Dietary carbohydrates are generally poorly utilised by teleosts(1), and the recommended inclusion level of carbohydrates in the feeds for cultured warm water carnivorous finfish should not exceed 10 %(Reference Gou, Chen and Xu2–Reference Li, Li and Zhang4). Aquafeeds thus tend to be rich in protein and lipid, which although efficiently utilised, represent a source of contention from the perspective of the overall environmental and economic sustainability of the aquaculture sector(Reference Turchini, Trushenski and Glencross5). An excess of carbohydrates in the diet is associated with prolonged raised post-prandial levels of blood glucose and persistent hepatic glycogen accumulation, which can have negative effects with respect to hepatocyte functions and the growth and health status of fish(Reference Goodwin, Lochmann and Tieman6–Reference Xu, Chen and Liu8). Aquafeeds do, nevertheless, need to contain a certain amount of starch, as it acts as a binder and facilitates extruded pellet expansion(Reference Sørensen, Nguyen and Storebakken9). However, given that the minimum starch content required for the manufacture of aquafeeds is close to, and may even exceed, the glucose tolerance capacity of carnivorous fish, this presents something of nutritional–technological conundrum that potentially jeopardises the environmental sustainability and economic viability of some aquaculture sectors. For example, in China, cultivation of the largemouth bass (Micropterus salmoides) (approximately 0·5 MT in 2018)(10) is unsustainability dependent on up to just 60 % of the total production of fresh/thawed raw fish, owing to hepatic metabolic diseases attributable to the high starch contents in compounded feeds.
Starch is a polysaccharide consisting of amylose (a linear polymer) and amylopectin (a branched polymer), the latter of which is more susceptible to enzymatic cleavage(1). Amylose can undergo transformation to resistant starch, characterised by complex crystalline structures (retrograded recrystallised amylose), which nutritionally mimics dietary fibre and is notably resistant to digestion(Reference Tester, Karkalas and Qi11). Accordingly, the amylose to amylopectin ratio of starch and the content of resistant starch have a pronounced influence on the digestibility and nutritional utilisation of different sources of dietary starch(Reference Svihus, Uhlen and Harstad12).
In mammals, the insulin pathway plays a central role in the maintenance of glucose homeostasis through the regulation of glucose metabolism(Reference Saltiel and Kahn13,Reference Titchenell, Lazar and Birnbaum14) and is affected to differing extents by different sources of dietary starch(Reference Jun, Daiwen and Bing15). For example, slowly digestible starch commonly results in moderate glycaemic and insulinaemic responses(Reference Jun, Daiwen and Bing15–Reference Regmi, van Kempen and Matte17). From the perspective of the aquaculture of teleosts, considerable research effort has focussed on identifying starch sources that can effectively provide the required rheological properties for extruded feed manufacturing, without negatively influencing the overall glycaemic status of the fish(Reference Liu, Ye and Ye18–Reference Li, Sang and Wang21). Unfortunately, given that the effects of different starch sources have tended to be evaluated using different fish species, the results of studies conducted to date are often not directly comparable, and at times can even be contradictory. Amongst the different starch sources that can be used in aquafeeds, pea starch, which has a relatively high proportion of amylose to amylopectin, has shown promising results. When included in the diets of largemouth bass, pea starch has been found to promote more stable glycaemic responses and lower hepatic glycogen accumulation(Reference Song, Shi and Lin20,Reference Li, Sang and Wang21) compared with other starch sources (α-cassava starch (CS), potato starch and dextrin) that have lower amylose to amylopectin ratios. These results accordingly indicate that dietary starch with a high ratio of amylose to amylopectin might be beneficial to carnivorous fish and could improve glucose tolerance. Moreover, studies on mammals have shown that resistant starch can contribute to lowering post-prandial glucose levels and enhancing glucose homeostasis(Reference Johnston, Thomas and Bell22,Reference Maziarz, Preisendanz and Juma23) . Nevertheless, amylose and resistant starch contents are not the only characteristics of a given starch that influence starch nutritional properties. The granule size, crystalline structures, molecular complexity and physical state of starch can all, to varying extents, directly influence both the digestibility of dietary starch and starch utilisation efficiency(Reference Peres and Oliva-Teles24–Reference You, Lim and Lee26).
In this study, we sought to determine and potentially quantify the nutritional effects of dietary starch with high amylose and resistant starch contents in the diet for carnivorous fish, whilst excluding the effects of other starch-related factors such as the physical characteristics of different starch sources. To this end, as a reference, we selected a starch source with a low amylose to amylopectin ratio (α-CS) and examined its inclusion in the diets for largemouth bass at three different levels, as either unmodified or processed starch, the latter of which was obtain by treatment with a starch de-branching enzyme (pullulanase) that cleaves amylopectin branches, thereby increasing the amylose and resistant starch contents. It is envisaged that the findings of this study will facilitate a more efficient utilisation of starch in fish diets, and in doing so, contribute towards resolving the dilemma between the technological and nutritional requirements of aquaculture feeds, and ultimately enable the aquaculture sector to fulfil its environmental and economic sustainability expectations and potentials.
Materials and methods
Experimental diets
To modify the amylose to amylopectin ratio of α-CS, and specifically to increase the amylose and resistant starch contents at the expense of reducing the amylopectin content, we performed enzymatic amylopectin de-branching using pullulanase, as previously described(Reference Pongjanta, Utaipatanacheep and Naivikul27,Reference Zhao and Lin28) . Briefly, a 20 % (w/v) solution of CS was prepared in sodium acetate buffer solution (pH 4·5) and sterilised at 120°C for 10 min in an autoclave. After cooling to room temperature, pullulanase enzyme (1350 NPUN/g; Novozymes) was added to the mixture at an enzyme:substrate ratio of 6 g/kg, and enzymatic hydrolysis was conducted for 8 h at 55°C and pH 4·5. The enzymatic reaction was terminated by thermal enzyme denaturation at 95°C for 10 min. After cooling to room temperature, the mixture was stored at 4°C for 24 h, prior to being oven-dried at 60°C for 28 h. The contents of amylose, amylopectin, digestible starch and resistant starch in untreated CS and the de-branched starch (DS) are presented in online Supplementary Table S1.
Six isonitrogenous and isolipidic diets were formulated based on graded inclusion levels of CS (4, 8 and 12 %; CS4, CS8 and CS12, respectively,) or DS (4, 8 and 12 %; DS4, DS8 and DS12, respectively). Zeolite powder was included as an inert filler to compensate for differences in the amounts of added starch. With the exception of the two different starch sources and zeolites, all other experimental diet ingredients and their inclusion rates were identical (online Supplementary Table S2). The experimental diets were prepared following the method described by Li et al.(Reference Li, Sang and Wang21). Briefly, all the dry ingredients were mixed thoroughly after having been ground and sieved through a 75-μm mesh and then blended with a prepared mixture of the oily ingredients. Thereafter, water was added to produce a stiff dough, which was then extruded through a 25 mm die. The extruded pellets were steamed in an oven for 15 min at 105°C to gelatinise the starch and then dried in a ventilated oven at 55°C. The dried diets thus obtained were then stored at –20°C until use.
In vivo trial and sampling
The proposed animal study was reviewed and approved by the Animal Care and Use Committee of Shanghai Ocean University. Juvenile largemouth bass (Micropterus salmoides) were sourced from Jinchengfu Fisheries Technology Co., Ltd and transported to an experimental recirculating aquaculture system at Shanghai Ocean University (Shanghai, China). After a 2-week acclimation in the experimental facility, 735 fish of similar weight (10·07 (sem 0·30) g), which had been fasted for 24 h, were selected following anaesthesia with eugenol (1:10 000) (Shanghai Reagent). Among these fish, fifteen were randomly collected as the initial sample, and the remaining 720 fish were randomly distributed (forty fish per tank) in eighteen tanks (800 litres). Each of the six experimental diets was then randomly allocated to each of three tanks. The feeding trial lasted for 12 weeks, and during this period, fish were fed to apparent satiation twice daily. All tanks were maintained under a natural photoperiod and were provided with a continuous flow of aerated (dissolved oxygen ≥ 6 mg/l) sand-filtered freshwater (2·0 litres/min). The water temperature and pH were maintained at 27 (sem 1)°C and 7·2 (sem 0·2), respectively.
Faecal samples were collected according to the method described by Lee(Reference Lee29) for analysis of the apparent digestibility coefficients (ADC) of nutrients. At the end of the 12-week in vivo experimental period, food was withheld for 24 h and then all fish were counted and weighed after being anaesthetised with eugenol (1:10 000). Five individuals from each tank were randomly sampled for subsequent body composition analysis, and a further ten fish were selected for tissue analysis. Among these latter ten fish, four were dissected, and weights of the liver and viscera were recorded for calculation the hepatosomatic index (HSI) and viscerosomatic index. Liver samples were then fixed with Bouin’s solution for histological analysis. The remaining six fish were dissected and used to obtain muscle and liver samples for biochemical composition analysis. The livers of all dissected fish were also photographed for morphological assessment. The remaining fish were returned to their original tanks and maintained on their respective experimental diet. After a further week, and after having observed that the fish had attained normal physiological and behavioural conditions and were feeding well, the post-prandial blood samples were collected at 0, 1, 4, 8, 12 and 24 h after feeding. At each time point, blood samples were collected from the caudal vasculature of four randomly selected fish in each tank using 1 ml syringes. The blood samples were subsequently centrifuged at 3000 g for 10 min (4°C) to separate the serum. Having collected blood from the remaining four fish in each tank at 24 h post-feeding, the fish were euthanized and dissected, and liver tissue samples were collected for gene expression analysis.
Chemical analyses
Proximate composition analysis was performed following standard procedures. Briefly, the moisture content was determined by drying samples to a constant weight(30), crude protein content was measured using the Kjeldahl method (N × 6·25) and ash was determined through combustion to a constant weight in a muffle furnace at 550°C(30). Cellulose content was estimated using a cellulose analyser (FT12; Gerhardt), whereas the amylose and amylopectin contents in the two starch sources (CS and DS) were measured using the dual-wavelength spectrophotometry method, following the protocol described by Shi et al.(Reference Shi, Hao and Fang31). Briefly, amylose and amylopectin were separated and purified using the n-butanol crystallisation method, and the contents were then determined at selected wavelengths of 624 and 538 nm with corresponding reference wavelengths of 440 and 750 nm. Serum glucose was measured spectrophotometrically at 505 nm after the reaction of samples with a glucose oxidase-peroxidase reagent. The contents of starch, digestible starch and resistant starch in the experimental diets and starch sources were measured based on a method described by McCleary et al. (Reference McCleary, McNally and Rossiter32). Glycogen content was determined using anthrone-sulphuric acid solution following the treatment of tissue samples with KOH solution(Reference Seifter, Dayton and Novic33). Chromic oxide content of experimental diets and faecal sample was measured for the analysis of ADC of nutrients with the wet-acid digestion method(Reference Furukawa and Tsukahara34).
Histology
After immersion in Bouin’s solution for 24 h, liver samples were washed, transferred to a clean glass vial and preserved in 70 % ethanol solution. Samples were dehydrated with a standard ethanol series to 100 % and hyalinised using xylene, prior to embedding in paraffin wax and subsequent sectioning at 4-μm intervals, according to standard histological procedures. Haematoxylin–eosin staining was conducted following the procedures described by Hardy(Reference Hardy and Roberts35). Histological images at ×400 magnification were acquired using a digital imaging microscope (MQD42055; Nikon).
Gene expression
The relative expression of key genes involved in the insulin signalling pathway and glucose metabolism was determined following previously described procedures(Reference Li, Sang and Wang21). The selected target genes were insulin receptor (IR), insulin receptor substrate 1 (IRS1), phosphatidylinositol-3-kinase p85 alpha (PI3KR1), serine/threonine kinase (AKT1), glucokinase (GK), phosphofructokinase liver type (PFKL), pyruvate kinase (PK), glucose-6-phosphatase catalytic subunit (G6PC), fructose-1,6-bisphosphatase-1 (FPB1) and phosphoenolpyruvate carboxykinase (PEPCK). Total RNA was obtained from isolated liver samples using Trizol reagent (Takara, Japan), and the isolated RNA was subsequently used for the synthesis of first-strand cDNA using a Prime ScriptTM RT reagent Kit (Takara). The specific primers used have been reported previously(Reference Li, Sang and Wang21), the details of which are present in online Supplementary Table S3. β-Actin was used as the reference gene. PCR amplification was conducted using a quantitative thermal cycler with the following program: 95°C for 2 min, followed by forty cycles of 95°C for 10 s, 57°C for 10 s and 72°C for 20 s. After the reaction, melting curve analysis was conducted to confirm the presence of a single PCR product in the reactions. The relative expression of the selected genes was calculated based on the 2–ΔΔCt method(Reference Livak and Schmittgen36).
Results
Growth performance and feed utilisation
Throughout the entire in vivo trial, no fish mortality was recorded, thereby indicating the adequacy of the husbandry conditions and all experimental diets, and that the two dietary starch sources had no direct adverse effects on fish survival at the examined inclusion levels. Additionally, we observed that all fish grew well, achieving a weight gain exceeding 650 % at the end of the trial. However, we did detect statistically significant interactions between starch sources and inclusion levels with respect to final body weight, weight gain and specific growth rate of fish (Table 1). Compared with the untreated CS, these three growth parameters were significantly increased in fish fed DS-containing diets, although decreases were observed with an increase in the content of dietary starch. Fish fed the DS4 diet obtained the highest weight gain and specific growth rate, with the values being significantly higher than those recorded in other treatments (Table 1). Although we detected no statistically significant effects of starch inclusion level on the fish condition factor, differences were observed with respect to starch source. The indices HSI and viscerosomatic index were found to be modified by the dietary treatments, with DS being associated with a significant decrease in both indices. In the case of both starch sources, however, HSI and viscerosomatic index showed marked increases with an increase in the levels of dietary starch (P < 0·05) (Table 1).
Table 1. Growth performance of largemouth bass fed the diets with graded levels of α-cassava starch (CS) and de-branched starch (DS) for 12 weeks
(Mean values with their standard errors; n 3)†
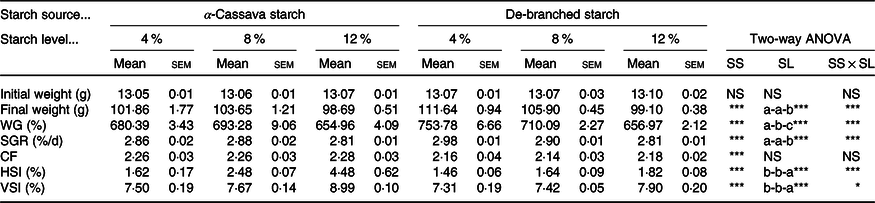
NS, no significant differences; WG, weight gain; SGR, specific growth rate; CF, condition factor; HSI, hepatosomatic index; VSI, viscerosomatic index.
* P < 0·05, ** P < 0·01 and *** P < 0·001.
† For two-way ANOVA, the same lowercase letters indicate no significant differences (P > 0·05) as dietary starch increased.
Fish fed DS-containing diets showed a significantly higher feed intake compared with those fish fed diets containing CS. For both starch source, however, feed intake decreased (P < 005) concomitant with an increase in the level of dietary starch (Table 2). Significant interactions between starch source and dietary inclusion level were observed with respect to both the feed efficiency ratio and protein efficiency ratio (Table 2). Compared with fish fed CS-containing diets, those fed diets containing DS showed lower feed efficiency ratio and protein efficiency ratio (P < 005), and for both starch sources, the two indices showed significant increases with increasing dietary starch levels. We also detected a statistically significant interaction between starch source and dietary inclusion level with respect to the protein retention rate, although not for the lipid retention rate (Table 2). A similar trend was noted with respect to starch source, with the protein retention rate being significantly increased in fish fed diets containing CS, independent of its inclusion levels, whereas no significant effects were observed for lipid retention rate.
Table 2. Feed utilisation of largemouth bass fed the diets with graded levels of α-cassava starch (CS) and de-branched starch (DS) for 12 weeks
(Mean values with their standard errors; n 3)†
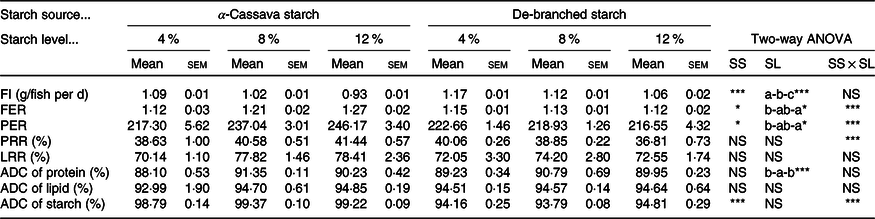
NS, no significant differences; FI, feed intake; FER, feed efficiency ratio; PER, protein efficiency ratio; PRR, protein retention rate; LRR, lipid retention rate; ADC, apparent digestibility coefficient.
* P < 0·05, ** P < 0·01 and *** P < 0·001.
† For two-way ANOVA, the same lowercase letters indicate no significant differences (P > 0·05) as dietary starch increased.
Results obtained for the ADC are presented in Table 2. Independent of starch source, protein ADC values were higher in fish fed the two intermediate diets formulated with 8 % starch inclusion (CS8 and DS8), compared with fish fed diets containing higher or lower levels of starch, whereas lipid ADC values did not appear to be affected by dietary treatment. In contrast, starch ADC values showed notable variations, with the values of fish fed in diets formulated with DS being approximately 5 % lower than those in fish fed diets containing untreated CS, the differences in which were statistically significant. However, starch dietary inclusion levels did not appear to have any significant effect on the recorded starch ADC values (Table 2).
Nutritional composition analysis
The results of the nutritional and proximate composition analysis of fish whole body, liver and muscle are presented in Table 3. With the exception of whole-body protein content, which, independent of starch source, was significantly reduced in response to an increasing level of dietary starch, none of the other proximate composition parameters recorded in the whole bodies of fish were affected by the dietary treatment. Similarly, in fish fillets, only the protein content showed certain statistically significant differences. The glycogen content of fish muscle also appeared to be unaffected by dietary treatment.
Table 3. Body composition of largemouth bass fed the diets with graded levels of α-cassava starch (CS) and de-branched starch (DS) for 12 weeks (% live weight)
(Mean values with their standard errors; n 3)†
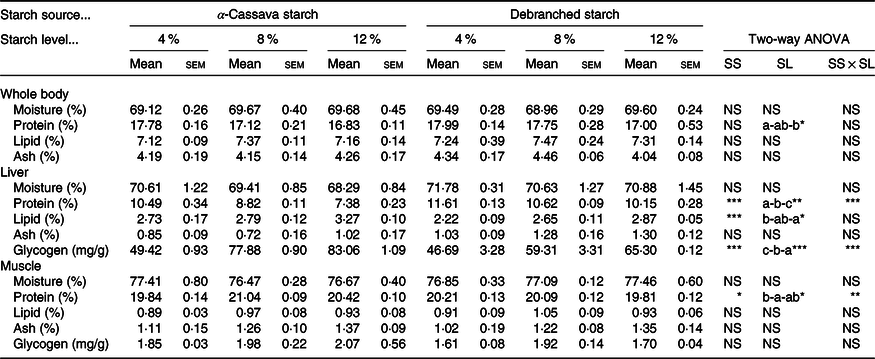
NS, no significant differences.
* P < 0·05, ** P < 0·01 and *** P < 0·001.
† For two-way ANOVA, the same lowercase letters indicate no significant differences (P > 0·05) as dietary starch increased.
More pronounced effects of dietary treatments on tissue nutritional composition were recorded in liver tissues (Table 3). With the exception of moisture and ash contents, which were not appreciably affected by the dietary treatments, statistically significant effects of dietary starch sources and their inclusion levels were observed for the other liver parameters. Both dietary starch sources and their inclusion levels significantly affected hepatic protein and lipid content, which showed clear opposite trends, with protein levels being higher and lipid levels lower in fish fed DS-containing diets than in those fed diets containing CS, and with protein levels decreasing and lipid levels increasing in response to an increase in dietary starch inclusion levels.
Furthermore, we observed that the hepatic glycogen content was significantly affected by dietary treatments, with respect to both starch source and inclusion levels, and also to the interactions between the two (Table 3). Specifically, we observed significant increases in hepatic glycogen levels in response to an increase in the level of dietary starch. Moreover, levels were significantly higher in the livers of fish fed CS-containing diets than in the livers of fish fed diets containing DS. Interestingly, however, the highest hepatic glycogen content observed in fish fed DS diets was 65·3 mg/g (DS12), which was lower than the content (77·8 mg/g) in fish fed a diet containing the intermediate level untreated CS (8 %) (Table 3).
Histopathological analysis
Simple macroscopic observations of the livers of fish fed the six experimental diets containing starch of different types and inclusion levels revealed clear modifications (Fig. 1). Notably, we observed marked increases in the size of livers in response to an increase in dietary CS, which was consistent with the HSI values we obtained (Table 1), and there was a parallel reduction in the intensity of the colour of liver tissues. In contrast, we detected no obvious modification in the size or colour of the livers of fish in response to an increase in dietary DS level (Fig. 1). Histologically, the results of haematoxylin–eosin staining clearly revealed that most of the hepatocytes in the livers of fish fed the CS12 diet were swollen and that the nuclei in these cells had re-localised to the cell periphery (Fig. 2). In contrast, the hepatocytes of fish fed the CS4 and CS8 diets appeared normal. Similarly, we detected no manifest modifications in the hepatocytes of fish fed the DS diets at any of the three inclusion levels (Fig. 2).
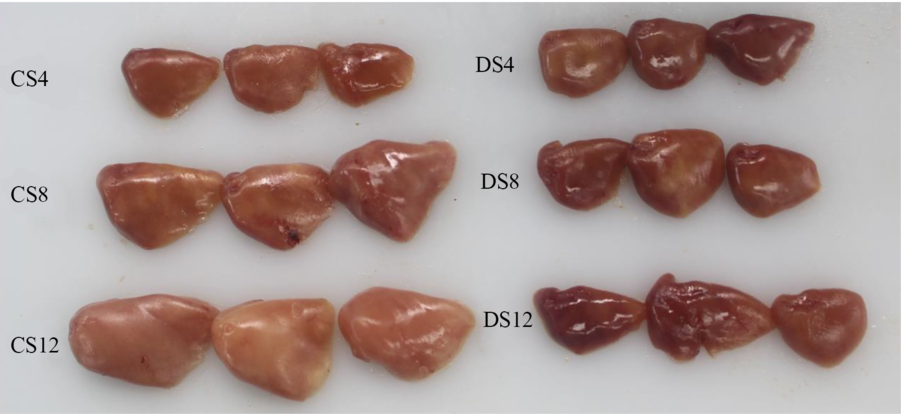
Fig. 1. The livers of largemouth bass fed the diets with graded levels of α-cassava starch (4, 8 and 12 %, CS4, CS8 and CS12, respectively) and de-branched starch (4, 8 and 12 %, DS4, DS8 and DS12, respectively) for 12 weeks.
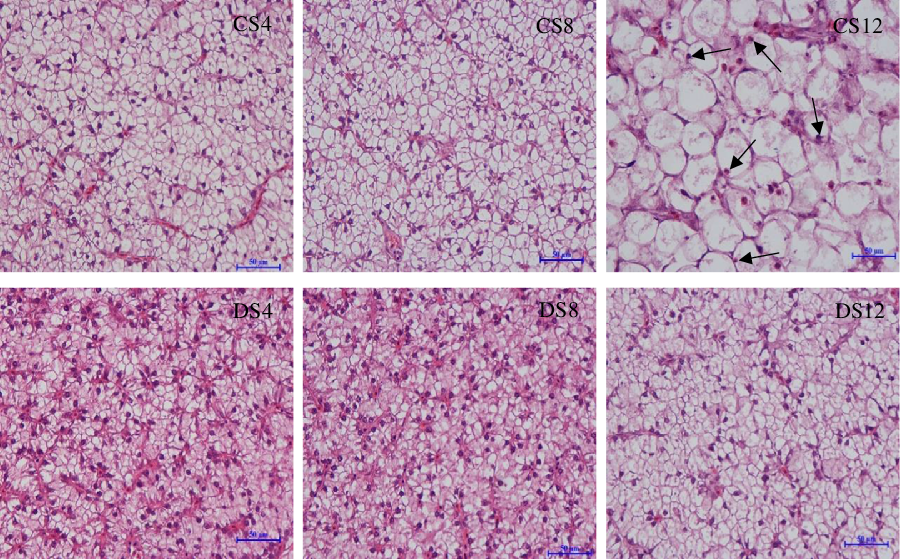
Fig. 2. The morphology analysis of the liver from largemouth bass (haematoxylin–eosin staining, bar = 50 μm) fed the diets with graded levels of α-cassava starch (4, 8 and 12 %, CS4, CS8 and CS12, respectively) and de-branched starch (4, 8 and 12 %, DS4, DS8 and DS12, respectively) for 12 weeks.
Post-prandial glucose levels
The effects of dietary treatments on serum glucose levels at 0, 1, 4, 8, 12 and 24 h post-feeding are shown in Fig. 3. In all groups, post-prandial glucose levels peaked at 12 h post-feeding. Independent of dietary starch type, post-prandial glucose levels increased significantly with an increase in the level of dietary starch (Fig. 3). Notably, however, compared with CS, the inclusion of DS in diets significantly reduced the serum glucose levels of fish at 0, 8 and 24 h post-feeding.
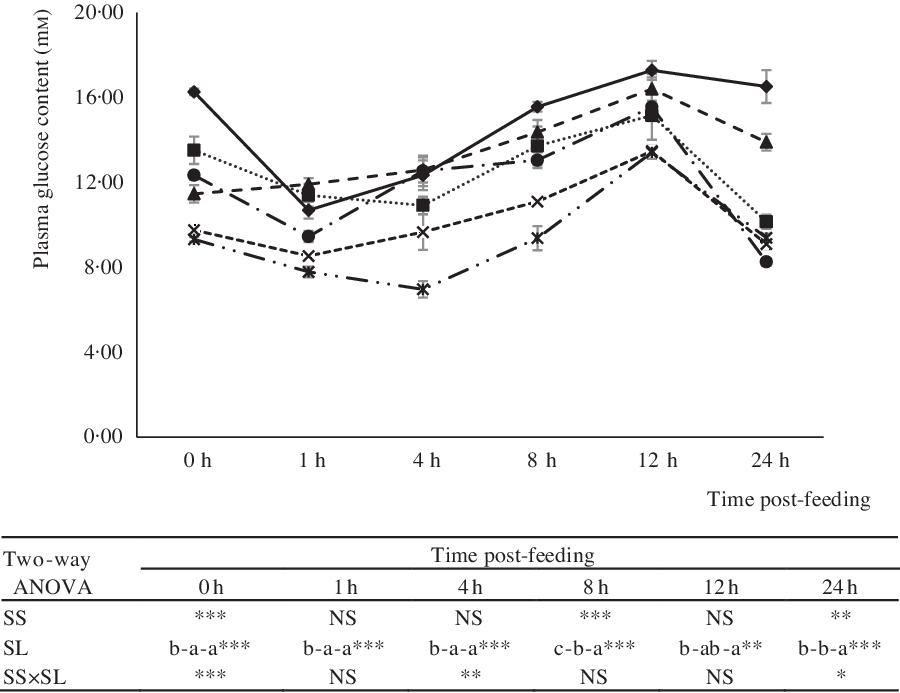
Fig. 3. Serum glucose of largemouth bass fed the diets with graded levels of α-cassava starch (CS) and de-branched starch (DS) for 12 weeks. Values are means (n 3), with their standard errors represented by vertical bars. For two-way ANOVA, the same lowercase letters indicate no significant differences (P > 0·05) as dietary starch increased and asterisks indicate significant differences as: * P < 0·05, ** P < 0·01 and *** P < 0·001. NS indicates no significant differences. ![]() , CS4;
, CS4; ![]() , CS8;
, CS8; ![]() , CS12;
, CS12; ![]() , DS4;
, DS4; ![]() , DS8;
, DS8; ![]() , DS12.
, DS12.
Gene expression
The transcription of IR was significantly affected by dietary treatments (Fig. 4), with the expression of this gene being reduced in response to an increase in the level of dietary starch. Moreover, clear differences in expression were observed between the fish fed diets supplemented with CS and DS at the 8 or 12 % inclusion levels, with higher expression being detected in fish fed DS. In contrast, dietary treatment appeared to have no significant effect on the transcription of IRS1. The expression of PI3KR1 was, however, significantly affected by dietary starch source, and this was particularly evident in fish fed the two diets containing the highest levels of starch (CS12 and DS12), with the expression of PI3KR1 being significantly down-regulated in the former (Fig. 4). A similar pattern was observed for AKT1, the expression of which was increased in fish fed diets containing DS, independent of inclusion level (P < 005) (Fig. 4).
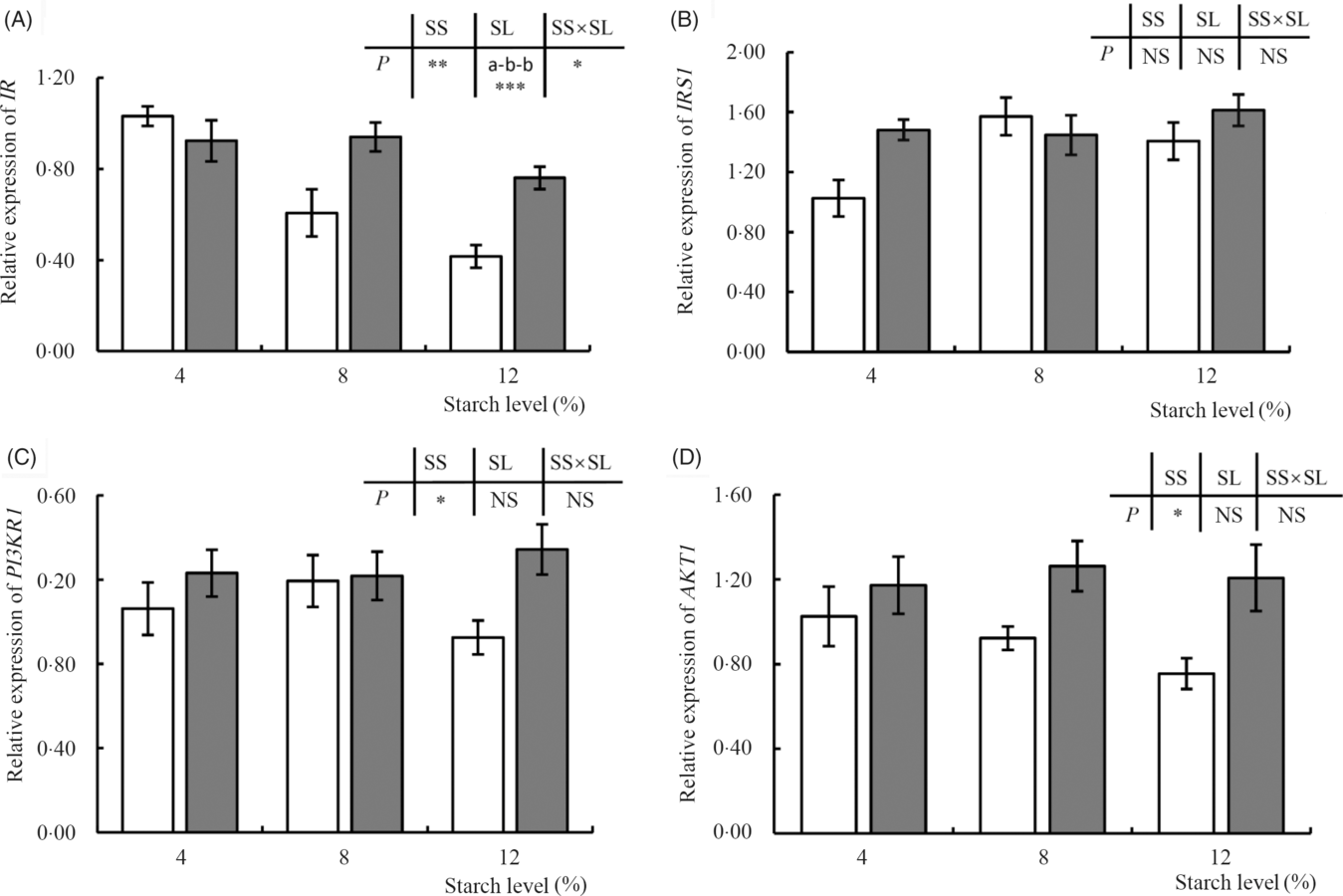
Fig. 4. Expression of insulin pathway-related genes, insulin receptor (IR) (A), insulin receptor substrate 1 (IRS1) (B), phosphatidylinositol-3-kinase p85 alpha (PI3KR1) (C) and serine/threonine kinase 1 (AKT1) (D), in response to graded levels of α-cassava starch (CS) and de-branched starch (DS) for 12 weeks. Values are means (n 3), with their standard errors represented by vertical bars. For two-way ANOVA, the same lowercase letters indicate no significant differences (P > 0·05) as dietary starch increased and asterisks indicate significant differences as: * P < 0·05, ** P < 0·01 and *** P < 0·001. NS indicates no significant differences. ![]() , CS;
, CS; ![]() , DS.
, DS.
The transcription of PFKL was unaffected by dietary treatments, with no effects of starch source, inclusion level or interactions between the two being observed (Fig. 5). In contrast, we detected statistically significant interactions between dietary starch source and inclusion level with respect to the expression of GK and PK (Fig. 5). Independent of inclusion levels, the expression of GK was up-regulated (P < 005) in fish fed diets containing DS. Similarly, compared with fish fed CS-containing diets, PK was significantly up-regulated in those fed diets containing DS, in which expression levels in fish fed the DS8 diet were significantly higher than in those fed the DS4 and DS12 diets (Fig. 5). Among the three gluconeogenesis-related genes we assessed, FPB1 and PEPCK showed no statistically significant modification associated with dietary treatment, whereas the expression of G6PC was down-regulated (P < 005) in fish fed the diets containing DS as the starch source, independent of its inclusion level (Fig. 5).

Fig. 5. Expression of glycolysis, glucokinase (GK) (A), phosphofructokinase liver type (PFKL) (B) and pyruvate kinase (PK) (C), and gluconeogenesis, glucose-6-phosphatase catalytic subunit (G6PC) (D), fructose-1,6-bisphosphatase-1 (FPB1) (E) and phosphoenolpyruvate carboxykinase (PEPCK) (F), related genes in response to graded levels of α-cassava starch (CS) and de-branched starch (DS) for 12 weeks. Values are means (n 3), with their standard errors represented by vertical bars. For two-way ANOVA, the same lowercase letters indicate no significant differences (P > 0·05) as dietary starch increased and asterisks indicate significant differences as: * P < 0·05, ** P < 0·01 and *** P < 0·001. NS indicates no significant differences. ![]() , CS;
, CS; ![]() , DS.
, DS.
Discussion
In this study, we compared the effects of different dietary inclusion levels of untreated (CS) and pullulanase-treated (DS) α-CS on largemouth bass, independent of the confounding effects associated with the use of different starch sources. Notably, in addition to increasing the amylose content of starch, pullulanase-induced de-branching also elevated the resistant starch content, primarily due to the retrogradation of amylose(Reference Raigond, Ezekiel and Raigond37). Furthermore, to mimic commercial manufacturing processes, we subjected the extruded experimental diets to thermal treatment to gelatinise the starch, thereby achieving the necessary plasticity and stability of the pellets, as well as enhancing starch digestibility. Consequently, we also measured the contents of resistant and digestible starch in all experimental diets to better clarify the major nutritional differences between diets formulated with the two different forms of starch.
Previously, it has clearly been demonstrated that, similar to other carnivorous teleosts, the largemouth bass has limited glucose utilisation capabilities(Reference Gou, Chen and Xu2) and consistent with previous findings(Reference Gatesoupe, Huelvan and Le Bayon19–Reference Li, Sang and Wang21), where fish growth performance and feed intake were increasingly compromised with an increase in dietary starch content. Given our experimental design objectives, and in particular our desire to obtain iso-nitrogenous and iso-lipidic feeds, those diets with a higher starch content also had a higher total energy content. It is therefore plausible that the fish in the present study modified their feed intakes in order to compensate for differences in total energy intake, as has previously been suggested(Reference Li, Li and Zhang4). However, we detected decreases in both the feed efficiency rate and protein efficiency rate in fish fed DS-containing diets, independent of dietary starch inclusion level. In this regard, it has been well documented that a high amylose content reduces the susceptibility of starch to enzymatic hydrolysis(Reference Svihus, Uhlen and Harstad12,Reference Regmi, van Kempen and Matte17,Reference Sasaki, Kohyama and Suzuki38) , and resistant starch in diets is not digested as ordinary starch and resembles non-starch polysaccharides and non-digestible oligosaccharides in evading small intestinal digestion(Reference Raigond, Ezekiel and Raigond37,Reference Muir and O’Dea39) . Accordingly, the high contents of amylose and resistant starch in DS may have contributed to reduced digestibility, thereby impairing the energetic efficiency of the diets, and consequently resulting in reductions in feed efficiency ratio and protein efficiency ratio. Moreover, it is plausible that the lower digestible energy of the DS diets stimulated feed intake and further led to a general trend toward improved performance in fish fed the DS-containing diets. Similar observations have been made in studies on mammals(Reference Li, Li and Zhang40), and in previous studies on largemouth bass, in which the effects of different starch sources were examined, and dietary pea starch was found to increase growth performance(Reference Song, Shi and Lin20,Reference Li, Sang and Wang21) . Although resistant starch acts as a dietary fibre with limited absorption, we found that higher levels in the DS treatments did not further depress starch digestibility, as all measured ADC values were relatively high and exceeded 90 %. It has been suggested that, although resistant starch cannot be absorbed in the intestine, it may function as a prebiotic that is fermented by anaerobic gut microbiota to SCFA, mainly acetate, butyrate, and propionate, which are beneficial for maintaining the health status of the gut, and even organisms as a whole(Reference Fuentes-Zaragoza, Sánchez-Zapata and Sendra41,Reference Topping and Clifton42) . Therefore, the prebiotic functions of resistant starch could partially account for the relatively high ADC values we recorded, and thus contribute to a slower/delayed absorption, which might prove to be beneficial for carnivorous fish species.
It is well established that excessive dietary starch is associated with hepatic glycogen deposition in carnivorous fish(Reference Wang, Li and Han3,Reference Li, Li and Zhang4) , and consistently, in the present study, we observed significant increases in hepatic glycogen in response to an increase in dietary starch content. In fish fed the untreated CS, the continual accumulation of hepatic glycogen resulted in significantly enlarged livers and a higher HSI. Additionally, at the histological level, provision of feed containing the highest level (12 %) of untreated CS (CS12) resulted in the swelling of hepatocytes, along with a displacement of nuclei to the cell periphery, clearly indicating hepatic damage due to excessive glycogen accumulation, as has previously been observed(Reference Goodwin, Lochmann and Tieman6,Reference Xu, Chen and Liu8) . Importantly, we found that the use of DS had a marked effect in alleviating these phenomena, with normal levels of hepatic glycogen, normal HSI values, and anatomically and histologically normal livers being observed in fish fed diets containing the highest level of de-branched starch (DS12). In previous studies focusing on dietary starch sources and glycogen deposition in largemouth bass, it was shown that provision of diets containing starch sources with a high ratio of amylose to amylopectin, such as pea starch and high amylose maize, led to significant reductions in hepatic glycogen deposition compared with other sources richer in amylopectin(Reference Song, Shi and Lin20,Reference Li, Sang and Wang21) . Nevertheless, the experimental designs of these studies limited the ability to interpret the results with respect to the specific effects of different proportions of dietary starch amylose and amylopectin, as different starch sources were used. In light of the clear results obtained in the present study, in which there were no possible confounding effects attributable to the use of different raw materials, it is possible to conclude that the high contents of amylose and resistant starch in DS can reduce the likelihood of excessive glycogen deposition.
In mammals, the insulin pathway plays important roles in maintaining glucose homeostasis(Reference Saltiel and Kahn13,Reference Titchenell, Lazar and Birnbaum14) , and sources of dietary starch characterised by differing contents of resistant starch and ratios of amylose to amylopectin have been demonstrated to directly affect this pathway(Reference Regmi, Matte and Van Kempen43–Reference Yin, Yin and Zhang45). The binding of insulin to IR on the exterior of cells induces activation of the insulin signalling pathway(Reference Lizcano and Alessi46). In fish, a deficiency in the number of IR molecules is considered to be one of the major reasons for their limited glucose utilisation capacities(Reference Navarro, Leibush and Moon47). Notably, from the perspective of analysing insulin in teleosts, previous studies have confirmed the importance of considering sampling time post feeding(Reference Sundby, Eliassen and Blom48,Reference Papatryphon, Capilla and Navarro49) , and in the present study, we conducted gene expression analyses in liver samples at 24 h post-feeding. We accordingly detected significant reductions in IR expression with increases in the content of dietary starch, thereby confirming the limited glucose utilisation capabilities of carnivorous fish. However, a key finding of the present study was that, compared with those fish fed diets containing unmodified CS, the expression of IR was up-regulated in fish fed DS-containing diets, which we suspect contributed to the enhanced glucose tolerance observed in the latter fish. Furthermore, we also detected the up-regulated expression of PI3KR1 and AKT1 in fish fed DS-containing diets, which is indicative of higher insulin pathway activity. These findings are consistent with previous observations indicating a significantly higher expression of PI3KR1 and AKT1 in fish fed a diet containing pea starch compared with starches richer in amylopectin. In mammals, however, amylose-rich and resistant starch sources have been reported to depress the secretion of insulin(Reference Regmi, van Kempen and Matte17,Reference Regmi, Matte and Van Kempen43,Reference Scribner, Pawlak and Ludwig50,Reference Ren, Zhang and Jiang51) , which clearly contrasts with our observations of higher insulin pathway activity in DS-fed fish, compared with fish fed CS-containing diets. As an explanation to account for this apparent discrepancy, it could be conjectured that the insulin pathway was at normal/physiological levels in fish fed DS-containing diets, but was substantially depressed (down-regulated/compromised) in fish fed diets containing CS, as a consequence major hepatic damage caused by dietary CS. This would confirm that, compared with amylopectin-rich starch sources, dietary starch richer in amylose enhances glucose tolerance in carnivorous fish,
The insulin pathway in mammals is also involved in the regulation of glucose homeostasis through the promotion of glycolysis and inhibition of gluconeogenesis(Reference Saltiel and Kahn13,Reference Titchenell, Lazar and Birnbaum14) . In teleosts, however, and in carnivorous fish particularly, a relatively poor regulation of gluconeogenesis-related genes has been observed in response to increases in dietary carbohydrate(Reference Li, Li and Zhang4), which would partially account for the limited ability of these fish to utilise glucose(Reference Kamalam, Medale and Panserat52). Consistently, in the present study, we found that increasing the levels of dietary carbohydrate did not result in any significant changes in the expression of the gluconeogenesis-related genes G6PC, FBP1 and PEPCK, as has previously been observed(Reference Li, Li and Zhang4,Reference Panserat, Médale and Breque53–Reference Panserat, Plagnes and Breque55) . Furthermore, consistent with previous findings(Reference Li, Sang and Wang21), we detected no significant influence of dietary starch source on the expression of FBP1 and PEPCK. It has, nevertheless, been found that dietary starch with a high ratio of amylose to amylopectin can significantly elevated the activity of GK in European seabass(Reference Enes, Panserat and Kaushik56), whereas in contrast, a higher amylose content has been shown to significantly reduce GK activity in the obscure puffer(Reference Liu, Ye and Ye18). In the present study, we found that the largemouth bass responded similarly to European seabass, with expression of the glycolysis-related genes GK and PK being up-regulated in fish fed diets containing DS (with a high ratio of amylose to amylopectin and content of resistant starch) as a starch source. This modified gene expression pattern may, in turn, partially account for alleviation of the heightened hepatic glycogen accumulation observed in fish fed the de-branched CS, compared with those fed untreated starch. Indeed, it is plausible that DS as a starch source in diets had the effect of promoting glycolysis, which could be related to the elevated expression of genes associated with the insulin pathway. Recently, teleost microRNA (miRNA; small non-coding RNA) have attracted considerable research attention on account of their roles in multiple physiological processes, including growth and general metabolism, through the regulation of endogenous genes(Reference Bizuayehu and Babiak57). Previous studies on farmed carp (Labeo bata) have identified liver-specific microRNA linked with glucose metabolism(Reference Rasal, Iquebal and Jaiswal58,Reference Rasal, Iquebal and Pandey59) and have provided further evidence in support of the limited capacity of fish species to utilise glucose. Thus, further research on the expression of miRNA is warranted to gain a better understanding of the mechanisms whereby dietary DS enhances the glucose tolerance capacity of largemouth bass.
In conclusion, the increased levels of dietary starch negatively affected the performance of largemouth bass, which is assumed to be associated with the limited glucose utilisation capability of this species. Using the same starch source, either unmodified or treated (de-branched) α-CS, we found that dietary starch with relatively high amylose and resistant starch contents can significantly enhance growth and simultaneously alleviate the likelihood of excessive hepatic glycogen accumulation, which is associated with the reduced ADC of starch and upregulation of genes involved in the insulin pathway and glycolysis. In addition to providing novel insights into the carbohydrate metabolism of carnivorous finfish, the findings of this study also have important practical applications, notably with respect to providing a foundation for developing future aquaculture feeds, by showing how it may be possible to resolve the nutritional–technological conundrum related to the use of starch in compounded extruded feed for carnivorous species.
Acknowledgements
The authors are grateful to Yijun Liu and Min Dai for their assistance in the feeding trial and sampling.
This work was financially supported by National Natural Science Foundation of China (31802308), China Agriculture Research System (CARS-46), Shanghai Talent Development Fund (no. 2019097) and ‘Chenguang program’ supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (19CG56).
N. C. and S. L. designed the research; S. L. and C. S. conducted the experiment and analysed the data; A. W. and J. Z. provided assistance on the chemical analysis. G. M. T. provided suggestions on the experiment design; S. L. and G. M. T. wrote the paper.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520002214











