtipilis, De Leon, Reference De Leon1958, and N. saxatilis Halbert, Reference Halbert1923 have setae d short on genua I–III but distinctly long on genua IV. N. farrieri De Leon, Reference De Leon1958, N. fissus De Leon, Reference De Leon1967, and N. niloticus Bolland, Reference Bolland1991 all have setae d on genua I–IV very long. N. armenica Bolland, Reference Bolland1991 and N. sycomorus Zaher and Gomaa, Reference Zaher and Gomaa1979 have the setae d long on genua I, III, IV but short on genua II. In some other genera of Camerobiidae—Bisetulobius, Camerobia, Decaphyllobius, and Tillandsobius—these setae are always short, that is, the same length as the genu or slightly longer (Bolland, Reference Bolland1986). However, in Tycherobius, there may also be a different pattern of setae length, and Acamerobia has some setae d distinctly longer (Fan and Walter Reference Fan and Walter2006, Reference Fan and Walter2011). Both N. electrus n. sp. and N. glaesus n. sp. have short dorsal setae of each genu I–IV. It is noticeable that the same pattern is present in N. succineus; hence, this feature is common for all known fossil Camerobiidae. It may be a plesiomorphic state of that characteristic, but a sample error is also likely, so the next findings may or may not falsify this hypothesis. If these setae have begun to lengthen in the Cenozoic era, determining the primary cause and function of the final product could be an interesting task. Dorsal setae d are simple setae, not morphologically distinct from other leg setae, so a more probable function of them is sensory or/and protective rather than chemosensory. Minute setae κ of genua I–II, which are probably present in all species but indiscernible in fossils, may be chemostimuli-responsive.
Neophyllobius, the most numerous and the oldest genus
Mites of the genus Neophyllobius have been found on all continents except Antarctica (Bolland, Reference Bolland1991). Their worldwide distribution can be due to their exceptional dispersal abilities or their long evolutionary history (camerobiids are slow-moving mites, and no dispersal forms or behaviors have been observed). The three known inclusions of Camerobiidae are evidence that the characteristics of the genus Neophyllobius were well developed in the Eocene, and the degree of morphological difference between the fossil species is comparable to that of species living today. Organic inclusions in Baltic amber come from ecosystems of Eocene Fennoscandia (northern Europe of that time). The presence of extant Camerobiidae in northern-central Europe—N. bialagorensis Bolland, Reference Bolland1991 (northern Poland), N. aesculi Bolland, Reference Bolland1983, N. vandebundi Bolland, Reference Bolland1991 (the Netherlands), N. plumifer Bolland, Reference Bolland1991 (central Poland), N. saxatilis Halbert, Reference Halbert1923 (Ireland)—may indicate that Neophyllobius returned to central and northern Europe after the last Pleistocene glacial period, which ended ca. 12,000 years ago (Weitschat and Wichard, Reference Weitschat and Wichard2002; Lomolino et al., Reference Lomolino, Riddle and Brown2010).
Acknowledgments
I thank A. Christian and K. Franke (Senckenberg Museum of Natural History Görlitz) for making the amber sample available for this study. I am grateful to J. Dunlop (Museum für Naturkunde, Berlin) and an anonymous reviewer for their suggestions, which improved the quality of the original manuscript. Scientific work was financed from the budget for science in the years 2018–2021, as a research project under the ‘Diamond Grant’ program (no. DI2017 002547).
















