Introduction
Intertidal habitats are characterized by a cyclical change of abiotic stress factors (temperature, desiccation, wave action) and biotic risks, primarily predation (Branch, Reference Branch1981; Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983; Chapman & Underwood, Reference Chapman, Underwood, John, Hawkins and Price1992; Raffaelli & Hawkins, Reference Raffaelli and Hawkins1996). Hence for mobile organisms, time is typically partitioned into periods of activity, and periods in refuges that offer relative shelter from predators and environmental stressors, when risks are greater. Foraging activity, therefore, represents trade-offs between the requirement for food resources, the quality of the food available, and the risks associated with acquiring these resources (Burrows et al., Reference Burrows, Santini and Chelazzi2000; Clark & Mangel, Reference Clark and Mangel2000; Santini et al., Reference Santini, Ngan, Burrows, Chelazzi and Williams2014).
The direct effects of abiotic and biotic factors in influencing activity are well documented for a wide range of intertidal species (Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983; Chapman & Underwood, Reference Chapman, Underwood, John, Hawkins and Price1992; Raffaelli & Hawkins, Reference Raffaelli and Hawkins1996; Little et al., Reference Little, Williams and Trowbridge2009 for reviews). These influences may be further modified as a result of the indirect effects of other organisms. In some cases such effects may be relatively predictable (e.g. a dense algal canopy can provide shelter from desiccation and refuge from predators, Moore et al., Reference Moore, Hawkins and Thompson2007), but sometimes relatively subtle differences in the abundance of one species can have dramatic and often unpredictable indirect effects on the behaviour and abundance of another species (Anderson, Reference Anderson1999; Trussell et al., Reference Trussell, Ewanchuk and Matassa2006; O'Connor et al., Reference O'Connor, Emmerson, Crowe and Donohue2013; Rashidul Alam & Noda, Reference Rashidul Alam and Noda2016). Moreover, the costs and benefits of activity out of the shelter are variable in both time and space, and to maximize benefits and minimize risk at any given time it is important for an organism to be able to react to locally changing conditions and modify its behaviour. The ability to do so varies among individuals and among species according to the relative importance of their endogenous rhythms and the ability to override them in response to exogenous cues (Little, Reference Little1989; Santini et al., Reference Santini, Thompson, Tendi, Hawkins, Hartnoll and Chelazzi2004, Reference Santini, Tendi, Righini, Thompson and Chelazzi2005).
Limpets are an important component of rocky intertidal assemblages worldwide and their grazing is known to have a key role in the ecology of these habitats (Hawkins, Reference Hawkins1981; Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983; Jenkins et al., Reference Jenkins, Coleman, Hawkins, Burrows and Hartnoll2005; Coleman et al., Reference Coleman, Benedetti-Cecchi, Åberg, Arenas, Arrontes, Castro, Hartnoll, Jenkins, Paula, Della Santina, Underwood and Hawkins2006; Burgos-Rubio et al., Reference Burgos-Rubio, De la Rosa, Altamirano and Espinosa2015). Reducing the density of these grazers is consistently followed by a proliferation of micro and then macroalgae and can lead to substantial changes in assemblage composition and ecosystem functioning on rocky shores (Hawkins et al., Reference Hawkins, Hartnoll, Kain, Norton, John, Hawkins and Price1992; Poore et al., Reference Poore, Campbell, Coleman, Edgar, Jormalainen, Reynolds, Sotka, Stachowicz, Taylor, Vanderklift and Duffy2012). Barnacles are sessile filter feeders, being major occupiers of space on exposed and moderately exposed rocky shores and are common worldwide (Lewis, Reference Lewis1964; Stephenson & Stephenson, Reference Stephenson and Stephenson1972). Due to the importance of both limpets and barnacles, investigating their interactions is essential for a better understanding of the dynamics of rocky shore communities (Hartnoll & Hawkins, Reference Hartnoll and Hawkins1985; Johnson et al., Reference Johnson, Burrows, Hartnoll and Hawkins1997, Reference Johnson, Burrows and Hawkins1998; Burrows & Hawkins, Reference Burrows and Hawkins1998; Hawkins et al., Reference Hawkins, Moore, Burrows, Poloczanska, Mieszkowska, Herbert, Jenkins, Thompson, Genner and Southward2008; Jenkins et al., Reference Jenkins, Moore, Burrows, Garbary, Hawkins, Ingólffson, Sebens, Snelgrove, Wethey and Woodin2008). Limpets are known to dislodge barnacle cyprids that have recently settled from the plankton (e.g. Hawkins, Reference Hawkins1983; Dungan, Reference Dungan1986; Jenkins et al., Reference Jenkins, Norton and Hawkins1999; Holmes et al., Reference Holmes, Walker and van der Meer2005), but information on the effects of barnacles on limpets living among barnacles are scarcer. Growth rates and maximum sizes of limpets can be reduced where barnacles and other sessile organisms are abundant (Lewis & Bowman, Reference Lewis and Bowman1975; Choat, Reference Choat1977; Thompson, Reference Thompson1980; Hawkins & Hartnoll, Reference Hawkins and Hartnoll1982; Dunmore & Schiel, Reference Dunmore and Schiel2003) and there is anecdotal evidence that the rugosity of barnacle dominated surfaces may impede the ability of limpets to deposit the mucous trails along which they glide to facilitate locomotion, limiting their ability to adhere to the substratum and hence to resist dislodgement by predators and wave action (Smith, Reference Smith1991). Fraser et al. (Reference Fraser, Coleman and Seebacher2014) demonstrated that topographic complexity associated with barnacles affects the resting orientation of limpets on vertical surfaces. Finally, there is also evidence that barnacles may provide a greater resource of microalgae, on which limpets feed, than that present on adjacent areas of open rock (Thompson et al., Reference Thompson, Wilson, Tobin, Hill and Hawkins1996). Understanding the effect of the presence of barnacles on limpet behaviour may help clarify the mechanisms underlying recruitment of algae to rocky shores. For example, it is known that barnacles may promote fucoid recruitment (e.g. Hawkins, Reference Hawkins1981), but it is not yet clear whether this effect is entirely due to an increase of refugia for algal propagules or if a modification of limpet behaviour may play a role.
Here we examine the effects of barnacles on the behaviour of the homing limpet Patella vulgata (Linnaeus, 1758). This species is a putative keystone grazer on North-east Atlantic rocky shores (Jenkins et al., Reference Jenkins, Coleman, Hawkins, Burrows and Hartnoll2005; Coleman et al., Reference Coleman, Benedetti-Cecchi, Åberg, Arenas, Arrontes, Castro, Hartnoll, Jenkins, Paula, Della Santina, Underwood and Hawkins2006), often being used as a model organism to investigate the behaviour of intertidal grazers and its influence on the dynamics of algal-grazer interactions (e.g. Thompson et al., Reference Thompson, Johnson and Hawkins1997, Reference Thompson, Norton and Hawkins2004; Burrows & Hawkins, Reference Burrows and Hawkins1998; Johnson et al., Reference Johnson, Burrows and Hawkins1998; Jenkins et al., Reference Jenkins, Åberg, Cervin, Coleman, Delany, Della Santina, Hawkins, Lacroix, Myers, Lindegarth, Power, Roberts and Hartnoll2000; Jonsson et al., Reference Jonsson, Granhag, Moschella, Aberg, Hawkins and Thompson2006). Previous studies on the behaviour of P. vulgata have revealed considerable variability in the timing of activity. Populations from different localities have been shown to be active during different phases of the tidal and day/night cycles (e.g. Hawkins & Hartnoll, Reference Hawkins and Hartnoll1982, Reference Hawkins and Hartnoll1983; Little, Reference Little1989; Williams et al., Reference Williams, Little, Morritt, Stirling, Teagle, Miles, Pilling and Consalvey1999); but variability has also been described among limpets belonging to the same population but resting in different micro-habitats (i.e. vertical vs horizontal surfaces) (e.g. Della Santina et al., Reference Della Santina, Santini and Chelazzi1995; Santini et al., Reference Santini, Thompson, Tendi, Hawkins, Hartnoll and Chelazzi2004). Several factors, such as desiccation stress, dislodgement risks and/or predation have also been invoked to explain the observed patterns at different sites, season of the year, micro-habitat or level on the shore. Preliminary work by Hawkins & Hartnoll (Reference Hawkins and Hartnoll1982) indicated that barnacle cover can influence tide-out foraging behaviour on a vertical barnacle dominated surface, where limpets were observed foraging when the tide was out as well as during both day and night provided conditions were damp, but not when raining. In this paper, the behaviour of limpets was investigated using autonomous radio-telemetry on the same vertical harbour wall described by Hawkins & Hartnoll (Reference Hawkins and Hartnoll1982). We compared their activity patterns on plots characterized by dense barnacle cover and plots from which the barnacles were experimentally removed. Given that it is known that the behaviour of limpets may change with height on the shore, we explored the effect of barnacle cover at two different tidal heights.
We hypothesized that barnacle cover may affect limpet behaviour in two different ways. The first detectable effect should be on the temporal budget of activity. Since barnacles may offer greater resources of microalgae than smooth surfaces, while at the same time exposing limpets to greater risks of dislodgement, we hypothesized that limpets on barnacles would be time-minimizers (Evans & Williams, Reference Evans and Williams1991; Santini & Chelazzi, Reference Santini and Chelazzi1996) when compared with limpets on smooth concrete. A second hypothesized effect is that the presence of barnacles influences the distribution of foraging effort among each of the four available activity windows (tide-out daytime, tide-in daytime, tide-out night-time, tide-in night-time). Dense barnacle cover is likely to affect the ability to adhere to the substratum when out of the home scar, hence increasing the risk of dislodgement and vulnerability to predation, forcing limpets to be active during less risky time periods. In particular we expect that limpets living on vertical barnacle-covered surfaces will be more active during tide-out periods (lower risks of dislodgement and predation), especially at night (reduced desiccation stress), than limpets on smooth surfaces. As small limpets are easier to prey upon than larger ones and lose water more readily, we expect that this pattern would be more pronounced in smaller than larger limpets.
Materials and methods
The study was carried out at Port Erin, Isle of Man (UK). Data were collected during three periods each of ~2 weeks over the spring-neap cycle during spring 1999 (first from 21 April to 4 May; second from 6 May to 19 May; third from 29 May to 11 June). The study area consisted of a vertical harbour wall, The Raglan Pier, which is faced in concrete, was also used by Hawkins & Hartnoll (Reference Hawkins and Hartnoll1982). The base of this pier wall was covered by coarse sand just above mean low water springs (MLWS) (0.75 m above lowest astronomical tide, LAT) and the wall extends in height to well above mean high water springs (MHWS). The study was conducted between about mid tide level (~2.8 m above LAT) and mean high water neaps (MHWN) (4.6 m above LAT, Figure 1) in the region of the pier covered with barnacles (>95% cover). Tides are semidiurnal in this location with maximum height of around 5.0 m above LAT during spring tides. The experiments were carried out on the exposed side of the pier. However, the site can be considered sheltered from wave action, as it is protected by a breakwater which lies to the seaward side of the pier.
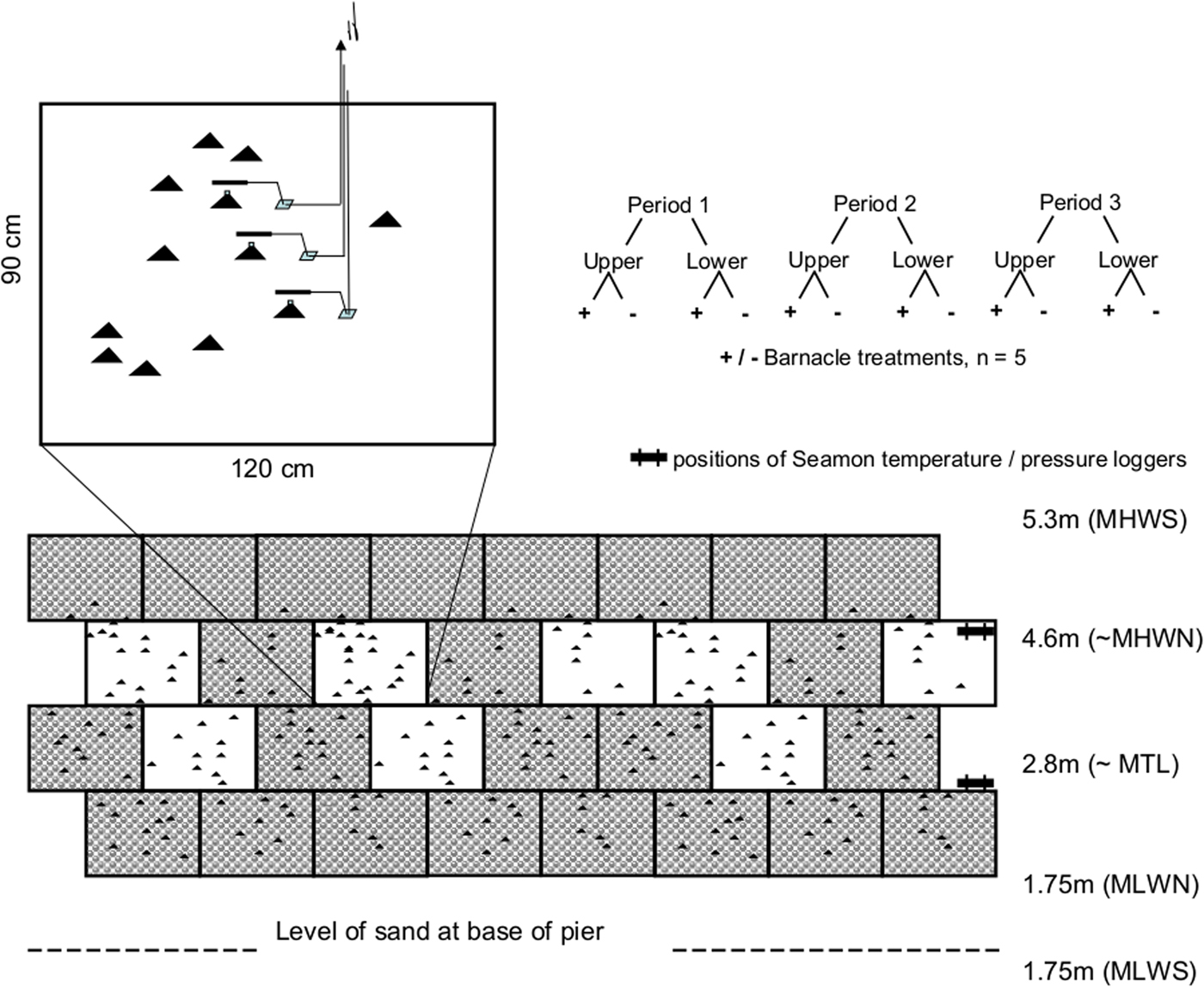
Fig. 1. Schematic representation of the study site and sampling design. Grey blocks are barnacle-covered areas, whilst white blocks represent smooth surfaces.
The concrete face of the pier wall had conveniently been constructed with 120 cm by 90 cm blocks. The study area was arbitrarily subdivided into two elevation levels (referred to as ‘lower’ and ‘upper’, respectively), whose height corresponded to the height of one block. During 22–28 February 1999 barnacles were scraped from the entire surface of several blocks at each of two tidal levels. Scraped blocks were interspersed with unscraped ones. Care was taken not to dislodge limpets during the removal of barnacles. A period of one month was allowed for the limpets and the biofilm on the scraped surface to recover from this disturbance before behavioural recording started. The recordings ended 2.5 months from disturbance; thus there should have been sufficient time for recovery of microalgae (Hill, Reference Hill1990). One month was sufficient for new limpet shell growth to occur to fit the concrete around the home scar. However, we cannot exclude the possibility that microalgae were still in the recovery phase when the recordings were carried out.
The response, in terms of limpet activity, to the following factors was considered:
1. Type of substratum (‘substratum’, SU): fixed factor with two levels (‘barnacle’, ‘smooth’).
2. Height on the shore (‘height’, HE): a fixed factor with two levels ‘upper’ and ‘lower’, each corresponding to one of the two rows of blocks used (Figure 1).
Limpet behaviour was monitored using the telemetric technique described by Santini et al. (Reference Santini, Righini and Chelazzi2001) that allows the long-term recording of activity rhythms for homing species. Briefly, the system consisted of 30 reed switches each mounted on metal support arms, which were distributed among the different height and substratum levels. Individuals in the central portion of each block were used wherever possible so as to minimize the influence of any edge effects. Reed switches were positioned so as to overhang the home scars of these individuals, but sufficiently far away so as not to interfere with access by the limpets to and from their home scars. Small magnets (weight ~1.5 g) were attached with Milliput epoxy putty to the shell of the limpet on each of these home scars and the length of each individual recorded. Each of the 30 individual switches was connected via a cable to a transmitter positioned well above MHWS. Sea level was monitored at 1 min intervals using Seamon TD water pressure loggers, positioned at the average tidal height of the limpets in both upper and lower tidal levels (Figure 1). Every five minutes the equipment assigned each limpet a home or away signal. This information was transmitted to a Televilt RX-900 scanner/logger, located at the Port Erin Marine Laboratory of the University of Liverpool (now closed). The data were then combined with information on the state of the tide obtained from the Seamon loggers and the time of dawn and dusk, obtained from tide tables, to give the activity status of each individual in relation to the four temporal windows described. After two weeks the reed switches were repositioned over different individuals and magnets attached to the new individual as before. Thus each 2-week period, encompassing a spring-neap tidal cycle, provided data on a different set of ~30 animals. In some cases an animal did not yield sufficient useful information, for example because it relocated home scar or went missing during the experiment. Data from these individuals were discarded from subsequent analyses. The final fully balanced design included 15 individuals in each experimental condition (upper and lower shore, plus and minus barnacles). Limpets used in the analyses were within the size range 25–36 mm shell length (mean 29.6) and the upper shore limpets were slightly larger (average values ± SE: upper = 30.2 ± 0.1, lower = 28.8 ± 0.1 mm, t-test P = 0.036), reflecting vertical gradients in size in this species.
At the beginning of the sampling periods limpets that were to be monitored with the telemetric apparatus, together with a further 20 individuals in each experimental treatment, were uniquely labelled with micro-marker number tags. The home scars of these individuals were also labelled. Records of the position of the limpets as being home or away were made by visual observation twice during each time window, either by accessing the site from the sand below the harbour wall at low tide, or by scuba at high tide. For tagged individuals that were active at these times the substratum on which the animal was moving was noted. These data were used to cross check the data provided by the Televilt equipment, but also to monitor if limpets living on barnacles had moved to forage on smooth surfaces or the reverse. The presence of any predators was also recorded during these visual observations. Previous observations showed that the main tide-in predators were the crabs Necora puber (Linnaeus, 1767), Carcinus maenas (Linnaeus, 1758) and Cancer pagurus (Linnaeus, 1758) (Thompson et al., Reference Thompson, Jenkins and Bussell2000; confirmed by more recent work by Silva et al., Reference Silva, Hawkins, Boaventura and Thompson2008, Reference Silva, Hawkins, Boaventura, Brewster and Thompson2010a, Reference Silva, Hawkins, Clarke, Boaventura and Thompson2010b). Predation by floating gulls on emersed limpets at the waterline occurred very occasionally (RCT personal observation).
Chippings from the surface of the pier were collected from each experimental treatment (six replicates each) to obtain an estimate of microbial food resources by chlorophyll-a extraction (Thompson et al., Reference Thompson, Tobin, Hawkins and Norton1999). In the case of the barnacle-covered surfaces, the barnacle plates were scraped from the concrete and then the soft body parts of each individual barnacle were removed with forceps so as to obtain an estimate of the microbial biomass from the outside of the barnacle plates only. This was only undertaken at the end of the final monitoring period because of its destructive nature.
Two distinct aspects of behaviour were compared among treatments:
1. Long-term activity budget, for which the following variables were computed from individual time series:
a. Proportion of time active (RT), computed as the ratio between the time spent by each individual away from home and the total recording time;
b. Average duration of activity bouts (DU, hours);
c. Number of bouts/day (NB) performed by each limpet.
2. Selection for given tidal and diel phases, for which the following variables were computed:
a. Proportion of activity performed during emersion periods (ET), computed as the ratio between the time spent active during tide-out and total activity time;
b. Proportion of activity performed during night-time (NT), computed as the ratio between the time spent active during the night and total activity time.
The effect of the two factors considered for the long-term budget of activity was investigated through two-way ANOVA (Underwood, Reference Underwood1997) using the GMAV software package. The relationship between activity, limpet length, height on the shore and surface rugosity was analysed using ANCOVA and linear regression (Sokal & Rohlf, Reference Sokal and Rohlf1995). The joint variation in the proportion of activity performed during emersion periods and the proportion of activity during night-time (ET and NT, respectively) was assessed through non-parametric multivariate analysis of variance (PERMANOVA), according to Anderson (Reference Anderson2001).
Results
The analysis of chlorophyll content confirmed that barnacle-covered surfaces (irrespective of tidal height) had considerably greater standing stock of microalgal food than concrete surfaces (average chlorophyll content, 13.56 ± 0.67 vs 1.23 ± 0.178 µg cm−2, F 1,20 = 291.06, P < 0.001). There was no detectable effect of height on the shore on chlorophyll content (F 1,20 = 0.01, P = 0.937).
In terms of activity across all possible tidal windows, the average proportion of the total time recorded during which limpets were active (proportion active, RT) was affected both by rugosity and height but not by their interaction (Table 1; Figure 2). In particular, time active was greater on smooth surfaces than on barnacles (Figure 2A) and on the lower shore than the upper shore (Figure 2B). The comparison of the number of foraging excursions per day revealed a similar effect of both substratum type (Table 1; Figure 2C) and tidal height (Figure 2D), but not of their interaction. Finally the average duration of each foraging excursion (Table 1; Figure 2E, F) was affected by substratum but not by height on the shore or their interaction.

Fig. 2. Variation in the proportion of time active, mean number of excursion per day and average duration of excursions, across surface type (A, C, E) and tidal level (B, D, F), respectively. Average values and standard errors are shown. Asterisks show P values: *P < 0.05; **P < 0.01, ***P < 0.001, otherwise non-significant.
Table 1. ANOVA on activity of Patella vulgata on surfaces with and without barnacles at each of two tidal levels on the Raglan Pier, Isle of Man, UK

C, Cochran's test for homogeneity of variances; n.s., not significant.
Data were transformed as indicated at the bottom of the table.
A summary of the analysis of the choice of activity phases is shown in Figure 3 and Table 2. Timing of activity, measured as the joint change in the fraction of activity performed during emersion (ET) and during night-time (NT), was very variable but, in general, limpets were either predominantly active during diurnal high tides or diurnal low tides. A few limpets were also active during diurnal low tides, especially on damp days. Whereas nocturnal high tides were always avoided (Figure 3A). Choice of the activity phase was affected by a first-order interaction between presence/absence of barnacles and height on the shore, as revealed by PERMANOVA (Table 2) and was evident from the phase-space portrait of average NT and ET values from the different groups of limpets (barnacle upper-shore, smooth upper-shore, barnacle lower-shore and smooth lower-shore) reported in Figure 3B. Individuals on barnacles on the upper shore were generally more active during low water (ET ~70%) at night (NT ~60%); whilst individuals on smooth surfaces on the upper level and all individuals on the lower shore tended to have more similar behaviour and favoured excursions centred in daylight hours (NT ~40%) with a reasonably equal distribution of activity between periods of low and high water (ET ~50%).

Fig. 3. (A) Mean individual proportion of activity performed during emersion (ET) and night (NT) by limpets in the different groups (closed circles = barnacle top shore; closed triangles = barnacles low shore; open circles = smooth surface top shore, open triangles = smooth bottom shore). (B) Mean (±SE) group ET and NT values.
Table 2. Results of PERMANOVA on the joint variation in the proportion of activity performed during emersion periods and the proportion of activity during night-time on surfaces with and without barnacles at each of two tidal levels

Euclidean distance dissimilarity was used and 4999 random permutations of residuals under the reduced model were performed. Variable names as in Table 1.
Closer inspection of individual average activity reveals more subtle details on the strategy followed by each individual limpet (Figure 3A). Limpets on barnacles on the upper shore showed the greatest variability in the choice of activity phase and despite the majority (N = 9) being active during nocturnal low tides a few were clearly more active during diurnal high tides (bottom left part of the plot), whereas others seemed to adopt a mixed strategy (characterized by individual ET and NT values close to 0.5). Individual, but less pronounced, variability was also present in other groups.
The size range of the limpets used in these experiments was relatively narrow; there were, however, significant relationships between the size of individuals and their behaviour. ANCOVA showed that the fraction of activity performed during emersion (ET) was influenced by the interaction between size of the limpets and height on the shore, but not by the type of substratum (Table 3; Figure 4). In particular, ET values decreased with increased size for limpets living on the high shore; but not for those living on the lower part of the wall (F 1,28 = 0.38, P > 0.05; F 1,28 = 16.35, P < 0.001, respectively).

Fig. 4. Relationship between the proportion of activity performed during emersion (ET) and limpet shell length, according to height on the shore and substratum type (closed circles = barnacle top shore; closed triangles = barnacles low shore; open circles = smooth surface top shore, open triangles = smooth bottom shore). Continuous line = relationship observed for limpets high on the shore; dashed line = relationship for low-shore limpets.
Table 3. Results of ANCOVA to evaluate the relationships between the size of limpets and their behaviour

Variable names as in Table 1.
Finally, the direct observation of limpet behaviour revealed that none of the limpets on barnacles moved to forage on nearby smooth areas, whereas the reverse was true. In fact, ~27% of excursions by limpets living on smooth surfaces were observed to occur on barnacle-covered areas.
Discussion
We revealed a significant effect of barnacle cover on the behaviour of Patella vulgata. Effects were detected for many of the components of its behaviour (overall activity budget, choice of specific time window) and varied between limpets of different size.
Limpets living on barnacles spent a lower proportion (~19%) of their time active than limpets on smooth surfaces (~26%), performed a lower number of excursions each day and these were shorter than those of limpets on smooth surfaces, thus confirming they were time-minimizers. The most likely determinant of this pattern is the difference in microalgal standing stock, which was more than 10 times higher on barnacles than on smooth surfaces: clearly the more rich the food supply available the lower the time needed to obtain a specific energy level. A similar pattern has been described previously when comparing the differences in standing stock and grazing between sheltered and exposed shores (e.g. Jenkins & Hartnoll, Reference Jenkins and Hartnoll2001). That barnacle-covered areas are more energy-rich and hence attractive to limpets seems to have been confirmed by direct observations, which showed that 27% of tagged limpets on smooth surfaces moved to forage on barnacle-covered blocks. In contrast, movements in the opposite direction were never observed. Direct comparison of standing crop between smooth and barnacle covered surfaces may, however, be difficult as the exact surface area to be grazed is not known. On one hand, barnacles increase the rugosity of the substratum and hence the surface available for algal growth. On the other, however, it is likely that not all of the microbial material living in small pits and cracks amongst the barnacle mosaic is available to the limpets (Hill & Hawkins, Reference Hill and Hawkins1991; Thompson et al., Reference Thompson, Wilson, Tobin, Hill and Hawkins1996; Hutchinson et al., Reference Hutchinson, Nagarkar, Aitchison and Williams2006). It is important to stress that inferences on foraging activity and food ingestion obtained from the observation of the time spent out of the home scar are risky and may potentially lead to erroneous conclusions. The distribution of microalgae and bacterial mucilage is very patchy, and limpets can actively select different patches (e.g. Jackson et al., Reference Jackson, Murphy and Underwood2013) and, hence, detailed observation of limpets' behaviour and cross-checking with gut contents analysis are recommended to infer the actual amount of ingested material. Moreover, although we know that most of the time limpets spend out of the home scar is devoted to foraging (Chelazzi et al., Reference Chelazzi, Parpagnoli and Santini1998), radula activity may sometimes stop, and limpets can move or rest without scraping the substratum (Hartnoll & Wright, Reference Hartnoll and Wright1977; Coleman et al., Reference Coleman, Underwood and Chapman2004).
The second likely determinant of the difference in time budgets between limpets on barnacle and non-barnacle covered surface is represented by increased risks. Limpets are known to adhere to the substratum using a combination of suction and gluing (Smith, Reference Smith1991; Denny, Reference Denny2000). Suction is suggested to be the main mechanism whilst foraging (Smith, Reference Smith1992), but for suction to work, the edge of the foot must be sealed to the substratum. This is hard to achieve when moving on an irregular surface. In addition, suction may provide good resistance to hydrodynamic lift, but provides poor resistance to shear forces (Ellem et al., Reference Ellem, Furst and Zimmerman2002). On vertical surfaces, where individuals are subject to a constant downward gravity force, the reduction of adherence may easily become critical, making limpets more susceptible to dislodgement by waves and predation (e.g. Coleman et al., Reference Coleman, Goss-Custard, Le, Dit Durell and Hawkins1999; Thompson et al., Reference Thompson, Jenkins and Bussell2000; Silva et al., Reference Silva, Hawkins, Boaventura and Thompson2008; Fraser et al., Reference Fraser, Coleman and Seebacher2014).
Choice of the diel/tidal window for activity showed a preference for moving either during daytime submersion or low-tide at night, a finding broadly in line with previous work on the behaviour of this species (e.g. Hartnoll & Wright, Reference Hartnoll and Wright1977; Hawkins & Hartnoll, Reference Hawkins and Hartnoll1982; Williams et al., Reference Williams, Little, Morritt, Stirling, Teagle, Miles, Pilling and Consalvey1999; Santini et al., Reference Santini, Thompson, Tendi, Hawkins, Hartnoll and Chelazzi2004). This pattern is dictated by a trade-off between different types of stresses and risks, for example, desiccation during daytime tide-out periods and predation during nocturnal tide-in periods. During the unused temporal window of high water at night (consistent with Hawkins & Hartnoll, Reference Hawkins and Hartnoll1982), predatory crabs (Necora puber, Carcinus maenas and Cancer pagurus) were commonly observed on the experimental blocks and on the seabed beneath the pier wall (see also work by Silva et al., Reference Silva, Hawkins, Boaventura and Thompson2008, Reference Silva, Hawkins, Boaventura, Brewster and Thompson2010a, Reference Silva, Hawkins, Clarke, Boaventura and Thompson2010b, Reference Silva, Boaventura, Thompson and Hawkins2014, on crab predation on limpets and foraging in the intertidal zone). Marks from crab chelae (Thompson et al., Reference Thompson, Jenkins and Bussell2000) were also observed on the surface of the resin used to fix magnets on the limpets and on one occasion a crab was seen eating a limpet.
Whilst confirming previous findings, our study provides new insights on the choice of the temporal window for activity in P. vulgata. Limpets living on barnacles on the upper shore clearly differed from all other groups, being the only group spending most of their activity during nocturnal low tides: although considerable within-group, inter-individual, differences were evident. Hawkins & Hartnoll (Reference Hawkins and Hartnoll1982) working at mid-shore showed that barnacle removal affected limpet behaviour, reducing their foraging activity during emersion. Our results support this finding but only for limpets on the upper shore and not for those on the lower shore, which behaved similarly to limpets on smooth surfaces. Limpets from other treatment groups appeared to be more day-active, although more unselective than previously reported (e.g. Santini et al., Reference Santini, Thompson, Tendi, Hawkins, Hartnoll and Chelazzi2004). For these groups, in fact, the average proportion of activity performed during emersion (ET) and night-time (NT) were in fact close to 0.5, meaning that an ‘average’ limpet had no specific preference for either high or low tide or day and night. This average pattern was, however, dictated by a mix of inter-individual differences (where each limpet had a preference for a specific temporal window but individual limpets adopting different behaviours coexisted within a group) and intra-individual variation (when the same limpet is active during different times). Interestingly, part of this variability is due to size-related effects although, contrary to our expectations, no effect of substratum type was detected. However an effect of height on the shore was evident, and a clear negative relationship between the proportion of activity during low tide and size was detected in high shore limpets. Size-related differences in the foraging of P. vulgata have been described in previous studies (e.g. Little et al., Reference Little, Williams, Morritt, Perrins and Stirling1988; Della Santina et al., Reference Della Santina, Santini and Chelazzi1995; Santini et al., Reference Santini, Chelazzi and Della Santina1995) but results of the present investigation add to this knowledge and reveal finer details not described in previous studies. It is important to underline that the range of sizes used in this study was relatively narrow (25–36 mm) if compared with that explored in previous studies (e.g. a range 25–56 mm was explored by Della Santina et al., Reference Della Santina, Santini and Chelazzi1995) thus suggesting greater influence than previously reported. Of course, large limpets incur comparatively greater costs than smaller ones to be active during emersion, given they are heavier and taller (and hence subject to a greater downward drag). However, why this was evident only for those high but not low on the shore is not yet clear. Higher up they may be subject to less risk from tide-in crab predation.
In conclusion, our study showed the importance of indirect effects of biologically mediated substratum type – the presence of barnacle cover – on the behaviour of P. vulgata. It also showed that individual differences in behaviour exist and can be important. Such a variability may challenge the notion of Potential Activity Phase (PAP, Evans & Williams, Reference Evans and Williams1991), at least intended as a single time window available to each limpet for being active (see also Santini et al., Reference Santini, Ngan and Williams2011). Despite this the concept of PAP may be useful to understand the determinants of population level behaviour within a specific time window, and may be a reasonable simplification for solving optimization problems (Burrows et al., Reference Burrows, Santini and Chelazzi2000). Finally, our findings may potentially be extended to the behaviour of other intertidal grazers. Given the importance of both limpets and barnacles on rocky shores throughout the North-east Atlantic (see Hawkins et al., Reference Hawkins, Hartnoll, Kain, Norton, John, Hawkins and Price1992; Jenkins et al., Reference Jenkins, Moore, Burrows, Garbary, Hawkins, Ingólffson, Sebens, Snelgrove, Wethey and Woodin2008 for reviews), information on their indirect and direct interactions is of considerable importance in understanding the dynamics of these systems.
Acknowledgements
Many thanks are due to Paolo Della Santina, Cosimo Tendi and Nicoletta Righini for their help and encouragement during fieldwork. Author contributions: RCT and SJH designed the experiment, RCT and SRJ collected the data; RCT and GS analysed the data; GS, RCT, SJH wrote the manuscript with input from SRJ, RGH and GC; GC developed the concepts and techniques and with RGH provided equipment and logistical support. Many thanks are due to two anonymous referees, whose comments greatly improved the manuscript.
Financial support
Fieldwork was funded by the European Union [Eurorock project (contract MAS3-CT95-0012)]. RCT, SJH and RGM were also supported by a Leverhulme Grant (F/180/AO) on limpet foraging behaviour.









