Infectious diarrhoea is a severe problem in newborns in many parts of the world and remains one of the leading causes of morbidity and mortality in children under 5 years( Reference Kosek, Bern and Guerrant 1 , Reference Liu, Oza and Hogan 2 ). Enteric infections can be caused by various bacteria and viruses, including enterotoxigenic Escherichia coli (ETEC)( 3 ). ETEC is a diverse group of pathogens that colonise the small intestine (SI) in many mammalian species and produce enterotoxins, which perturbs the intestinal microbiota and stimulates secretion of electrolytes and water and results in diarrhoea( Reference Nagy and Fekete 4 ). The intestinal microbiota is increasingly acknowledged as being important for gut health. A healthy bacterial colonisation may provide a barrier against pathogens and is important for development and homoeostasis of the immune system( Reference Guarner and Malagelada 5 , Reference Clemente, Ursell and Parfrey 6 ). Conversely, dysbiosis and alterations in the composition of the microbiota are associated with gastrointestinal diseases such as infectious diarrhoea, inflammatory bowel disease( Reference Barbosa and Rescigno 7 ) and necrotising enterocolitis (NEC) in paediatric patients( Reference Carlisle and Morowitz 8 ).
Following enteric infections in children, probiotics (defined as ‘live microorganisms that confer health benefits to the host when administered in adequate amounts( Reference Morelli and Capurso 9 )) have been administered to restore a healthy and well-balanced microbiota. However, effects on various patient populations are inconsistent and the optimal timing, dose and type of probiotics remain unknown. Among the most commonly used probiotics are lactic acid bacteria (LAB). A recent systematic review showed that probiotics given to children with acute gastroenteritis reduced the hospital stay with 1 d( Reference Freedman, Ali and Oleszczuk 10 ), and probiotics may support resistance against persistent (>14 d) diarrhoea( Reference Bernaola Aponte, Bada Mancilla and Carreazo 11 ). Preventive treatments with probiotics may also be effective and could potentially support the initial bacterial colonisation after birth to help establish a well-balanced gut microbiota within the 1st days or weeks. In turn, such early treatments may confer a competitive advantage and increase bacterial tolerance and immune maturation and thereby support barrier functions against enteric pathogens and their toxins. Further, gastric acidity is low in newborns( Reference Sangild, Cranwell and Hilsted 12 ) and the pH-reducing effect of lactic acid, a common LAB metabolite, is one of the ways whereby probiotics can inhibit growth of potential pathogens( Reference Alakomi, Skyttä and Saarela 13 ).
In newborn and newly weaned pigs, infectious diarrhoea is a common problem in pig herds. Probiotics have successfully been used as experimental treatments in pig feeds to prevent this( Reference Shu, Freeman and Gill 14 – Reference Taras, Vahjen and Macha 16 ). Infectious diarrhoea in weaning pigs is often caused by ETEC such as E. coli F18 (F18)( Reference Fairbrother, Nadeau and Gyles 17 ). Susceptibility towards F18 depend on the host expression of the FUT-1 gene that is necessary for the formation of the intestinal F18 receptor( Reference Frydendahl, Kåre Jensen and Strodl Andersen 18 ). We have recently demonstrated FUT-1 to be expressed in both preterm and term newborn pigs and documented that F18 inoculation effectively induces diarrhoea already during the first 5 d of life in newborn caesarean-delivered and colostrum-deprived pigs( Reference Jensen, Cilieborg and Østergaard 19 , Reference Cilieborg, Sangild and Jensen 20 ). Delivery by caesarean section allows a high degree of standardisation and controlled interventions affecting the initial bacterial colonisation in the gut. Such pigs are highly susceptible to enteric and systemic infections because they are deprived of the passive immunity provided via ingestion of sow’s colostrum( Reference Sangild, Thymann and Schmidt 21 ). In our model, this sensitivity is further increased with administration of parenteral nutrition, which is often a part of the treatment of vulnerable newborn infants. On this background, we hypothesised that an early postnatal administration of probiotics would reduce the severity of F18-induced diarrhoea in newborn, caesarean-delivered, colostrum-deprived term piglets on parenteral nutrition for the first 15 h. For our studies, we chose to test a relatively well-known probiotic strain, Lactobacillus paracasei F19 (LAP) and a probiotic candidate strain, Pediococcus pentosaceus (PEP, GenBank accession no. JX409638). The LAP strain has been shown to reduce Clostridium difficile in infants, and diarrhoea in irritable bowel syndrome patients( Reference Di Cerbo and Palmieri 22 ), and it has been shown to reduce the progression of NEC from stage II (proven NEC) to stage III (advanced NEC) in preterm infants( Reference Zampieri, Pietrobelli and Biban 23 ). The PEP strain was isolated from fermented whey( Reference Manurung 24 ). This species has been granted Qualified Presumption of Safety status by the European Food Safety Authority( 25 ), and was initially selected based on its ability to grow and reduce pH in whey permeate, a well-known diet supplement in both piglet and paediatric diets( Reference Manurung 24 ). Preliminary studies show that PEP inhibits the growth of several pathogen indicator strains in vitro (Streptococcus suis, Listeria monocytogenes, Salmonella typhimurium)( Reference Manurung 24 ).
Clinical status in piglets (growth, diarrhoea, blood parameters, haematology) were assessed together with parameters of intestinal structure (villus height, mucosal mass), function (digestive enzyme activities), intestinal permeability and microbiology (mucosa-adhering bacteria, bacterial composition, SCFA levels). These parameters have previously been shown to respond to probiotic interventions and reflect clinical status in newborn preterm, caesarean-derived, colostrum-deprived pigs( Reference Cilieborg, Thymann and Siggers 26 ).
Methods
Preparation of Lactobacillus paracasei F19, Pediococcus pentosaceus and Escherichia coli F18
The commercially freeze-dried probiotic strain LAP (provided by Arla Foods Amba) was used by mixing the powder into the milk replacer at a concentration of 2×106 colony-forming units (CFU)/ml. The other strain, PEP, isolated from fermented whey( Reference Manurung 24 ), was freshly grown and harvested around the time of birth for each litter in the experiment. In brief, PEP from a −80°C glycerol stock solution was grown in MRS broth (De Man, Rogosa and Sharpe agar; National Veterinary Institute) and bacteria were isolated, washed in sterile PBS, resuspended in the milk replacer (108 CFU/ml) and stored at 4°C. The storage viability and bacterial concentrations of LAP and PEP were verified by daily cultivations of milk samples.
F18 (9910297-2STM, O138:F18( Reference Frydendahl, Kåre Jensen and Strodl Andersen 18 )) was grown overnight at 37°C on blood agar plates. Approximately 50 µl of colony material was subsequently suspended in 4 ml of sterile PBS and poured onto an iso-sensitest agar (Oxoid), supplemented with Alizarin yellow 0·06 % w/v (Merck), chosen for optimal expression of F18 fimbria( Reference Wittig, Prager and Stamm 27 ). Excess suspension was discarded before culturing overnight at 37°C in 10 % CO2. The next day, 10 ml PBS was poured onto the plate with F18. Bacteria were gently scraped loose and vortexed into the PBS solution, corresponding to approximately 1010 CFU/ml, of which the pigs received 1 ml/d from days 1–4, with the first challenge provided 3 h after the initial LAB administration.
Animal protocol and experimental design
All experimental procedures were approved by the National Ethics Committee on Animal Experimentation (protocol no. 2012-15-2934-00193). Four sows (Danish Landrace×Large White×Duroc) were selected for homozygosity (M307 GG ) of the FUT-1 gene to ensure susceptibility to F18 adhesion and infection in the offspring( Reference Jensen, Cilieborg and Østergaard 19 ). A total of sixty-one pigs were delivered near to term (114 d, term=116 d) by caesarean section and piglets were immediately transferred to individual incubators with heating and O2 supply until euthanasia on day 5, as described previously( Reference Jensen, Sangild and Lykke 28 ). One litter (n 12–17) at a time was reared in the facility where the piglets were stratified according to birth weight and sex, and randomly allocated within each stratum to one of six experimental groups: untreated controls (CONT, n 9), LAP (n 10), PEP (n 10), F18 (n 10), F18–LAP (n 10) and F18–PEP (n 10). Although still sedated from the caesarean section, pigs were fitted with umbilical catheters (infant feeding tube 4 F; Portex) and oro-gastric feeding tubes (6 F; Portex), both secured to the skin with sutures. The initial gut colonisation was standardised by providing a maternal faecal suspension via the feeding tube (1 ml, 2×107 CFU), followed by a single 1 ml bolus of a milk replacer with no bacteria or containing one of the two LAB tested (108 CFU) to ensure immediate inoculation of LAB before full enteral feeding. Parenteral nutrition (7 ml/kg per h of Kabiven, Fresenius Kabi) was given for the first 15 h to increase intestinal sensitivity. To meet piglet nutrient requirements 274 of 1053 ml glucose and 80 of 400 ml fat was withdrawn, whereas 140 ml sterile water and 214 ml Vamin 18 Novum (amino acids; Fresenius Kabi) was added to reach the following composition: Energy, 3138 kJ/l; amino acids, 45 g/l; glucose, 72 g/l; lipids, 31 g/l; calcium chloride, 0·21 g/l; sodium glycerophosphate, 1·46 g/l; magnesium sulphate, 0·46 g/l; potassium chloride1·75 g/l; sodium acetate, 1·41 g/l. Mother´s plasma was co-infused (2×10·5 ml/kg) with the parenteral nutrition for passive immunisation, as described previously( Reference Sangild, Thymann and Schmidt 21 ). From day 2, parenteral nutrition was discontinued and replaced by full enteral feedings of 16 ml/kg per 3 h. All pigs were given milk replacer (Milex; Arla Foods), containing additional whey protein to support normal piglet growth (50 g/l Lacprodan DI-9224; Arla Foods) to reach the following composition: Energy, 3604 kJ/l; protein, 62 g/l; carbohydrate, 72 g/l; and fat, 37 g/l. Nutritional intake was adjusted according to body weights recorded daily together with core body temperatures. Group diets differed only with respect to addition of the probiotic bacteria. From day 2, LAB was given via the milk replacer in daily doses of 2·6×108 and 1·3×1010 CFU/kg for LAP and PEP, respectively. For LAP, the chosen dose was based partly on cost considerations for commercial use, which prevented supplementation to formula at a dose higher than 108 CFU/kg per day (discussions with our LAP supplier; Arla Foods). PEP was a new candidate probiotic, isolated from fermented whey, and a slightly higher dose was used for PEP to ensure a full response. The doses for LAP and PEP were both within the range of doses typically used for probiotic supplementation to formula for infants (107–1011 CFU/kg per day).
To prevent bacterial cross contamination between the treatment groups, high hygienic standards were maintained at all times. Incubators from the same treatment groups were clustered together within the experimental unit and feeding was separated. Diarrhoea was assessed twice a day from day 2 at 09.00 hours to day 5 at 09.00 hours resulting in a total of seven observations per pig. The following criteria were used to score the faeces: 0=no faeces, 1=firm faeces, 2=pasty faeces, 3=droplets of watery faeces/diarrhoea, 4=moderate amounts of diarrhoea and 5=large amounts of diarrhoea.
Intestinal permeability, organ and tissue sampling
Intestinal permeability was determined as the ratio of lactulose:mannitol in urine collected at euthanasia on day 5, 3 h after oral administration (15 ml/kg of 5 % lactulose and 5 % mannitol)( Reference Jensen, Sangild and Lykke 28 ). All pigs were fed half a bolus of their normal feed (8 ml/kg), 90 min before euthanasia to standardise the feeding state of the collected tissues. Blood was drawn (10 ml) by cardiac puncture into heparin- and EDTA-containing tubes before each piglet was killed with an intra-cardiac injection of sodium pentobarbital. Blood samples were immediately analysed for blood gases, haematocrit, glucose, lactate and pH (GEM Premier 3000; Instrumentation Laboratory) and blood cells were counted by an automatic cell counter (ADVIA 2120i; Hematology System). Internal organs (liver, kidney, lung, heart, spleen, colon and SI) were immersed in crushed ice, removed and weighed. The SI, caecum and colon were gently emptied of their contents by squeezing before weighing and recording of the SI length. On this basis, the SI was divided into proximal, middle and distal parts of equal size. Tissues from the middle of all three SI parts (each 4 cm) together with caecum and colon contents were collected, snap frozen in N2 and stored at −80°C for analyses of brush border enzymes (SI tissue), microbiota composition by high-throughput quantitative PCR (qPCR) analyses (caecum content) and SCFA concentration (colon contents). Moreover, sampling of mucosa-associated bacteria was performed. The most distal 35 cm of the SI was opened longitudinally and gently scraped with a sterile plastic slide and samples were stored at −20°C for analyses a few days later by high-throughput qPCR. Sections of 2 cm from the middle of the proximal, mid and distal SI and colon was collected and stored in paraformaldehyde for later histomorphology of SI and fluorescent in situ hybridisation (FISH) for SI and colon. Finally, mucosa was scraped from 10 cm sections from the last third of both the proximal and the distal SI and dried at 60°C for 72 h. Dry weight of total tissue and of mucosa alone was measured to determine the dry weight proportion of mucosa. To prevent degradation of intestinal tissue, organs and tissues were kept on a cooling table (2–4°C) during the entire sampling procedure. All samples were collected within 15 min of euthanasia.
Intestinal microbiology
Paraformaldehyde-fixed sections of the proximal, middle and distal SI and colon were embedded in paraffin and sectioned (3 µm). For each pig, the three SI sections and one section of colon were mounted on the same glass slide. FISH with oligonucleotide probes 5'-labelled with CY3 (Eurofins MWG Operon) targeting bacterial 16S ribosomal RNA for all bacteria (S-D-Eub-0338; 5'-GCT GCC TCC CGT AGG AGT-3') was performed. Sections were scanned (ArrayWoRx microarray scanner; Applied Precision to visualise bacterial micro-colonies and the scanning pictures were used for evaluation of bacterial abundance. Each tissue section were given a FISH score where 1=no bacteria, 2=few micro-colonies, 3=abundant bacteria located in the mucosal periphery and 4= extensive colonisation with bacteria closely associated with the mucosa. The used method cannot detect bacteria in the mucus layer, as this layer is not preserved after paraformaldehyde fixation.
Bacterial DNA was extracted from approximately 200 mg of caecum content and mucosal scrapings of distal SI, using the Maxwell LEV Blood DNA Purification Kit (Promega Corporation), according to the manufacturer’s instructions. This was performed after an additional pre-treatment with lysozyme for 2 h at 37°C, 2 min of bead beating at 20 Hz with 5 mm steel beads, and proteinase K treatment for 1 h at 56°C. DNA concentration was subsequently quantified on a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies).
Relative bacterial composition of mucosa-associated F18 was assessed in mucosal scrapings of distal SI by qPCR( Reference Ståhl, Kokotovic and Hjulsager 29 ). Caecum contents and SI mucosa were investigated for relative bacterial composition using a high-throughput in-house qPCR assay( Reference Hermann-Bank, Skovgaard and Stockmarr 30 ). This assay was set up as a relative 48×48 dynamic array integrated fluidic circuit system (Fluidigm) using primer sets targeting main phyla and taxonomically related subgroups in the porcine intestinal microbiota and normalises these against a primer set specific for all bacteria( Reference Hermann-Bank, Skovgaard and Stockmarr 30 , Reference Hermann-Bank, Skovgaard and Stockmarr 31 ). In all, twenty-two phylo groups were included in the assay, as described previously( Reference Hermann-Bank, Skovgaard and Stockmarr 30 , Reference Hermann-Bank, Skovgaard and Stockmarr 31 ), however archae bacteria was replaced by a primer set targeting the 16S ribosomal DNA gene in PEP (forward 5'-CTT-CCG-TTA-ATT-GAT-TAT-GAC-3', and reverse 5'-TAT-CAC-TGC-CTT-GGT-GAG-CCT-3').
Microbial metabolic activity was assessed by measuring SCFA concentrations (mmol/kg wet sample) by GC in samples of colon contents, as previously described( Reference Canibe, Hojberg and Badsberg 32 ).
Intestinal histomorphology and brush border enzymes
The above scanning pictures of paraformaldehyde-fixed sections of the proximal, middle and distal SI were used for measuring villous heights using the ImageJ Software (version 1.22c US; National Institute of Health). In all, ten representative villi were measured in each section, and a mean value was reported.
Samples of the proximal, middle and distal SI were extracted in 1·0 % Triton X-100 and homogenised. Homogenates were analysed for activities of lactase, sucrase, maltase, aminopeptidase N (ApN), aminopeptidase A (ApA) and dipeptidyl peptidase IV (DPPIV) using lactose, sucrose, maltose, glycyl-l-proline-4-nitroanilide, l-alanine-4-nitroanilide, and α-l-glutamic acid 4-nitroanilide, respectively, as substrates and determined spectrophotometrically as described previously( Reference Thymann, Møller and Stoll 33 ).
Statistical analyses
Determination of the sample size for the study population was based on a power calculation using the estimated mean 88 (sd 12) % (n 43) for F18-induced diarrhoea in previous pig studies( Reference Jensen, Cilieborg and Østergaard 19 , Reference Cilieborg, Sangild and Jensen 20 ) and an expected 20 % reduction of incidence with probiotic treatment based on three pig studies where early probiotic supplementation lead to a 17–26 % reduction of infectious diarrhoea incidence( Reference Shu, Freeman and Gill 14 – Reference Taras, Vahjen and Macha 16 ). Consequently, the estimated sample size for 90 % power was n 10.
Stata 12.0 was used for statistical analyses. Normality of data was evaluated by the histogram and qnorm command and by inspection of model residuals. Total leucocytes, lymphocytes and neutrophils were log transformed before statistical testing. This was also the case for propionic acid, butyric acid, formic acid, lactic acid and total SCFA. Group differences in the prevalence of diarrhoea were examined using Fisher’s exact test. The impact of F18 challenge (yes/no) and LAB (LAP, PEP or NoLAB) was assessed and data were analysed with two-way ANOVA models adjusted for sex and litter as control variables. All models included the interaction term F18×LAB. Correspondingly, P values related to the interaction and main effects of F18 or LAB are from these full models. In cases of significant F18×LAB interaction, differences between treatment groups were subsequently evaluated using the pwcompare command, which uses the least significant difference method. When no significant interaction but significant main effects were found, these were further investigated using the same method. Bacterial phylo group differences, relative to the CONT group, were assessed using the Mann–Whitney U test. To assess overall differences in the composition of the microbial communities, principal component analysis plots based on the presence or absence of signal in the qPCR analyses from the microbiota assay were performed using Unscrambler version 9.8 (CAMO). Values are means with their standard errors, unless otherwise stated. For all analyses, P<0·05 was used as the level of significance. P<0·10 was considered as a tendency to a difference between means.
Results
Of the sixty-one enrolled pigs, fifty-nine completed the study protocol with two pigs euthanised on day 2 because of the poor clinical condition (one F18 and one CONT pig). One CONT pig (11 %) had diarrhoea (score≥3) at one observation among the seven observations with an overall group score of 0·4 (sem 0·1). Conversely, 40 % (4/10) of the pigs in the F18 group had diarrhoea at some point. For three of the pigs this was transient with only one observation of diarrhoea while the last pig had persistent diarrhoea from day 3 (five observations). This resulted in a mean faecal score of 0·8 (sem 0·3). Despite fewer observations of diarrhoea in the F18 pigs than expected, a larger proportion of the faecal scores were categorised as diarrhoea for F18 v. CONT pigs (P<0·05, Fig. 1(A)). When administered alone, neither of the LAB affected diarrhoea scores, compared with CONT, as no LAP pigs had diarrhoea and one PEP pig (10 %) had diarrhoea for three observations resulting in a group score of 0·6 (sem 0·1). On the other hand, when co-inoculated with F18, LAB significantly increased diarrhoea compared with F18 pigs (P<0·05, Fig. 1(A)). Of the F18–LAP pigs, 40 % (4/10) had five to six observations of diarrhoea per pig (faecal scores 1·4 (sem 0·5)) and while 60 % (6/10) of the F18–PEP pigs had two to six diarrhoea observations per pig (faecal scores 1·6 (sem 0·4)), with no difference between the two groups (P 0·71).

Fig. 1 Proportion (%) of diarrhoea observations (faecal score≥3) out of total seven observations during the 5-d study period (A) and daily relative weight gain on day 5 (B). Values are means with their standard errors represented by vertical bars. NoLAB, no lactic acid bacteria; LAP, Lactobacillus paracasei F19; PEP, Pediococcus pentosaceus; F18, Escherichia coli F18; P
LAB, P value for effect of LAB inoculation; P
F18, P value for effect of F18 inoculation; P
LAB×PF18, P value for interaction of LAB and F18; ![]() , NoLAB;
, NoLAB; ![]() , LAP;
, LAP; ![]() , PEP. n 10 for all groups except for controls, where n 9. a,b,c Mean values with unlike letters were significantly different (P<0·05).
, PEP. n 10 for all groups except for controls, where n 9. a,b,c Mean values with unlike letters were significantly different (P<0·05).
All groups had similar birth weights (1249 (sem 304) g), and the daily weight gain was similar among treatment groups (Fig. 1(B)), except for the pigs receiving PEP, where growth rates were reduced after challenge with F18 (P<0·05). There was no difference in body temperature between groups, but temperatures increased from days 1 to 2 (36·2 (sem 0·03) v. 38·3 (sem 0·06)) and remained stable until euthanasia on day 5. By day 5, the intestinal permeability did not differ between groups (Fig. 2(A)) and the groups showed similar relative organ weights (g/kg) for colon (8·0 (sem 0·2)), lungs (18·4 (sem 0·8)), kidneys (7·9 (sem 0·1)), liver (24·1 (sem 0·4)), spleen (1·50 (sem 0·04)) and heart (7·7 (sem 0·1)). Across all F18-challenged pigs, the relative SI weight was higher than in non-infected pigs (37·3 (sem 0·9) v. 34·2 (sem 0·6) g/kg, P<0·01). Values for pH, pO2, haematocrit, glucose and lactate were similar between groups (Table 1), whereas values for pCO2 were decreased for F18 inoculated pigs (Table 1, P<0·01). Total leucocyte and neutrophil counts were not affected by treatments (Table 1) but monocyte and lymphocyte counts were increased across the F18-challenged pigs, relative to all non-challenged pigs (both P<0·05). Further, thrombocyte counts were lower (−20 %) in piglets receiving PEP, alone or in combination with F18, relative to pigs receiving LAP and NoLAB (Table 1, both P<0·05).

Fig. 2 Lactulose:mannitol (LM) ratio (A), mucosa proportion in the distal small intestine (B) and villus lengths in the distal small intestine (C). Data were obtained after euthanasia on day 5. Values are means with their standard errors represented by vertical bars. NoLAB, no lactic acid bacteria; LAP, Lactobacillus paracasei F19; PEP, Pediococcus pentosaceus; F18, Escherichia coli F18; P
LAB, P value for effect of LAB inoculation; P
F18, P value for effect of F18 inoculation; P
LAB×P
F18, P value for interaction of LAB and F18; ![]() , NoLAB;
, NoLAB; ![]() , LAP;
, LAP; ![]() , PEP. n 10 for all groups except for controls, where n 9. a,b Mean values with unlike letters were significantly different (P<0·05).
, PEP. n 10 for all groups except for controls, where n 9. a,b Mean values with unlike letters were significantly different (P<0·05).
Table 1 Blood parameters and blood haematology counts (cells 109/l) as affected by either lactic acid bacteria (LAB) supplementation or Escherichia coli F18 (F18) challenge at euthanasia on day 5Footnote * (Mean values with their standard errors)

LAP, Lactobacillus paracasei F19; PEP, Pediococcus pentosaceus.
a,b Mean values with unlike superscript letters were significantly different.
* P LAB, P F18 and P F18×LAB are P values from ANOVA models adjusted for litter and sex.
F18 challenge reduced the proportion of mucosa in the distal SI for the LAP pigs (Fig. 2(B)), but otherwise there were no marked differences in proximal or distal SI mucosal proportions among groups. Across all groups, the villi were longer in the middle SI (relative to the proximal and distal regions, P<0·05), with no differences between groups for any region. In the distal SI, F18–PEP pigs had numerically shorter villi than the other groups (Fig. 2(C)). Fig. 3 shows the enzyme activities in the proximal, middle and distal SI. Sucrase and maltase activity were generally highest in the proximal SI while lactase activity was higher in the proximal and middle SI. Peptidase activities were similar across all three SI regions. Inoculation with F18 decreased the activity of lactase in the middle and distal SI (P<0·001, Fig. 3(I) and (O)), and decreased sucrase activity in the middle SI (P<0·05, Fig. 3(G)). Similarly, also ApA and ApN in the middle and distal SI (P<0·001, Fig. 3(J), (K), (P), (Q)), and DPPIV in the distal SI (P<0·05, Fig. 3(R)) were decreased following F18 inoculation, whereas maltase activity in the distal SI was increased (P<0·05, Fig. 3(N)). LAP and PEP pigs had lower lactase activity in the proximal SI, compared with pigs not receiving probiotics (P<0·05, Fig. 3(C)), and PEP pigs had lower ApA activity in the middle and distal SI, compared with pigs not receiving probiotics (P<0·05, Fig. 3(J) and (P)). On the other hand, activity for sucrase in the distal SI was higher for LAP, compared with PEP and pigs not receiving probiotics (Fig. 3(M)). LAP also increased DPPIV activity in the proximal SI, compared with PEP (P<0·05, Fig. 3(F)).

Fig. 3 Brush border enzyme activities in the proximal (A–F), middle (G–L) and distal small intestine (M–R). Values are means with their standard errors represented by vertical bars. NoLAB, no lactic acid bacteria (n 19); LAP, Lactobacillus paracasei F19 (n 20); PEP, Pediococcus pentosaceus (n 20); F18, Escherichia coli F18 (n 30); NoF18, no Escherichia coli F18 (n 29); ApN, aminopeptidase N; ApA, aminopeptidase A; DPPIV, dipeptidyl peptidase IV; ![]() , NoLAB;
, NoLAB; ![]() , LAP;
, LAP; ![]() , PEP;
, PEP; ![]() , NoF18;
, NoF18; ![]() , F18. a,b Mean values with unlike letters were significantly different (P<0·05).
, F18. a,b Mean values with unlike letters were significantly different (P<0·05).
The relative abundance of mucosa-associated F18 bacteria in the distal SI was 103 times higher in F18-challenged pigs, relative to non-challenged pigs (P<0·001), and LAB administration did not affect the abundance (data not shown). Abundance of bacterial DNA is relatively low in the distal SI mucosa, relative to the high amounts of eukaryotic DNA and RNA. This probably led to the low and variable signals for other bacteria after tissue DNA extraction, and it was therefore not possible to assess the effect of LAB or F18 inoculation on these samples. PEP was not detected in the distal SI mucosa of any PEP-inoculated pigs, but it was present in the caecum content samples, both with and without F18 inoculation (P<0·05 relative to the CONT group, Fig. 4(a)). For the caecum content samples, the principal component analysis plots from the relative microbiota qPCR assay did not reveal any differences in the bacterial composition among the treatment groups (data not shown). However, a few differences were observed in the relative abundances of few bacterial groups, particularly for pigs administered PEP (Fig. 4(b)-(h)). Without F18 inoculation, PEP pigs had more Lactobacillaceae than CONT pigs (P<0·05, Fig. 4(f)). After F18 inoculation, F18–PEP pigs had more E. coli (P<0·05, Fig. 4(h)) and tended to have more βγ-proteobacteria (Fig. 4(e)) and Enterobacteriaceae (Fig. 4(G)), and less Firmicutes (Fig. 4(b)) and Actinobacteria (Fig. 4(d)) than CONT pigs (all P<0·10). Neither the LAP nor F18–LAP pigs differed significantly from CONT pigs for any of the bacterial groups. Scanning pictures of SI and colon tissues (Fig. 5(A) and (B)) showed, as expected, that the mucus layer had not been preserved during the paraformaldehyde fixation. FISH analyses of all bacteria showed that the bacterial abundance in the proximal, middle and distal SI mucosa was very low and many sections were negative for bacteria and it was not possible to assess the effect of LAB or F18 inoculation. Bacteria were more abundant along the colonic mucosa and pigs challenged with F18 had higher colon FISH scores than non-challenged pigs (P<0·001, n 29–30, Fig. 5(C)). No effect of LAB was observed.

Fig. 4 Selected phylo groups from caecum content, as assessed by the high-throughput quantitative PCR microbiota assay for Pediococcus pentosaceus (PEP) (a), Firmicutes (b), Bacteroidetes (c), Actinobacteria (d), βγ-proteobacteria (e), Lactobacillaceae (f), Enterobacteriaceae (g) and Escherichia coli (h). Values are means with their standard errors represented by vertical bars. Data were obtained after euthanasia on day 5 and values were normalised against a universal bacterial primer to obtain mean relative bacterial abundance, relative to total bacteria. NoLAB, no lactic acid bacteria; LAP, Lactobacillus paracasei F19; F18, Escherichia coli F18; ![]() , NoLAB;
, NoLAB; ![]() , LAP;
, LAP; ![]() , PEP. n 10 for all groups except for controls (CONT), where n 9. * P<0·05; † P<0·1: relative to samples from CONT pigs.
, PEP. n 10 for all groups except for controls (CONT), where n 9. * P<0·05; † P<0·1: relative to samples from CONT pigs.

Fig. 5 Representative fluorescent in situ hybridisation (FISH) staining of all bacteria in the mid small intestine and colon from a Lactobacillus paracasei F19 (LAP) pig (A and B, respectively) and bacterial abundance (FISH score) in the colon from pigs across all groups (C). Values are means with their standard errors represented by vertical bars. Data were obtained after euthanasia on day 5. NoLAB, no lactic acid bacteria; PEP, Pediococcus pentosaceus; F18, Escherichia coli F18. n 10 for all groups except for controls, where n 9. a,b Mean values with unlike letters were significantly different (P<0·001).
Total SCFA concentration in colon contents was increased by PEP, compared with LAP (Table 2). Among the SCFA, butyric and acetic acid concentrations were increased in PEP pigs, compared with LAP and CONT pigs (all P<0·05). F18 inoculation specifically increased succinic acid levels (P<0·05).
Table 2 Concentration of SCFA (SCFA) (mmol/kg) in colon content at euthanasia on day 5 as affected by either lactic acid bacteria (LAB) supplementation or Escherichia coli F18 (F18) challengeFootnote * (Mean values with their standard errors)
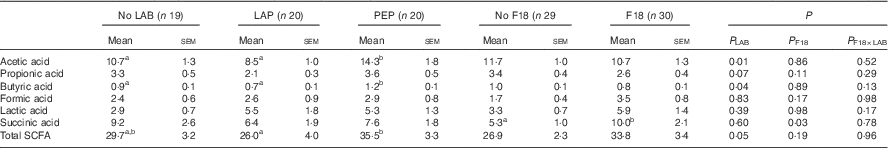
LAP, Lactobacillus paracasei F19; PEP, Pediococcus pentosaceus.
a,b Mean values with unlike superscript letters were significantly different.
* P LAB, P F18 and P F18×LAB are P values from ANOVA models adjusted for litter and sex.
Discussion
Administration of probiotic bacteria just after birth may be an attractive way to support the initial gut bacterial colonisation and thereby increase the resistance towards enteric pathogens in sensitive newborns. In contrast to our hypothesis, we failed to show that the two LAB strains chosen in this experiment supported intestinal health in newborn, caesarean-derived, colostrum-deprived pigs on parenteral nutrition, with or without administration of ETEC F18. In fact, both the LAP and PEP supplementations further increased the F18-induced diarrhoea.
In this study, the overall diarrhoea incidence was lower than that observed in our earlier studies with F18 challenge( Reference Jensen, Cilieborg and Østergaard 19 , Reference Cilieborg, Sangild and Jensen 20 ) but overall the data still support that the piglets were clinically affected by the F18 inoculation, both when given alone and in combination with LAB. The variation in clinical responses to F18 challenge among different studies reflects the common difficulty in getting consistent pathological response to enteric pathogen challenge in animal models. The challenge with F18 had limited or no effect on mucosal structure (e.g. proportion of mucosa, villous height) but the relative SI weight increased, probably reflecting oedema. The negative F18 effects were supported by decreased lactase and peptidase activities in the middle and distal SI. The gut microbiota composition was not markedly affected but F18 challenge increased the total number of bacteria adhering to the surface epithelium in the colon. Increased exposure of the gut epithelium to F18 may be related to the increase in colonic succinic acid level which may or may not be beneficial for the epithelium( Reference Lin 34 ). The high exposure to F18 may also induce systemic immune responses, as evidenced by increased monocyte and lymphocyte counts.
All these seemingly negative effects of F18 challenge were not prevented by LAB or PEP supplementation to formula. The presence of PEP bacteria was confirmed in caecum contents of PEP pigs and the presence of LAP after supplementation has been studied previously( Reference Di Cerbo and Palmieri 22 ). For intestinal enzymatic activities, we observed no difference or decreases (proximal lactase activity for LAP and PEP, ApA activity for PEP in the middle and distal intestine), and only one increase (distal sucrase activity for LAP). Specifically for the PEP probiotics, increased nutrient fermentation was indicated by higher levels of colonic acetic and butyric acid that may be detrimental for newborns( Reference Lin 34 ). We observed limited effects on the microbiota composition for both probiotics although PEP increased the proportion of Lactobacillaceae without F18 inoculation (potentially beneficial), and increased E. coli after F18 inoculation (potentially negative). Decreased thrombocyte counts for the PEP group also support a potential negative clinical effect. It is not clear if the higher (50-fold) daily dose of PEP v. LAP played a role for the tendency to more negative effects of PEP v. LAB for some parameters, but both strains were given in doses common for infant probiotic supplementations. We conclude that probiotic bacteria, as shown by LAP and PEP in this study, should be used with caution for sensitive newborns during their initial bacterial colonisation and intestinal immaturity. More studies are required to show if other doses, or later administration of LAP, PEP or other probiotic bacteria, induce more favourable effects in normal and pathogen-challenged newborns.
Our conclusions may be relevant for both pigs and infants. Still, it cannot be excluded that newborn caesarean-delivered, colostrum-deprived piglets on parenteral nutrition are more sensitive to suboptimal diets and enteric infections than normal newborn infants( Reference Sangild, Thymann and Schmidt 21 ). We have previously shown that F18 challenge of newborn caesarean-delivered, colostrum-deprived pigs increases diarrhoea more than in vaginally delivered, 3–20 day-old naturally suckled pigs, despite similar expression of the receptor required for F18 adhesion in both groups( Reference Jensen, Cilieborg and Østergaard 19 ). The sensitivity of the current model may to a large degree depend on the absence of protective factors in mother’s own colostrum and milk. Using a similar model, we recently showed that addition of the human milk oligosaccharide, α1,2-fucosyllactose (2'FL) to formula, had limited effects on diarrhoea in newborn pigs, despite in vitro studies showing reduced bacterial adhesion to intestinal cells after 2’FL-addition( Reference Cilieborg, Sangild and Jensen 20 ). This suggests that F18 may elicit diarrhoea also without specific intestinal adhesion. Nevertheless, in the present study F18 clearly colonised the distal SI mucosa in the F18-challenged groups, but none of the LAB managed to reduce clinical symptoms or F18 bacterial adhesion.
In pig farms, probiotics have successfully been given to pregnant sows( Reference Taras, Vahjen and Macha 16 ), newborn pigs( Reference Zeyner and Boldt 15 , Reference Taras, Vahjen and Macha 16 ) and weaning piglets( Reference Shu, Freeman and Gill 14 ) to prevent infectious diarrhoea. These studies showed a 17–26 % reduction in diarrhoea prevalence. There are no previous reports on effects of probiotics in infected, caesarean-derived, colostrum-deprived newborn pigs on parenteral nutrition, but older, suckled pigs have been used to assess probiotic effects upon challenge with ETEC strain E. coli F4( Reference Trevisi, Casini and Coloretti 35 – Reference Li, Zhu and Zhang 38 ). Interestingly, Lactobacillus rhamnosus GG (1010 CFU/d) reduced the daily weight gain and tended to increase diarrhoea and decrease intestinal villus heights in piglets challenged with E. coli F4 (a single bolus with 1010 CFU of E. coli F4( Reference Trevisi, Casini and Coloretti 35 )). In another study, Lactobacillus sobrius (1010–1011 CFU/d) reduced the pathogen density, but increased diarrhoea in E. coli F4-inoculated piglets (1010 CFU)( Reference Konstantinov, Smidt and Akkermans 36 ). When high doses of L. rhamnosus were compared with moderate doses (1011 v. 109 CFU/d( Reference Zhu, Li and Zhang 37 ), or 1012 v. 1010 CFU/d( Reference Li, Zhu and Zhang 38 )), both dose regimens ameliorated the diarrhoea induced by E. coli F4. Still, the higher doses were associated with more diarrhoea before E. coli F4-challenge, compared with lower dose( Reference Zhu, Li and Zhang 37 , Reference Li, Zhu and Zhang 38 ). Both doses of L. rhamnosus affected leucocyte sub-populations in the lamina propria and Peyer’s patches and circulating cytokine levels, with a more proinflammatory profile in the pigs given high-dose probiotics( Reference Zhu, Li and Zhang 37 , Reference Li, Zhu and Zhang 38 ). The low dose increased the expression of bacterial receptors Toll-like receptor (TLR) 2, TLR9 and nucleotide-binding oligomerization domain-containing protein 1 (NOD1) together with TNF-α ( Reference Li, Zhu and Zhang 38 ), suggesting doses below 1011 CFU/d may be advantageous( Reference Zhu, Li and Zhang 37 , Reference Li, Zhu and Zhang 38 ). Increased proinflammatory cytokine responses after administration of the presumed probiotic strain Pediococcus acidilactici (109 CFU/d) in weaned pigs challenged with E. coli F4 has been reported( Reference Daudelin, Lessard and Beaudoin 39 ), but most studies in weaned pigs have shown positive rather than negative effects of probiotics on growth and infection resistance( Reference Trevisi, Casini and Coloretti 35 – Reference Li, Zhu and Zhang 38 ).
In clinical practice, the dose chosen for probiotic administration will often be based on a combination of factors, for example biological, practical and commercial considerations. In the literature, a wide range of doses have been used for various probiotic strains (107–1012 CFU/d) and generally these doses are much below the total amount of bacteria in the normal colonised gut (1011–1012 CFU/g intestinal content). Different strains have different doses for their maximal efficacy and potential harm. In this study, we chose the doses for LAP and PEP based on considerations related to both feasibility and presumed biological response. For the commercially available LAP strain, its current price only makes a dose of 108 CFU/d feasible for use in practice. For the less known experimental strain, PEP, we chose a higher dose (1010 CFU/d), however still within the range of normal doses for probiotic administration, to better secure efficacy and colonisation. Ideally, a full range of doses (107–1010 CFU/d), times of administration after birth, and types of milk diets should be tested before using probiotic bacteria for infants. On the other hand, this does not affect our overall conclusion that probiotics administered in clinically relevant doses may have adverse effects in immune-compromised newborn pigs and infants.
An immature intestinal colonisation and immune system of pigs in this study may explain why introduction of moderate to high amounts of bacteria with probiotic properties may disturb a delicate microbial balance and trigger adverse effects. The parenteral feeding and lack of maternal-derived immunity via colostrum ingestion further compromises postnatal immunity, despite that systemic passive immunity was provided via infusion of maternal plasma over the 1st day after birth. In preterm pigs reared in a similar manner, we have previously shown that relatively high doses of probiotics (1010–1011 CFU/d), provided to assist the early gut colonisation during the 1st days after birth, resulted in either decreased or increased sensitivity to intestinal inflammation, dysfunction and NEC( Reference Cilieborg, Thymann and Siggers 26 , Reference Siggers, Siggers and Boye 40 ). Hence, seemingly beneficial bacteria, or mixtures of bacteria, may under some circumstances exacerbate intestinal dysfunction when given too early in too high doses to highly sensitive newborns. The high bacterial sensitivity of newborn formula-fed pigs is illustrated by the dramatic NEC-protective effects of delayed gut colonisation by antibiotics treatment( Reference Jensen, Thymann and Cilieborg 41 ) and absence of such symptoms when rearing preterm pigs in germ-free isolators( Reference Cilieborg, Sangild and Jensen 20 ).
Appropriate neonatal gut colonisation is an important consideration for infants, especially for those born prematurely. Together, intestinal immaturity, absence of mother’s milk and the hospital environment may facilitate an adverse bacterial colonisation that predisposes to intestinal problems and dys-regulated immunity. Stimulation of gut bacterial colonisation immediately after preterm birth is controversial and in most studies, probiotic supplementation does not start until a few days after birth, together with increasing enteral milk feeding. Across these studies, probiotics significantly reduce NEC risk in preterm infants( Reference Lau and Chamberlain 42 ) but the optimal strain(s), doses and timing remain unclear. It is possible that beneficial effects predominate if probiotics are provided in moderate doses (e.g. 108–109 CFU/d), together with mother’s own milk, and that adverse effects could be observed at higher doses, especially if provided immediately after birth and together with infant formula( Reference Repa, Thanhaeuser and Endress 43 ). Across the studies in preterm infants, it has been difficult to show a consistent change in the composition of the gut microbiota following probiotic administration and this is supported by the results of this study and with our own studies in preterm pigs( Reference Cilieborg, Thymann and Siggers 26 , Reference Siggers, Siggers and Boye 40 ).
Many factors vary in the studies on probiotic administration in both newborn infants and animal models, and this makes it difficult to make firm conclusions regarding the use of probiotics for newborns. Most previous pathogen challenge studies in animals were not done in newborns and often used lower doses of pathogens. We deliberately wished to study clinically relevant endpoints in the naïve, newborn intestine because of the potential to document a clear effect of adding presumed beneficial gut bacteria. With or without pathogen challenge, we could not demonstrate effects of LAB on bacterial density in the colon mucosa, but some significant effects were induced by PEP for bacterial composition in caecum content and SCFA levels in colon content (Lactobacillaceae, E. coli, acetic acid, butyric acid). Clinical endpoints were either not affected, or showed some negative effects, with the administered LAB. The total amount of administered microbes, together with bacteria-specific epithelial interactions, may be critical factors for the combined response to probiotics and pathogens in newborns. Thus, co-inoculation of pathogens and probiotic bacteria may under certain conditions lead to increased virulence, bacterial overload, excessive fermentation and diarrhoea in sensitive newborns( Reference Cilieborg, Thymann and Siggers 26 ).
In conclusion, newborn, caesarean-delivered, colostrum-deprived piglets on parenteral nutrition are highly sensitive to non-optimal milk diets and gut microbiota manipulations, as shown in this and other studies( Reference Jensen, Cilieborg and Østergaard 19 , Reference Cilieborg, Sangild and Jensen 20 ). The tested LAB were unable to improve clinical symptoms and intestinal indices in newborn pigs, regardless of F18 challenge. On the contrary, the LAB interventions appeared to increase the responsiveness to the ETEC F18. The apparent favourable effects of administering probiotic bacteria to preterm infants, and in alleviating diarrhoea and infections in older children, may partly be explained by the use of lower doses, later administration, together with better diets (e.g. mother’s own milk rather than infant formula). Caution is required when using relatively unknown probiotic strains, in relatively high doses immediately after birth in immune-compromised newborns.
Acknowledgements
The authors gratefully acknowledge Joanna Zeitman Amenuvor, Sophia Rasmussen and Elin Skytte for their technical assistance and Michael Ladegaard Jensen and Stine Ostenfeldt Rasmussen for assistance with animal procedures during the study.
The study was funded by Green Development and Demonstration Programme under the Ministry of Environment and Food of Denmark, the NEOMUNE project funded by the Danish Research Councils (grant no. 0603-00774B) and Arla Foods Amba. The latter contributed to the study design but not to the final preparation of the manuscript.
A. D. A., P. T. S., M. S. C. were responsible for the conception and design of the research; A. D. A., M. S. C. performed experiments; A. D. A., M. S. C. and C. L. analysed data; A. D. A., P. T. S., M. S. C., C. L. interpreted results of the experiments; A. D. A. and P. T. S. drafted the manuscript; A. D. A., P. T. S., M. S. C., A. L. M. and C. L. edited and revised the manuscript; A. D. A., P. T. S., M. S. C., C. L. and A. L. M. approved the final version of the manuscript.
A. L. M. is employed at Arla Foods.










