Schizophrenia (SCZ) is a chronic psychiatric disorder characterised by marked cognitive impairments that affect functional ability, predict poor interpersonal and socio-occupational outcomes and appears associated with structural and functional brain changes in this population (Green et al., Reference Green, Kern and Heaton2004; Barch and Ceaser, Reference Barch and Ceaser2012). Since cognitive deficits of patients with SCZ respond only moderately to pharmacotherapy, in the last 15 years, researchers have focused on behavioural interventions aimed at improving the patient's functioning in everyday life such as cognitive remediation (CR) (Wykes et al., Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). A growing number of studies showed durable effects of CR on cognition and global functioning as well as a positive effect on neural activity of patients with chronic SCZ (Wykes et al., Reference Wykes, Huddy, Cellard, McGurk and Czobor2011; Penadés et al., Reference Penadés, González-Rodríguez, Catalán, Segura, Bernardo and Junqué2017). Conversely, only a paucity of neuroimaging studies investigated the effect of CR in early SCZ and results are still sparse (Eack et al., Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010, Reference Eack, Newhill and Keshavan2016; Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017; Ramsay et al., Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017). Given that brain changes occurring in SCZ appear to worsen over time (Torres et al., Reference Torres, Duran, Schaufelberger, Crippa, Louzã, Sallet, Kanegusuku, Elkis, Gattaz, Bassitt, Zuardi, Hallak, Leite, Castro, Santos, Murray and Busatto2016), the application of CR in the early phase of the disease may play a crucial role in protecting against future neural alteration and its subsequent impact on cognitive abilities and functioning.
In this review, we aimed to describe neuroimaging studies that investigated the effect of CR on brain structures and activity in patients with SCZ in the early phase of the disease.
The bibliographic search was performed using PUBMED and Web of Science databases. The following keywords were used for the search: (cognitive) AND (remediation OR training OR rehabilitation) AND (early OR first episode) AND (psychosis OR schizophrenia) AND (magnetic resonance imaging). The inclusion criteria are: (i) original publication published in a peer-reviewed journal, (ii) English language, (iii) the use of a structured protocol for CR training, (iv) the inclusion of a comparison group undergoing a control therapy and (v) application of structural or functional neuroimaging techniques. After title and abstract screening, four studies were identified and included in the review, three of which used the same cohort of patients (Eack et al., Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010, Reference Eack, Newhill and Keshavan2016; Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017). Methodological characteristics and main findings from each study are shown in Table 1.
Table 1. Neuroimaging studies of CR in early SCZ
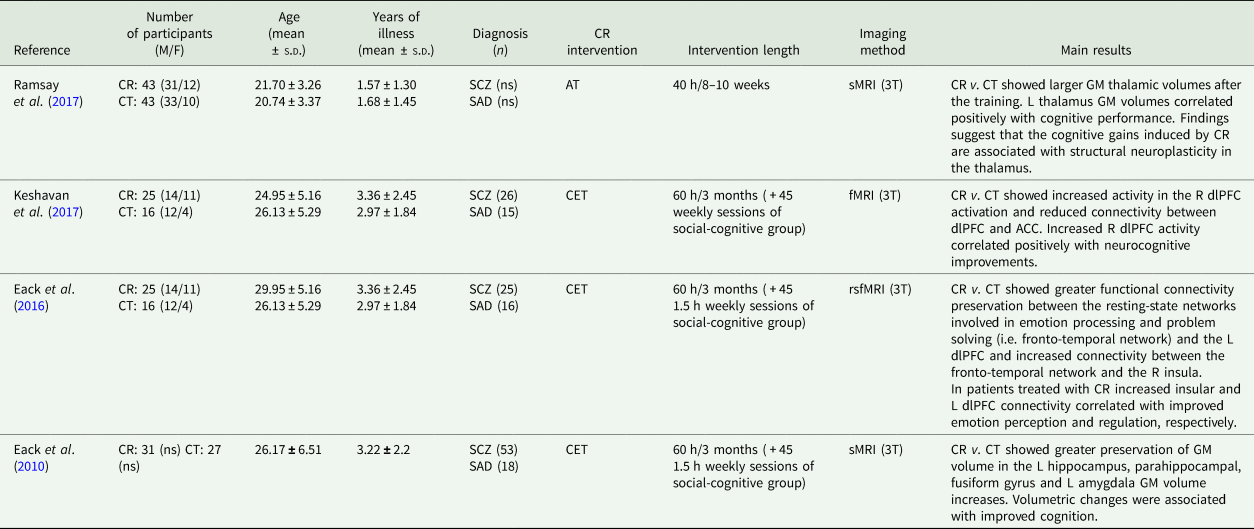
ACC, anterior cingulate cortex; AT, auditory training cognitive remediation; BOLD, blood-oxygenation level dependent; CET, cognitive enhancement therapy; CR, cognitive remediation; CT, control therapy; dlPFC, dorsolateral prefrontal cortex; F, females; fMRI, functional magnetic resonance imaging; GM, grey matter; L, left; M, males; ns, not specified; R, right; SCZ, schizophrenia; SAD, schizoaffective disorder; rsfMRI, resting state functional magnetic resonance imaging; sMRI, structural magnetic resonance imaging; T, tesla.
Ramsay et al. (Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017) conducted a structural magnetic resonance imaging (MRI) study to test the effect of a CR training v. a control therapy, on grey matter volumes of patients with early SCZ (Ramsay et al., Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017). After 40 h of training, the CR group showed larger thalamus than the control group. Moreover, within the CR group, left thalamus correlated positively with changes in cognitive performance (Ramsay et al., Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017). Additional three neuroimaging studies were conducted in early SCZ, by using the same sample of patients randomly assigned to a CR training (i.e. cognitive enhancement therapy –CET), or enriched supportive therapy (EST) and treated for two years (Eack et al., Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010, Reference Eack, Newhill and Keshavan2016; Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017). In the first study by Eack et al. (Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010) patients undergoing CET showed greater preservation of grey matter volume over the course of 2 years in the left hippocampus, parahippocampal gyrus and fusiform gyrus, and greater grey matter increases in the left amygdala than those receiving EST. Lower grey matter loss in the left parahippocampus and fusiform gyrus was significantly associated with improved neuro and social cognition, while greater grey matter increases in the left amygdala was associated with improved social cognition suggesting that amygdala may play a key role in social-cognitive processes (e.g. perspective-taking) (Lamm et al., Reference Lamm, Batson and Decety2007).
In a second study, Eack et al. (Reference Eack, Newhill and Keshavan2016) examined the effects of CET on frontotemporal brain connectivity, using resting-state functional MRI (Eack et al., Reference Eack, Newhill and Keshavan2016). Compared to the control group, patients treated with CET showed lower reduction of the left dorsolateral prefrontal cortex (dlPFC) connectivity and increased right insula connectivity with the resting state network. Moreover, within the CET group, increases in the connectivity between the right insula and the left dlPFC were associated with improvements in emotion perception and regulation, respectively (Eack et al., Reference Eack, Newhill and Keshavan2016).
In the third study, the authors aimed at exploring longitudinal changes in brain activations and connectivity in the CET v. EST group, and the association with performances at cognitive tasks (Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017).
Patients treated with CET exhibited a continuous increase of neural activity in the right dlPFC, that was associated with moderate improvements in neurocognition, suggesting a potential neuroprotective effect of CET. By contrast, patients treated with EST revealed a progressive reduction of task-related neural activation. Moreover, functional connectivity analysis showed decreases in connectivity between the dlPFC and the anterior cingulate cortex (ACC) in CET compared to EST over the two years of treatment, which was associated with neurocognitive improvement. Conversely, patients treated with EST showed no changes in functional connectivity over time (Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017).
Overall, available MRI studies in patients with early SCZ suggest that CR interventions are associated with structural and functional brain changes mostly allocated in frontal and limbic regions (i.e. hippocampus, amygdala, dlPFC and ACC) (Eack et al., Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010, Reference Eack, Newhill and Keshavan2016; Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017; Ramsay et al., Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017). In particular, CR appears to decelerate or partially reverse progressive brain volume deterioration occurring in the early phases of illness in areas known to be crucial for higher-order cognitive processes, including the frontal cortex, thalamus, hippocampus and the amygdala (Eack et al., Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010; Ramsay et al., Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017). Consistently, CR seems to have a ‘normalising' effect on resting-state networks and functional activity in the frontotemporal network, amygdala and ACC of patients with early SCZ (Eack et al., Reference Eack, Newhill and Keshavan2016; Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017). Moreover, neuroplastic changes associated with CR in early SCZ correlated with improvements in neuro and social cognition in all the presented studies (Eack et al., Reference Eack, Hogarty, Cho, Prasad, Greenwald, Hogarty and Keshavan2010, Reference Eack, Newhill and Keshavan2016; Keshavan et al., Reference Keshavan, Eack, Prasad, Haller and Cho2017; Ramsay et al., Reference Ramsay, Fryer, Boos, Roach, Fisher, Loewy, Vinogradov and Mathalon2017). It is plausible that these ameliorations can be obtained by increasing or decreasing signalling in neural circuits (Penadés et al., Reference Penadés, González-Rodríguez, Catalán, Segura, Bernardo and Junqué2017).
Even though CR effects in early SCZ resemble those observed in chronic patients, we can speculate that the neuroprotective effect of CR could be the highest the earliest the intervention is offered, as in the early phase of illness neurodevelopmental processes are still taking place (Corbera et al., Reference Corbera, Wexler, Poltorak, Thime and Kurtz2017). Moreover, early interventions proved to be effective in reducing negative symptoms and increasing socio-occupational functioning in SCZ (Bond et al., Reference Bond, Drake and Luciano2015; Murru and Carpiniello, Reference Murru and Carpiniello2018). Thus, given the efficacy of early interventions in other domains of SCZ, it is also plausible that intervening on cognitive impairments in the early stages of SCZ may lead to a better long-term functional outcome and possibly have a protective effect against neural alterations associated with the pathology.
To conclude, CR appears to be a promising approach in the treatment of cognitive deficits and neural alterations associated with the early phase of SCZ. However, evidence is still sparse, as only a paucity of studies has been conducted with limited control for confounding factors (e.g. heterogeneous patients' populations and methodology). Particularly, the long-term effect of CR interventions on brain plasticity has not been investigated yet. As such, future studies (using multimodal techniques) are needed to assess whether CR may be considered a sustainable approach with long-lasting positive effects on cognitive and neural alterations of patients with SCZ. Particularly, we warrant the conduct of longitudinal studies starting from the very early stage of the illness to clarify whether the application of CR in the early v. chronic phase of the disease may have incremental benefits.
Acknowledgements
This paper was partly supported by grants from the Italian Ministry of Health to CP (GR-2016-02361283) and PB (RF-2016-02364582)
Financial support
None.
Conflict of interest
None.



