Introduction
Measles is a highly contagious respiratory illness caused by the measles virus, a virus of the genus Morbillivirus in the family Paramyxoviridae. Measles can be a severe illness at any age, particularly in adults aged >20 years [Reference Gershon, Bennett, Dolin and Blaser1]. Complications such as sinusitis, hepatitis and otitis media are common, while pneumonia may occur in up to 30% of adult cases [Reference Gershon, Bennett, Dolin and Blaser1], post-infectious encephalitis occurs in 1–4 per 1000–2000 cases [Reference Strebel and Plotkin2] and subacute sclerosing panencephalitis occurs more commonly than previously thought in 1 per 2500–10 000 cases [Reference Strebel and Plotkin2, Reference Schönberger3].
Since measles vaccine was first registered in Australia in 1968, significant effort has been directed at achieving high levels of vaccination coverage. Funded measles vaccine for children aged 12–23 months was introduced in 1970. The measles, mumps and rubella (MMR) vaccine was introduced in 1989 and a second MMR vaccine for children aged 10–14 years was included in the national vaccination schedule in 1993. A national measles control campaign in 1998 focused on providing MMR to school-age children to ensure a second dose of measles-containing vaccine (MCV) and was followed in 2001 by a funded vaccination programme for young adults [Reference Gidding4]. The current National Immunisation Program Schedule in Australia includes MMR at 12 months and measles-mumps-rubella-varicella vaccine at 18 months, while funded MMR vaccine is offered to anyone born during or since 1966 without two documented doses of MCV [5].
National coverage of MMR dose 1 measured at age 24 months has remained steady at around 94% for over 10 years, while coverage for MMR dose 2 measured at age 60 months increased to 91% in 2012 from 82% in 2002 [Reference Gidding4]. In the state of Queensland (where Brisbane is located), coverage estimated using the now obsolete Australian Childhood Immunisation Register (ACIR, now Australian Immunisation Register) in December 2015 showed 94% of children aged 60 months had received MMR dose 2. The Australian national serosurveillance programme conducted by the National Centre for Immunisation Research and Surveillance provides an additional perspective on population immunity and results have been shown to be representative by age, sex and geographic location [Reference Kelly6]. The 2007 national serosurvey found 83% of those aged 1–34 years had detectable immunoglobulin G (IgG), while 92% had either detectable or equivocal IgG levels [Reference Gidding4]. Early analysis of a more recent 2012–2013 national serosurvey indicates that the proportion of seronegative (absence of detectable or equivocal IgG) individuals aged 1–49 years may have increased slightly to around 10% in recent years [Reference Gidding7]. Those born prior to 1966 are considered to have pre-existing immunity to measles due to the high likelihood of prior natural infection [8].
In March 2014, the World Health Organization (WHO) announced that Australia had achieved measles elimination status, having met the elimination criteria for the Western Pacific Region set by WHO in 2012 [Reference Gidding4]. Indeed, interruption of endemic measles transmission probably occurred in Australia in 1999, with Heywood et al. contending that Australia accomplished measles elimination in 2005 [Reference Heywood9]. Despite being free from endemic measles, cases continue to be reported in Australia each year, usually linked to the importation of measles from overseas. More than 600 cases were reported in Australia between 2011 and 2015 [10]. In 2015, Queensland had a population of 4.78 million people and recorded 21 measles cases (including cases from this outbreak), all of which were imported or directly linked to imported cases [11, 12].
This report describes the public health response to an outbreak of measles centred on a university in Queensland, Australia. The large size of the university population, extensive social interaction between students on and off campus, as well as the unique characteristics of a university community environment presented challenges for the control of this extremely infectious illness.
Methods
The setting was a university campus located in metropolitan Brisbane, Queensland. Around the time of the outbreak in August 2015, 38 668 students were enrolled in classes at the campus, accompanied by 5621 staff and faculty members. The university community comprised a high proportion (24%) of international students, staff and faculty members. Typical of university campuses, the age profile of the community was young, with only 1% of students and almost 30% of staff and faculty members aged >50 years.
Cases were defined according to the national case definition [8], whereby a confirmed case required laboratory definitive evidence involving at least one of the following: isolation of measles virus, detection of measles virus by nucleic acid testing (NAT), detection of measles virus antigen, IgG seroconversion or detection of measles virus-specific immunoglobulin M in a reference laboratory. Alternatively, case confirmation may have included clinical evidence, together with an established epidemiological link. Clinical evidence required fever, together with a maculopapular rash of >3 days duration and other symptoms such as cough, coryza, conjunctivitis or Koplik spots.
Vaccination records for domestic students were sought using ACIR and the Queensland Vaccine Information and Vaccine Administration System (VIVAS), both established in 1996. Written vaccination documentation was also sought for both domestic and international students where electronic records were unavailable. Those aged 50 years or more at the time of the outbreak were considered likely to have had naturally acquired immunity to measles [8].
Following identification of the index case, assignment of generation of cases was based on the known movements of the likely source case and known exposure profile of the subsequent case, as well as by estimating a plausible interval between period of communicability of the source case and minimum incubation period of 7 days in the subsequent generation. Period of communicability was considered 24 h prior to prodrome (or 4 days prior to rash onset if no prodrome) to 4 days after rash onset.
Results
The outbreak took place over a period of 8 weeks and involved 11 confirmed cases, of which eight were university students and three others had epidemiological links to the university cases (Fig. 1). All cases were laboratory confirmed by NAT. Genotyping was completed for seven cases, including the index case. Measles genotype D8 was identified in specimens from the seven cases, suggesting a common source for the outbreak.
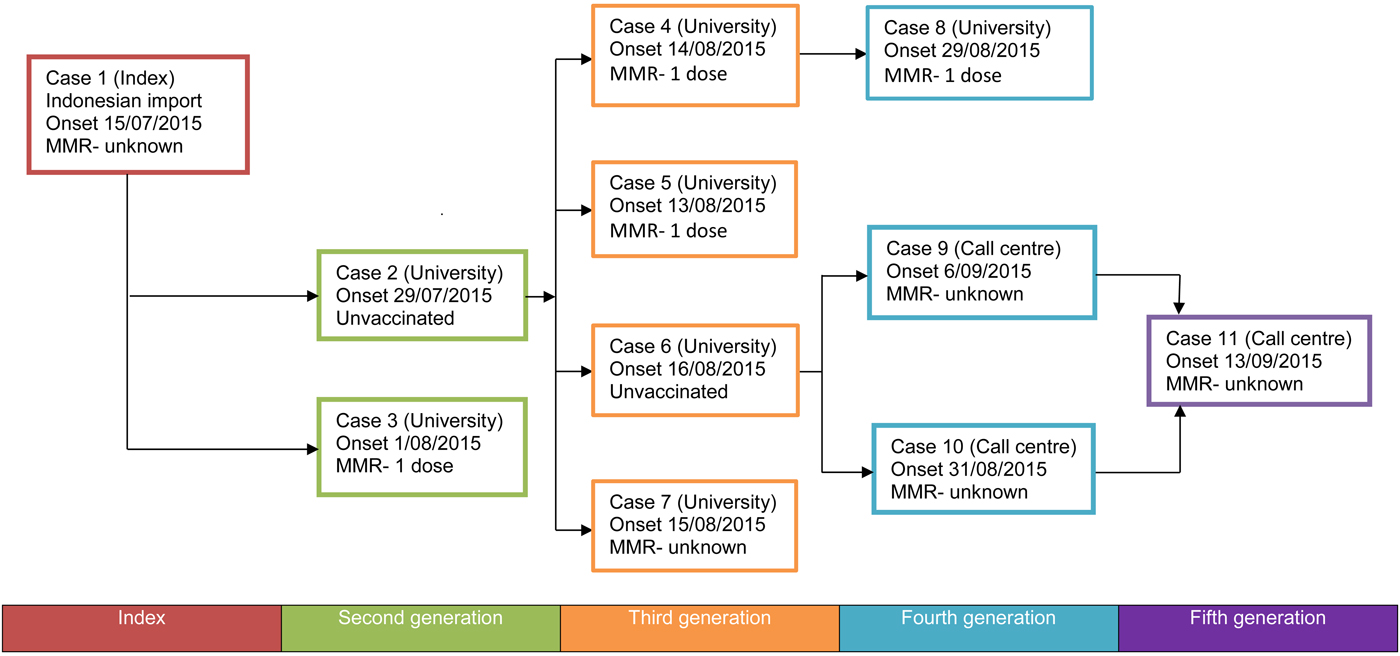
Fig. 1. Flowchart of disease transmission in a measles outbreak, Australia, 15 July 2015 to 13 September 2015. Case numbers were allocated in order of notification to public health units.
Consistent with a university student population, the majority of cases were aged in their late teens and early 20s, with a median age of 21 years (range 17–31 years). Just over half (six of 11 cases) of the cases were female. None of the cases identified as Aboriginal or Torres Strait Islander Australians. Although four of 11 cases presented to hospital emergency departments, notably none of the cases were admitted to hospital and all recovered without significant complications.
The index case was notified to health authorities on 20 July 2015, in a student who had recently travelled to Australia from Indonesia and who became unwell after arriving in Brisbane. While infectious, the student had extensive movements around the university campus. Exposure to other students and staff was compounded by the case's movements coinciding with university enrolment and orientation activities. The vaccination status of the index case was unknown.
Two second-generation cases were confirmed 2 weeks after the index case was notified (Figs 1 and 2). An unvaccinated student (case 2) was diagnosed with measles on 4 August 2015, with measles likely acquired inadvertently from the index case during orientation week. This case lived outside the campus but attended lectures while infectious and prior to the identification of measles. Case 3 was confirmed on 7 August 2015 and had previously received a dose of MMR vaccine in 1998 at age 5 years. It was assumed that case 3 had been in brief social contact with the index case during a meeting at the campus.
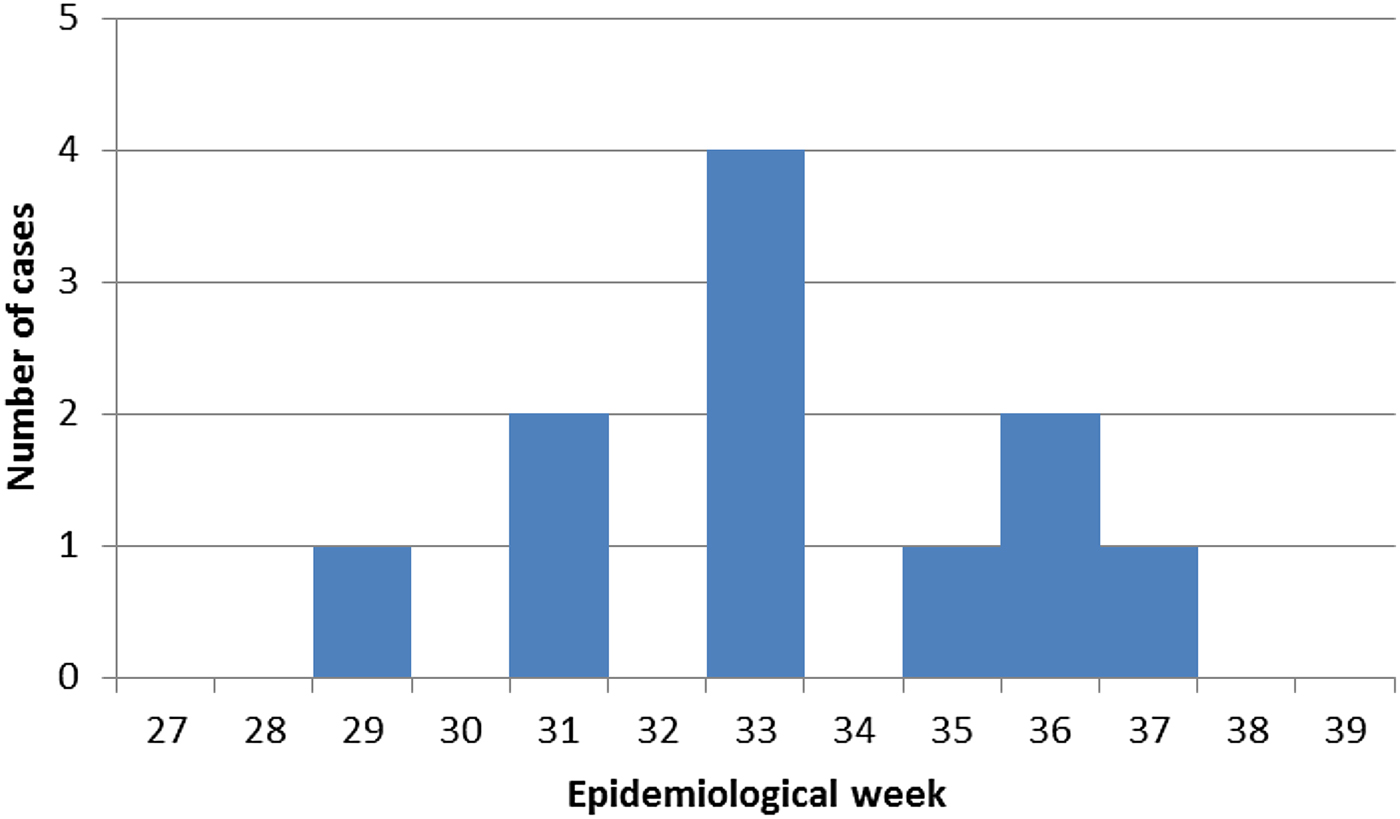
Fig. 2. Epidemic curve for measles outbreak, Australia, 15 July 2015 to 13 September 2015.
Four cases (cases 4–7) were subsequently identified around 2 weeks after the second-generation cases. These third-generation cases all appear to have acquired measles from case 2, as they were part of the same lecture group. Case 4 had received one prior dose of MMR in 1994 aged 1 year and lived in a hall of residence with 165 others. While infectious, case 4 also visited the university medical centre, library and social events. Case 5 had a prior dose of MMR in 1995 aged 1 year and lived outside of the campus. Case 5 had limited close contact with others while infectious. Case 6 was an unvaccinated student who worked at a telephone call centre together with more than 200 other students and young people. The call centre was located offsite from the main university campus. The vaccination status of case 7 was unknown. This person then travelled overseas while infectious.
Three further cases (cases 8–10) were thought to be fourth-generation cases. Case 8 was a university contact of case 4 and travelled to the Gold Coast, a nearby metropolitan centre, while infectious. While written vaccination records were unavailable for case 8, a verbal history of receipt of MCV in Malaysia in early childhood was reinforced by serology consistent with previous vaccination. Case 8 also received a dose of prophylactic MMR 2 days after exposure to case 4. Cases 9 and 10 were workplace contacts of case 6. A single fifth-generation case (case 11) was a workplace contact of cases 9 and 10. Case 11 did not work whilst infectious.
Cases were managed in accordance with national guidelines [8], including intensive contact tracing of identified household, workplace and healthcare contacts. Follow-up included review of electronic vaccination records where available, provision of information about measles and in many cases a recommendation for MMR vaccination for susceptible contacts. Susceptible contacts were defined as people born during or since 1966 without documentary evidence of receipt of at least two doses of MCV, documentary evidence of immunity or laboratory-confirmed history of prior measles. Susceptible household contacts were excluded from attendance at university or work if post-exposure prophylaxis was refused or was not given within acceptable timeframes of 3 days post-exposure for MMR and 6 days for normal human immunoglobulin (NHIG). In line with current national guidelines, NHIG was not used routinely for casual university contacts in the absence of a higher risk profile for more severe disease, such as immunocompromise or pregnancy.
Following identification of cases 4 and 5 in late August 2015, the public health incident response team added targeted vaccination clinics to measles control activities. The clinics were implemented in collaboration with staff from university health services. Two clinics were set up to specifically target students more likely to have been exposed to cases either in the hall of residence or in classes. In addition to the targeted clinics, the university health service provided funded MMR vaccine to all students and staff who presented to the clinics without two documented doses of MCV. By 10 September 2015, a total of 517 students and staff had been vaccinated through the targeted clinics.
Proactive and reactive media accompanied other control activities. Consistent with other measles incidents in Australia generally, this outbreak received substantial media attention at both state and national levels, with media messages focused on advising sick students, staff and faculty members to avoid university attendance and seek medical advice.
A review of immunisation records for classroom contacts of case 3 and those who attended one of the targeted vaccination clinics provided limited insight into pre-existing immunity in this population. Vaccination records were sought for 89 classroom contacts of case 3. The median age of classroom contacts was 22 years (range 20–35 years), while 86% were male. Forty-five of the classroom contacts were born in Australia and had electronic vaccination records, of whom 27 (60%) had two or more doses of MMR recorded and 18 (45%) had a single dose of MMR recorded. Of the 44 contacts born outside Australia, none had any available documentation of previous measles vaccination.
Among 157 people who attended a targeted vaccination clinic at a residential college, 113 (71%) were born in Australia. The median age of residents and staff members at the clinic was 21 years (range 19–56 years). Of those born in Australia, 86 (76%) were either considered likely to be immune due to being aged >50 years in 2015 or having at least two doses of MMR recorded. A further 19 (16%) had one MMR dose recorded.
Discussion
Preventing the spread of measles in a university setting was a public health challenge due to the high population density and close social interaction of the campus community, as well as limited information on vaccination coverage within the university population. Infected students travelled extensively to highly populated areas and attended close proximity social events including lectures, parties, bars and shopping centres. Many students also lived in shared accommodation, characterised by shared kitchen and hygiene facilities.
A single dose of MMR vaccine is expected to provide 95% protection against measles and be 92% effective at preventing the spread of measles to household contacts [13]. Notably, four (36%) of the 11 confirmed measles cases in this outbreak had one previous documented dose of MMR and one of the four had also received MMR for post-exposure prophylaxis. This is a greater proportion than expected. Between 2008 and 2012, 632 measles cases were notified in Australia, of which only 16% were partially vaccinated and 3% were fully vaccinated with two or more doses of MCV [Reference Gidding4]. Indeed, the observation of four cases with prior MCV vaccination may be an underestimate, as only two cases gave histories of not having been vaccinated due to parental objection, while the remaining five cases had no documentary evidence and were therefore classified as having unknown vaccination status. High proportions of vaccinated cases have been reported in previous measles outbreaks, an observation noted in a 1970–1971 outbreak in Texarkana, United States [Reference Landrigan14]. The observation is thought to represent a paradox, whereby a higher level of vaccine coverage within a population reduces the total number of expected cases, but increases the proportion of cases that have been vaccinated. In an outbreak in Pohnpei, a Western Pacific Island with approximately 36 000 residents, 71% of 251 cases had previously received MCV, and of those vaccinated 54% had received at least two doses of vaccine [Reference Hales15]. The pre-existing vaccination coverage for the population of Pohnpei was 85% for a single dose of measles vaccine and 72% for two doses.
At an individual level, primary or secondary vaccine failure may be responsible for development of measles in a previously vaccinated person and hence for the effect seen. In primary failure, the vaccine recipient does not develop a protective level of immunity after vaccination. A potential contributor to primary vaccine failure relates to historic inadequacy of vaccine storage and handling, or ‘cold chain breaches’. The cold chain ensures that vaccines are stored and transported within recommended temperature ranges to ensure maintenance of vaccine potency. Practices have improved significantly in Australia after the national vaccine storage guideline ‘Strive for Five’ was first published in 2003 [16]. All three cases who had previously been vaccinated in Australia received MMR vaccine before the first iteration of ‘Strive for Five’, when vaccine storage and handling practices were acknowledged to have been less rigorous. In secondary vaccine failure, the recipient develops an adequate initial immune response after which immunity wanes over time. De Serres et al. reviewed the largest measles outbreak in North America in recent times, involving 725 cases in Quebec, Canada in 2011 [Reference De Serres17]. Fifty-six per cent of cases were adolescents aged 12–17 years, of whom 25% had received at least one documented dose of MCV and a significant majority (88%) of these vaccine recipient cases had received at least two doses. Among those in a slightly older group aged 20–29 years, 22% had received at least one dose of MCV. The authors suggest secondary vaccine failure as a plausible explanation for residual adolescent susceptibility. The suggestion of waning immunity is supported by a German serosurvey of over 13 000 children aged 1–17 years, where significant waning effects were noted for all three components of MMR vaccine over time [Reference Poethko-Müller and Mankertz18, Reference Poethko-Müller and Mankertz19].
To interrupt endemic transmission and to eliminate measles from a population, a vaccination coverage of 93–95% with two doses of MCV is necessary [Reference Bester20]. Although the presence of an established early childhood measles vaccination programme in Australia may have suggested a high level of MMR coverage among domestic students, the presence of a high proportion of international students meant existing Australian serosurveys and coverage data would not have reliably predicted immunity in the university population. Reviews of vaccination records during the follow-up of university contacts provided only limited additional insight into vaccination coverage in the university setting. Given concerns about unknown levels of vaccination coverage and the associated risk of ongoing transmission, vaccination clinics were established to augment existing public health follow-up that relied on administration of MMR to identified contacts by primary health care. Due to delays between potential exposures and the vaccination clinics of greater than the 3-day timeframe for post-exposure MMR immunoprophylaxis, the purpose of these clinics was to provide protection to individuals in the event of further cases within the broader group rather than to provide post-exposure immunoprophylaxis.
Given the large size of the campus population and limited vaccination resources, a mass vaccination campaign directed at the wider university community was not considered feasible and would have been unlikely to have had a significant effect on overall coverage and hence ongoing transmission. Targeted clinics were therefore used in an attempt to limit further spread of the outbreak within defined groups most likely to have had exposure to measles. Access to vaccination for those at greater risk was optimised by holding the clinics in the buildings where exposure was likely to have occurred. Similar targeted vaccination campaigns have been used successfully in university settings to control both mumps and rubella outbreaks in the United Kingdom [Reference Stevenson21, Reference Kay22]. Students living in university accommodation were primarily targeted in these two outbreaks, with additional vaccine administered via student appointments at a health centre and through opportunistic vaccination at GP surgeries [Reference Kay22]. In this outbreak, the targeted vaccination response was strengthened within the broader university community through provision of opportunistic MMR vaccination by student health services.
A limitation of this outbreak investigation is the potential for under-reporting. All cases in the outbreak met the confirmed measles case definition. In the 2011 Quebec outbreak, active case finding involving questionnaires and telephone calls was undertaken to identify possible missed measles cases [Reference De Serres17]. Multiple additional cases were identified, including vaccinated and unvaccinated cases. Case finding identified 130% more cases among two dose MCV recipients. Some of these individuals met the Canadian national surveillance case definition, while others had milder symptoms and high measles-specific IgG levels, thought to indicate attenuated illness following prior vaccination. This Queensland investigation would have benefitted from more aggressive case finding to ascertain possible missed cases, such as through email questionnaires to students, staff and faculty members circulated via university distribution lists.
Financial costs associated with the response to this outbreak were not measured. Previous estimates of the cost of responding to similar sized measles outbreaks in the university setting in the United States have found such costs to be substantial [Reference Christmas, Mamolen and James23, Reference Birkhead24]. This outbreak placed a significant burden on public health and primary health care services over a protracted period and caused considerable disruption to university activities. The high cost of responding to such events must be acknowledged when considering approaches to the prevention of future university outbreaks.
In the United States, the Advisory Committee on Immunization Practices recommends two doses of MCV prior to matriculation and suggests refusal of potential students with fewer than two doses of vaccine [13]. A review of academic institutions with pre-matriculation immunisation requirements (PIRs) in the United States between 1988 and 1991 found those with state-mandated PIRs were around a third as likely to report measles outbreaks than schools without PIRs [Reference Baughman25]. Enforcement of PIRs is thought to be associated not only with prevention of outbreaks, but with reduced campus disruption and reduced costs associated with outbreak control [Reference Christmas, Mamolen and James23]. Unlike the United States, Australian universities have no policies mandating pre-matriculation immunisation. In 2016, the Queensland Government passed an amendment to the Queensland Public Health Act 2005, to give childcare centres the discretion to refuse enrolment of children on the basis of incomplete vaccinations, known as ‘No Jab, No Play’. However, mandatory vaccination is not a requirement for admission to childcare, primary or secondary school. In the absence of such mandatory requirements, we recommend both domestic and international students ensure their immunisations are up-to-date according to national recommendations, ideally prior to commencement of university. Various activities may be directed towards this goal, including immunisation promotion at university orientation programmes, immunisation recommendations and educational material contained in enrolment packs, ongoing opportunistic vaccination of students attending student health services and provision of funded vaccination clinics for students, staff and faculty.
As well as efforts directed at vaccination coverage, future outbreak responses would benefit from improved access to vaccination records. While vaccination records should be available for domestic students receiving vaccines since 1996 through electronic vaccination databases such as VIVAS, international students should be advised to retain copies of vaccination documentation, in the event that it may be required for public health or other reasons during their course of study. Consideration may also be given to mandating provision of vaccination records by prospective students at the time of enrolment. As occurs in other countries, Australian universities compete for the enrolment of fee-paying domestic and international students. Universities may be reluctant to introduce requirements like mandatory vaccination or vaccination record provision, which may be perceived as barriers to enrolment of potential students. While this outbreak supports the rationale for such measures, there would likely need to be nationwide agreement between universities for the measures, so that no individual university is disadvantaged by their implementation.
Lastly, this outbreak was contained to a small number of only 11 measles cases, despite several of the cases having extensive exposure to others in a range of social settings. While it is tempting to claim the success of intensive contact tracing and targeted immunisation clinics in containing the outbreak, it is likely that high levels of underlying immunity within a well-vaccinated student population had a substantial role in limiting wider spread of this highly infectious illness.
Acknowledgements
The authors acknowledge the assistance of the assistance of staff of the university health service and Queensland Health in responding to the outbreak, as well as Queensland Health Forensic and Scientific Services for assistance with laboratory investigations.
Declaration of Interest
None.
Ethical Standards
All data were collected under the auspices of the Queensland Public Health Act 2005, meaning human research ethics committee review was not required.




